Summary
Amputation induces substantial reorganization of the hand map in primary sensory cortex (S1 complex, hereafter S1) [1, 2], and these effects of deafferentiation increase with time [3]. Determining whether these changes are reversible is critical for understanding the potential to recover from deafferenting injuries. Earlier BOLD fMRI data demonstrate increased S1 activity in response to stimulation of an allogenically transplanted hand [4]. Here, we report the first evidence that the representation of a transplanted hand can actually recapture the pre-amputation S1 hand territory. A 54 year-old male received a unilateral hand transplant 35 years after traumatic amputation of his right hand. Despite limited sensation, palmar tactile stimulation delivered four months post-transplant evoked contralateral S1 responses that were indistinguishable in location and amplitude from those detected in healthy matched controls. Although commonly reported in amputees [5, 6], we find no evidence for persistent intrusion of representations of the face within the representation of the transplanted hand. Our results suggest that even after decades after complete deafferentiation, restoring afferent input to S1 leads to re-establishment of the gross hand representation within its original territory. Unexpectedly, large ipsilateral S1 responses accompanied sensory stimulation of the patient’s intact hand. These may reflect a change in interhemispheric inhibition that could contribute to maintaining latent hand representations during the period of amputation.
Results
Behavioral
Semmes-Weinstein filament testing revealed two areas on the transplanted hand within which the patient could both detect and localize touch in the absence of vision. One was located on the thenar eminence in the distribution of the median nerve (threshold = 8g), and the second was on the lateral base of the thumb in the radial nerve distribution (threshold = 1.4g). The rest of the hand remained insensitive at this stage of recovery.
fMRI Sensory Mapping in Controls
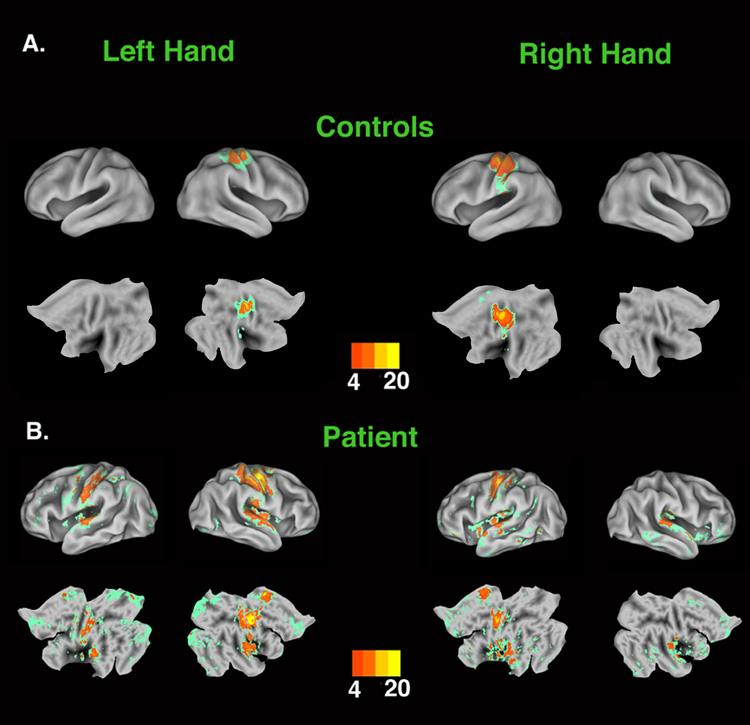
Stimulation of either palm in the control group resulted in increased activation of the contralateral hemisphere within S1 (Figure 1a). Peaks within these S1 clusters were located symmetrically in the central sulci of the two cerebral hemispheres (see Supplemental Data, Table 1), and activity extended rostrally into the precentral gyrus (preCG) and caudally into the postcentral gyrus (postCG). Additionally, stimulation of the left hand increased activity significantly in the contralateral parietal operculum (including putative S2 [7]), while stimulation of the right hand evoked bilateral operculum responses.
Figure 1.
Statistical parametric maps representing areas of increased activity associated with tactile stimulation versus rest. All areas in color showed significant increases in activity during movement (z > 2.3, p <.05 cluster-based correction for multiple comparisons). Areas of peak activation (i.e., z > 4.0) are represented in warm colors corresponding to values indicated in the color bar. Non-peak areas of significantly increased activity (z = 2.3 – 3.99) are represented in green. Mean control data are displayed on the PALs template brain (see Supplementary Data) that has been partially inflated (upper rows) or flattened (lower rows) to visualize activations located in sulcal folds. Patient D.S.’s data are displayed on inflated and flattened surface renderings created from high resolution anatomical images of his own brain. Panel A: Stimulation of the left or right palms of control subjects increased activity in contralateral SI with the peak located in the CS and activity extending rostrally into the the preCG and caudally into postCG. Contralateral activation is also detected in the parietal operculum (including putative SII). During right hand stimulation, left parietal operculum also showed a small but significant increase in activity (Supplementary Data, Table 1). However, due to individual variation in cortical topography, this ipsilateral cluster did not survive the multi-fiducial adjustment for individual differences in cortical topography and is therefore not visible in these surface renderings (see Methods). Lower Panel: Similarly, stimulation of the palms of the transplanted or intact hands evoked peak responses within contralateral SI (see text). In addition, stimulation of the intact left palm was associated with a large region of increased activity along the ipsilateral post-central gyrus. Bilateral responses were also observed in putative SII, CCZ and insula (see text).
fMRI Sensory Mapping in D.S
Similar to controls, stimulation of D.S.’s transplanted right palm produced a focal increase in contralateral S1 and bilateral operculum (Figure 1b; see Supplemental Data, Table 1). In addition, significant increases were detected in two other areas previously implicated in somatosensation, the caudal cingulate zone (CCZ) [8] and caudal insula [9]. Stimulation of D.S.’s healthy left hand was also associated with focal activation of contralateral S1, bilateral operculum, CCZ and insula. Unexpectedly, a large region of increased activity was detected along the length of the ipsilateral postCG (Figure 1b). The peak of this activation was located on the caudal bank of the postCG (−62, −18, 44), which corresponds to cortical areas 1 [10] and/or 2 [11] of the S1 complex defined probabilistically. This contrasts with previous findings of small ipsilateral S1 responses to tactile hand stimulation in healthy adults [12]. The coordinates of this peak are located lateral, anterior and inferior to those associated with stimulation of either D.S.’s (−40, −20, 54) or the control group’s (−38, −28, 58) right palms. Significant increases in ipsilateral activation of the lateral postCG were not observed during stimulation of the transplanted right hand, or of either hand in the control group. Transformation of D.S.’s data into a flattened standard space map illustrates the high degree of overlap with the contralateral activations detected in the control group during sensory stimulation of either hand (Figure 2). It can also be seen that relative to controls, substantially larger regions of the parietal operculum were engaged bilaterally during stimulation of either D.S.’s left or right palms.
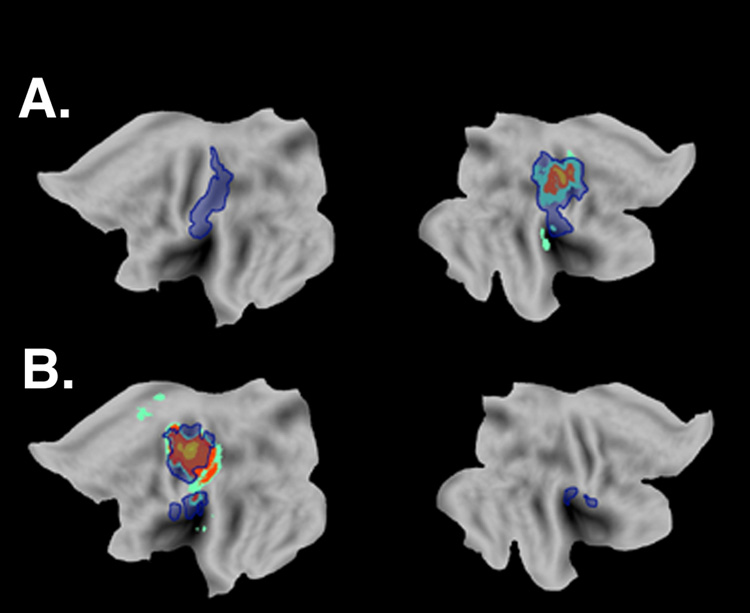
Figure 2.
Activations associated with hand stimulation in D.S. and controls represented in standard space. The multi-fiducial mapping procedure [29] was used to transform data from patient D.S. (blue overlay) into standard space for direct visual comparison with mean activations of the control group (see Supplementary Data). For D.S. all significant activations within preCG, postCG, parietal operculum and insula are included (see Figure 1). These flattened representations illustrate the high degree of overlap between D.S.’s contralateral SI responses and those of the control group. Panel A. Responses associated with stimulation of the left palm. In contrast to controls, stimulation of D.S.’s left palm elicited a strong ipsilateral response along the length of the postCG extending into the operculum. Panel B. Responses associated with stimulation of the right palm. Note the high degree of overlap between data from D.S. and control participants.
Activity Within the Normal Hand S1 Hand Representation
Activation foci in the control group sensory mapping data were used to define functionally the normative (i.e., pre-amputation) S1 hand representations. Stimulation of D.S.’s transplanted right hand evoked a response that is comparable to that of the control group within the right hand territory (Figure 3a). Stimulation of his left hand caused a modest ipsilateral increase in activity within this region that exceeded that of the controls. Stimulation of D.S.’s left hand likewise produced a response comparable to that of controls within the left hand representation (Figure 3b). Stimulation of the cheeks, however, did not evoke a strong response in either hand representation for D.S. or the control group.
Figure 3.
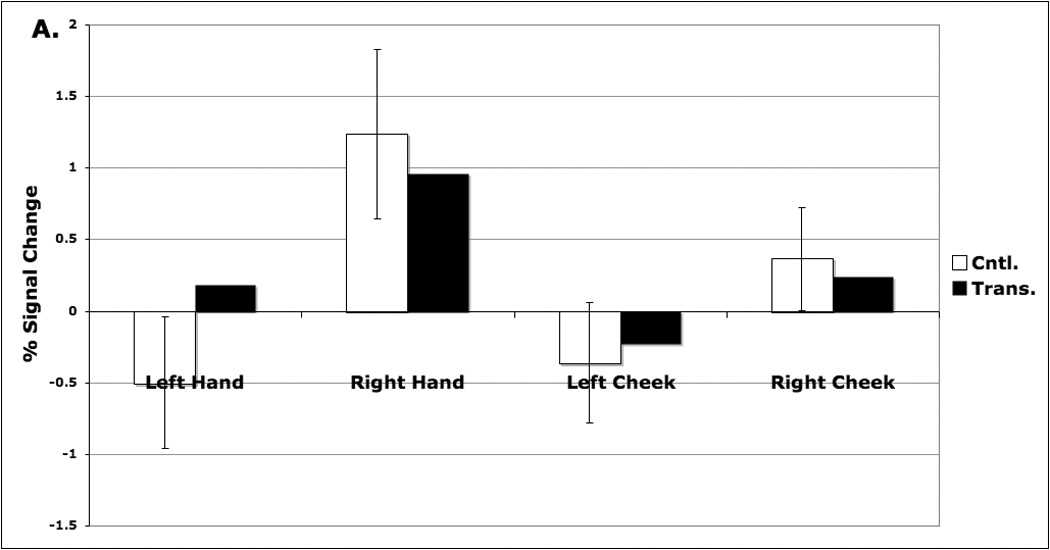
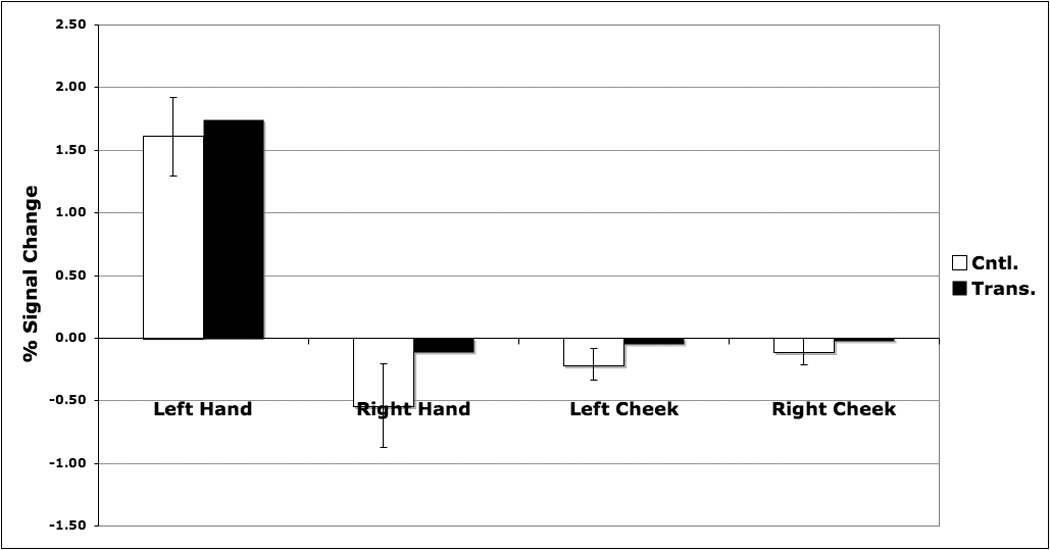
Percent signal change associated with sensory stimulation of the palms or cheeks within normal SI hand representations defined functionally on the basis of control data (see Methods). Panel A. Left hemisphere: S1 hand representation. Within the right hand S1 representation, D.S. and controls showed a strong increase during stimulation of the right hand. D.S. also showed a modest increase in activity during stimulation of the left hand (see text). Panel B. Right hemisphere: S1 hand representation. In the left hand S1 representation, both D.S. and controls showed comparable increases in activity during stimulation of the contralateral left hand. D.S. showed no greater response to stimulation of the cheeks than controls in either hemisphere.
Discussion
These experiments yielded two major findings. First, we found that even 35 years post-amputation, the gross S1 hand representation in the mature brain have a remarkable capacity to return to a state of organization that is indistinguishable from what would be expected prior to hand loss. This is extraordinary considering the well-established reorganizational changes that occur following deafferentiation [1, 2], and the fact that these effects increase with time [3]. Second, in addition to these apparently normal contralateral S1 responses, we found unexpectedly large responses in the ipsilateral preCG and/or postCG during stimulation of D.S.’s intact hand (Figure 1 & Figure 2). Both findings greatly extend our understanding of the ability of the cerebral cortex to respond adaptively to dramatic changes in stimulation even when fully mature. We discuss these two points in detail below.
Typical Sensory Cortical Organization
Previously, it was demonstrated that stimulation of a transplanted hand two years after amputation evoked a strong contralateral S1 response [4]. Control data was not used to functionally define the territory of the normative (i.e., pre-amputation) hand representation. However, the center of gravity (COG) of the S1 activation was reported to become increasingly disparate from that of healthy controls during the 4 months immediately following the transplant ([4]; their Table 2). This may indicate recruitment of sensory regions other than those formerly devoted to the patient’s birth hand. Unfortunately, the interpretation of this finding is complicated because the COG was computed across a large activation cluster that included not only S1, but also the parietal operculum and insula. By contrast, we used data from matched controls to functionally define the normative S1 hand territory. Four months after the procedure, we found that stimulation of the transplanted palm evoked responses that were comparable in peak location, spatial extent and amplitude to those detected in control participants (Figure 1 – Figure 3; see Supplemental Data, Table 1).
At the time of testing, D.S.’s sensitivity was limited to a relatively small portion of the grafted hand. This suggests that the recovery of the S1 hand territory may therefore precede increasing sensitivity in the grafted hand. It is important to note that at this stage of recovery we cannot rule the out possibility that the individual digit representations within the S1 hand map remain disorganized. In fact, this seems quite likely based on studies of non-human primates. The loss of topographical order in the peripheral nerves, caused by median nerve transection and surgical repair, leads the recovered cortical representation of the reinnervated hand in adult monkeys to be intrinsically disordered [13]. Additional work is underway to evaluate these predictions.
Responses within the normative S1 hand territory to stimulation of patient D.S.’s cheeks were minimal and did not differ from controls (Figure 3). This has not been previously studied in transplant patients. However, chronically deafferented monkeys [3] and human amputees [6, 14] do show evidence for encroachment of the face representation into the former hand territory. It is widely held that the somatotopic organization of S1 is maintained through competitive interactions [1, 2]. During the 4 post-operative months, increasing afferent input from the transplanted hand may therefore have effectively reclaimed the hand territory from the face. Due to the absence of pre-transplant data, however, we cannot rule out the possibility that for unknown reasons D.S.’s face representation simply did not shift into the hand territory following amputation. Reorganizational changes in S1 somatotopy appear to be more pronounced in amputees with significant phantom pain [5], and D.S. suffered minimally from this syndrome.
The mechanisms responsible for the recovery of a grossly normal S1 hand representation remain uncertain, and changes occurring subcortically in the thalamus and/or brainstem may play an important role [1]. One possibility is that, despite the dynamic functional rebalancing of inhibitory and excitatory synapses that occurs following deafferentiation [15], a latent representation of the gross organization of the hand is retained in the pattern of S1 neuronal connectivity. When afferent input is re-established post-transplant, this latent representation may be actively recovered. A parallel might be drawn here with the rodent sensory cortex where adult whisker amputation alters the receptive field properties of barrel cells, but appears to leave the architecture of the cortical map intact [16, 17]. Yet, peripheral injuries in primates are accompanied by growth of intracortical (but not thalamocortical) connections, which may play a role in map reorganization [18]. The impact of hand allotransplantation on these changes remains unknown.
Ipsilateral Sensory Responses
In addition to the normal contralateral responses, we found that sensory stimulation (Figure 1 & Figure 2) of D.S.’s intact hand evoked strong responses in the ipsilateral postCG. These activations were not, however, located predominantly within the region that would have represented the right hand prior to amputation (Figure 3a). This ipsilateral response was much larger than reported in previously in healthy adults [12], and was not apparent for D.S.’s transplanted hand or for either side in the control group.
It is possible that this reflects a functional adaptation to the chronic imbalance in the somatosensory system created by unilateral S1 deaffrerentiation. Some neurons in the S1 complex (area 2) have strong bilateral receptive fields and fire in response to stimulation of the ipsilateral hand via transcallosal connections [19]. Left and right S1 areas are known to also be connected via Inhibitory transcallosal pathways [20]. Previous results show that acute deafferentiation induces bilateral reorganizational changes in S1 [21, 22]. Long-standing amputation could decrease the normal level of interhemsipheric inhibition and thereby facilitate transcallosal neural activity coming from the primary representation of the intact hand. If so, then we predict that these ipsilateral responses will decrease as D.S.’s sensory recovery progresses.
In conclusion, allogenic hand transplantation provides an unprecedented opportunity to investigate the reversibility of changes in cortical organization that follow amputation. Our findings suggest that these changes are indeed highly reversible even in the mature brain; a fact that may have broad implications for our understanding of the brain’s response to deafferenting injuries.
Experimental Procedures
Subjects
D.S. is a male who suffered a wrist-level traumatic amputation of his dominant right hand at age 19 years, 7 months. Early in his recovery he experienced phantom sensations and pain, but these dissipated with time. He regularly wore a mechanical prosthesis for work. Thirty-five years after this accident, at age 54 years, he underwent a successful allogenic transplantation. D.S. underwent testing four months after the procedure. Data was also acquired from 4 age- (M = 54 years, SD = 4.9), sex-, and handedness-matched control participants. All subjects gave informed consent prior to their participation.
fMRI Sensory Mapping Task
Subjects each completed two 12:48 blocks with their eyes closed. Blocks were divided into four 3:12 cycles. Within each cycle, four body parts (left hand, right hand, left cheek or right cheek) were stimulated in pseudo-randomized order. Each period of stimulation lasted for 24s and was followed by a rest interval of equal duration. The palmar surface of each hand was stroked with a coarse sponge at a rate of 1Hz. Movements were unidirectional, sweeping from proximal (heel of hand) to distal (fingertips). Previous work shows that moving stimuli are an effective means of mapping human S1 [23], and this sponging technique has been used previously with healthy adults [24] and a hand transplant patient [4], and has been shown to stimulate S1 [25] and S2 [26] neurons in monkeys effectively. Unilateral cheek stimulation was delivered separately to each cheek at a rate of 1Hz.
fMRI Data Acquisition
A Siemens’ 3-Tesla Allegra MRI scanner was used to collect BOLD echoplanar images (EPI) using a T2*-weighted gradient echo sequence with prospective acquisition correction (PACE) [27].
fMRI Data Analysis
All data preprocessing (EPI dewarping, motion correction, brain extraction, spatial smoothing, registration and normalization) and modeling was conducted with FEAT (FMRI Expert Analysis Tool) Version 5.91 in the FSL image processing tools (http://www.fmrib.ox.ac.uk/fsl/). Surface reconstruction and visualization and was accomplished with version 5.5 of the CARET software http://brainmap.wustl.edu/caret/ [28].
Functionally Defining Normative Hand Representations
A multi-step procedure was used to define the spatial extent of normal hand motor and sensory maps conservatively on the basis of mean control group data. First, the overall maximum z-value was identified within conditions involving the hands. Second, statistical parametric maps for both left and right hand conditions were thresholded at 50% of this maximum z-value. All voxels surviving this thresholding procedure were defined as being within the normal left or hand representation.
Further methodological details are provided in the Supplemental Data online.
Supplementary Material
Acknowledgements
This work was supported in part by grants from the M.J. Murdock Charitable Trust, NIH/NINDS (NS053962), USAMRMC (W81XWH-07-2-0092 & W81XWH-07-1-0185), and the ONR (N000140610084). Opinions, interpretations, conclusions and recommendations are those of the authors and not necessarily endorsed by the U.S. Army or the U.S. Navy. We appreciate the helpful comments of Dr. Christina Kaufman and anonymous reviewers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones EG. Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Annual Review of Neuroscience. 2000;23:1–37. doi: 10.1146/annurev.neuro.23.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- 3.Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 4.Neugroschl C, Denolin V, Schuind F, Van Holder C, David P, Baleriaux D, Metens T. Functional MRI activation of somatosensory and motor cortices in a hand-grafted patient with early clinical sensorimotor recovery. Eur Radiol. 2005;15:1806–1814. doi: 10.1007/s00330-005-2763-4. [DOI] [PubMed] [Google Scholar]
- 5.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 6.Yang TT, Gallen CC, Ramachandran VS, Cobb S, Schwartz BJ, Bloom FE. Noninvasive detection of cerebral plasticity in adult human somatosensory cortex. Neuroreport. 1994;5:701–704. doi: 10.1097/00001756-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- 8.Kwan CL, Crawley AP, Mikulis DJ, Davis KD. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain. 2000;85:359–374. doi: 10.1016/S0304-3959(99)00287-0. [DOI] [PubMed] [Google Scholar]
- 9.Gelnar PA, Krauss BR, Szeverenyi NM, Apkarian AV. Fingertip representation in the human somatosensory cortex: an fMRI study. Neuroimage. 1998;7:261–283. doi: 10.1006/nimg.1998.0341. [DOI] [PubMed] [Google Scholar]
- 10.Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- 11.Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- 12.Hansson T, Brismar T. Tactile stimulation of the hand causes bilateral cortical activation: a functional magnetic resonance study in humans. Neurosci Lett. 1999;271:29–32. doi: 10.1016/s0304-3940(99)00508-x. [DOI] [PubMed] [Google Scholar]
- 13.Florence SL, Garraghty PE, Wall JT, Kaas JH. Sensory afferent projections and area 3b somatotopy following median nerve cut and repair in macaque monkeys. Cereb Cortex. 1994;4:391–407. doi: 10.1093/cercor/4.4.391. [DOI] [PubMed] [Google Scholar]
- 14.Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. Neuroreport. 1994;5:2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- 15.Kaas JH. The reorganization of somatosensory and motor cortex after peripheral nerve or spinal cord injury in primates. Prog Brain Res. 2000;128:173–179. doi: 10.1016/S0079-6123(00)28015-1. [DOI] [PubMed] [Google Scholar]
- 16.Fox K, Glazewski S, Schulze S. Plasticity and stability of somatosensory maps in thalamus and cortex. Curr Opin Neurobiol. 2000;10:494–497. doi: 10.1016/s0959-4388(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 17.Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 19.Iwamura Y, Iriki A, Tanaka M. Bilateral hand representation in the postcentral somatosensory cortex. Nature. 1994;369:554–556. doi: 10.1038/369554a0. [DOI] [PubMed] [Google Scholar]
- 20.Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calford MB, Tweedale R. Interhemispheric transfer of plasticity in the cerebral cortex. Science. 1990;249:805–807. doi: 10.1126/science.2389146. [DOI] [PubMed] [Google Scholar]
- 22.Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–938. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- 23.Bodegard A, Geyer S, Naito E, Zilles K, Roland PE. Somatosensory areas in man activated by moving stimuli: cytoarchitectonic mapping and PET. Neuroreport. 2000;11:187–191. doi: 10.1097/00001756-200001170-00037. [DOI] [PubMed] [Google Scholar]
- 24.Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418:1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Werner G, Whitsel BL. Topology of the body representation in somatosensory area I of primates. J Neurophysiol. 1968;31:856–869. doi: 10.1152/jn.1968.31.6.856. [DOI] [PubMed] [Google Scholar]
- 26.Whitsel BL, Petrucelli LM, Werner G. Symmetry and connectivity in the map of the body surface in somatosensory area II of primates. J Neurophysiol. 1969;32:170–183. doi: 10.1152/jn.1969.32.2.170. [DOI] [PubMed] [Google Scholar]
- 27.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.