Abstract
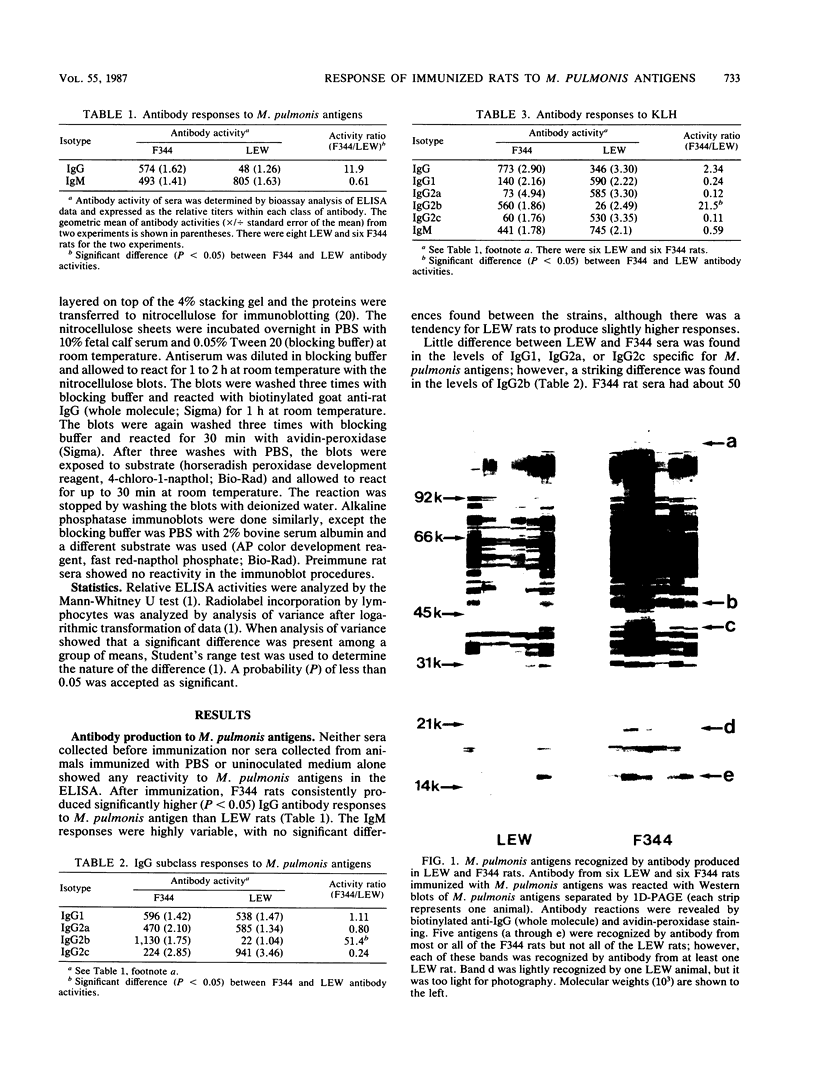
Mycoplasma pulmonis causes a chronic respiratory disease in rats which is more severe in LEW than in F344 rats. This study compared the ability of each of these rat strains to produce specific immune responses to M. pulmonis antigens. By an enzyme-linked immunosorbent assay, LEW rats were found to produce approximately 10 times lower levels of specific immunoglobulin G (IgG) after immunization with M. pulmonis antigens than F344 rats, while no significant difference was found in the levels of IgM. The difference in IgG levels was due to much greater levels of specific IgG2b (about 50 times) in F344 rats; no differences were found in other subclasses. Nonimmune LEW rats were found to have as much total IgG2b in their sera as unimmunized F344 rats by a single radial immunodiffusion test; thus, the difference was not due to the inability of LEW rats to produce IgG2b. In contrast to the antibody response to M. pulmonis antigens, anti-keyhole limpet hemocyanin IgG responses in LEW and F344 rats were similar, but F344 rats produced significantly more (about 21 times) IgG2b than was found in M. pulmonis responses. Antisera from F344 rats recognized several additional M. pulmonis antigens than antisera from LEW rats; however, this could not explain the differences in the level of IgG2b in LEW and F344 rats. In vitro stimulation of splenic lymphocytes with M. pulmonis antigens from immunized F344 rats produced much greater proliferative responses than in LEW and nonimmune F344 cells. Thus, the susceptible rat strain LEW produced lower cellular and humoral immune responses to M. pulmonis antigens than the resistant rat strain F344 after immunization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassell G. H., Davis J. K. Protective effect of vaccination against Mycoplasma pulmonis respiratory disease in rats. Infect Immun. 1978 Jul;21(1):69–75. doi: 10.1128/iai.21.1.69-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K., Davidson M. K., Brown M. B., Mayo J. G. Detection of natural Mycoplasma pulmonis infection in rats and mice by an enzyme linked immunosorbent assay (ELISA). Lab Anim Sci. 1981 Dec;31(6):676–682. [PubMed] [Google Scholar]
- Davis J. K., Cassell G. H. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol. 1982 May;19(3):280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Parker R. F., White H., Dziedzic D., Taylor G., Davidson M. K., Cox N. R., Cassell G. H. Strain differences in susceptibility to murine respiratory mycoplasmosis in C57BL/6 and C3H/HeN mice. Infect Immun. 1985 Dec;50(3):647–654. doi: 10.1128/iai.50.3.647-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Simecka J. W., Williamson J. S., Ross S. E., Juliana M. M., Thorp R. B., Cassell G. H. Nonspecific lymphocyte responses in F344 and LEW rats: susceptibility to murine respiratory mycoplasmosis and examination of cellular basis for strain differences. Infect Immun. 1985 Jul;49(1):152–158. doi: 10.1128/iai.49.1.152-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guidice R. A., Barile M. F. Immunofluorescent procedures for mycoplasma identification. Dev Biol Stand. 1974;23:134–137. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Jones J. M., Jensen F., Feldman J. D. Genetic control of immune responses to Moloney leukemia virus in rats. J Natl Cancer Inst. 1978 Jun;60(6):1467–1472. doi: 10.1093/jnci/60.6.1467. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Nash D. R., Holle B. Local and systemic cellular immune responses in guinea-pigs given antigen parenterally or directly into the lower respiratory tract. Clin Exp Immunol. 1973 Apr;13(4):573–583. [PMC free article] [PubMed] [Google Scholar]
- Rose F. V., Cebra J. J. Isotype commitment of B cells and dissemination of the primed state after mucosal stimulation with Mycoplasma pulmonis. Infect Immun. 1985 Aug;49(2):428–434. doi: 10.1128/iai.49.2.428-434.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]