Abstract
Background
Both heart failure (HF) and chronic kidney disease (CKD) are highly prevalent conditions that often coexist, however, the quality of care received by hospitalized patients with both is not known.
Methods
The Get With The Guidelines-HF registry and performance improvement program prospectively collects data on patients hospitalized with HF. Performance measures to improve HF patient treatment and in-hospital mortality were examined by kidney function based on glomerular filtration rate (GFR) categorized as normal (GFR≥90), mild (60≤ GFR<90), moderate (30≤GFR< 60), severe (15≤GFR< 30), and kidney failure (GFR<15 or dialysis).
Results
Nearly two-thirds of hospitalized patients with HF (15,560 patients from 137 hospitals) also had CKD: moderate CKD (43.9%), severe CKD (14.2%), and kidney failure (6.6%). Inpatient mortality was higher for patients with more severe renal dysfunction. Those with kidney failure were significantly less likely to receive nearly all guidelines-based therapies. In contrast, those with moderate or severe CKD often received similar care when compared to those with normal kidney function, except for lower use of angiotensin converting enzyme inhibitors or receptor blockers [OR 0.19 (0.13, 0.28) and 0.47 (0.36, 0.62), respectively] and lower proportions with blood pressure control [OR 0.70 (0.58, 0.85) and 0.52 (0.42, 0.63), respectively].
Conclusions
In a large contemporary cohort of patients hospitalized with HF, we found that renal dysfunction was a highly prevalent comorbidity. Despite higher mortality rates, patients with increased severity of renal dysfunction were less likely to receive important guideline-recommended therapies. Further efforts are needed to improve the care of patients with HF and CKD.
Keywords: heart failure, chronic kidney disease, quality of care, guidelines
INTRODUCTION
Heart failure (HF) continues to be a national epidemic with an increasing prevalence, affecting 5.2 million persons in the United States or 2.5% of the adult population,1 and an incidence that has not declined in the past two decades.2 Hospitalizations for heart failure continue to increase1 and HF is a leading cause of hospitalization among the elderly. The estimated direct and indirect cost of HF in the US for 2007 is $33.2 billion.1 Management of patients using evidence-based therapies is crucial to improving clinical outcomes and decreasing human and economic costs resulting from HF.3
Chronic kidney disease (CKD) is also a national epidemic with an increasing prevalence now affecting 16.8% of the adult population,4 and an incidence that continues to rise.4 CKD is a common comorbidity among patients with HF and is independently associated with increased morbidity and mortality among patients with HF.5 The co-existence of heart failure and CKD is believed to increase risk through a greater burden of comorbidities, increased toxicity from diagnostic and therapeutic procedures, accelerated atherosclerosis, or underuse of medications and non-pharmacologic treatments that have been studied extensively and recommended in national HF guidelines.3
Despite the higher mortality risk among patients with heart failure and CKD, several studies demonstrate that effective therapies are dramatically underused.5 In response, several quality improvement efforts have been launched.7 Get With The Guidelines-Heart Failure (GWTG-HF) is the American Heart Association’s in-hospital process for continuous quality improvement that seeks to optimize HF patient treatment.7 GWTG-HF focuses on care team protocols to ensure that patients are treated and discharged on appropriate medications and with risk modification counseling. However, a more broad assessment of the quality of care received by hospitalized patients with HF and CKD has not been performed. In addition, as HF management has improved over time, contemporary in-hospital outcomes for patients with HF and CKD are also not known.
METHODS
Overview of GWTG-HF
GWTG-HF is an ongoing Web-based registry and performance improvement initiative to enhance guideline adherence in patients hospitalized with HF. The overall GWTG-HF program objectives have been described previously in other publications and for the program’s predecessor, the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF).7,8 Briefly, the GWTG-HF registry is a voluntary quality improvement program that gathers data from the medical record through the use of a Web-based case report form on patient characteristics and elements of heart failure management that are provided during admission and at discharge. Previous studies of OPTIMIZE-HF and other HF registries have shown that patients admitted with HF have similar baseline characteristics to patients in datasets from the entire country, suggesting that data from registry hospitals are likely to be representative of national trends and practices.9
Patient characteristics, laboratories, diagnostic tests, treatments, and in-hospital outcomes were prospectively collected for 17,785 patients from 137 hospitals that participated in GWTG-HF and utilized the Patient Management Tool™ (Outcome, Cambridge, MA) for data collection between January 2005 and March 2006. Eligibility for the GWTG-HF registry required that patients be adults hospitalized with an episode of new or worsening HF as the primary reason for admission, or with significant heart failure symptoms that developed during hospitalization in which HF was the primary discharge diagnosis. The analysis sample included 15,560 patients after excluding patients who did not have the data needed to estimate renal function (age, gender, race, serum creatinine) (n=3,759).
Admission staff, medical staff, or both recorded data on consecutive eligible admissions according to established Joint Commission methods after the protocol was approved by each participating center’s institutional review board. The coordinating center for the registry was Outcome (Cambridge, MA) and study data were analyzed at the Duke Clinical Research Institute (Durham, NC).
Performance Measures
The heart failure performance measures utilized by GWTG-HF and the inpatient measure descriptions include the following:
Discharge instructions: “HF patients discharged home with written instructions or educational material given to patient or caregiver at discharge or during the hospital stay addressing all of the following: activity level, diet, discharge medications, follow-up appointment, weight monitoring, and what to do if symptoms worsen.” 10
Evaluation of left ventricular systolic function: “HF patients with documentation in the hospital record that left ventricular systolic function was assessed before arrival, during hospitalization, or is planned after discharge.” 10
Angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for left ventricular systolic dysfunction: “HF patients with left ventricular systolic dysfunction and without both ACEI and ARB contraindications who are prescribed an ACEI or ARB at hospital discharge.” 10
Adult smoking cessation advice/counseling: “HF patients with a history of smoking cigarettes, who are given smoking cessation advice or counseling during hospital stay.” 10
β-blocker use at discharge for HF patients with left ventricular systolic dysfunction without documented β-blocker contraindications or intolerance. 3
Three additional quality measures of interest were also assessed: 1) Anticoagulant at discharge for patients with atrial fibrillation: “HF patients with chronic/recurrent atrial fibrillation and without warfarin contraindications who are prescribed warfarin at discharge”;10 2) evidence-based β-blocker (bisoprolol fumarate, carvedilol, metoprolol succinate) prescribed at discharge for patients with left ventricular systolic dysfunction;11 and 3) the proportion of patients with a last recorded blood pressure less than 140/90 at discharge.3
The first four performance measures comprise those advanced by the Joint Commission,12 used and publicly reported by federal agencies such as the Centers for Medicare and Medicaid Services,13 and assessed by major insurers as criteria for pay-for-performance demonstration projects. The second and third performance measures were part of the Health Quality Alliance process measures that were included in the “starter set”.14 Although the fifth performance measure is not currently a national performance measure, recent evidence suggests that it should be considered. In a large prospective study of patients with HF, the performance measures with the strongest process-outcome links in the first 60–90 days after hospital discharge were use of ACEI or ARB and β-blockers in eligible patients.7
Performance measures were constructed using the numerator and denominator definitions defined by clinical performance measures for adults with HF10 and Joint Commission ORYX specifications11 assessing use rates among eligible patients without documented contraindications, intolerance, or other physician documentation. An example of such an exclusion for performance measurement included the documented presence of hyperkalemia among patients with an indication for ACEI or ARB therapy. Patients with HF who had left ventricular function assessed and left ventricular ejection fraction of less than 40% or moderate to severe systolic dysfunction were included for the ACEI or ARB and β-blocker performance measures. The measures for complete discharge instructions, smoking cessation counseling, blood pressure control and anticoagulation for atrial fibrillation apply to eligible patients irrespective of left ventricular function. A composite performance measure was created to describe 100% compliance among eligible patients with the first four performance measures that comprise those advanced by the Joint Commission.
Kidney Function
Estimates of glomerular filtration rate (GFR) were calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) formula:15 Estimated GFR (mL/min/1.73 m2) = 186 × [serum creatinine]− 1.154 × age−0.203 × [0.742 if female] × [1.21 if black]. If the race variable was reported as any of the following, estimation of GFR was performed with white race by default: Asian/Pacific Islander (n=273; 1.75%), Hispanic (n=925; 5.94%), American Indian/Alaskan (n=59; 0.38%), or other (n=69; 0.44%). We then categorized patients according to kidney function (GFR in mL/min/ 1.73 m2) by using modified definitions from the National Kidney Foundation – Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines:15 normal (GFR ≥ 90), mild (60 ≤ GFR < 90), moderate (30 ≤ GFR < 60), severe (15 ≤ GFR < 30), and kidney failure (GFR < 15).
Statistical Analysis
For descriptive analyses, medians and interquartiles (the 25th and 75th percentiles) were reported for continuous variables and percentages for categorical variables. Comparisons between patients who were in groups of different levels of kidney function were made using Pearson chi-square test for categorical variables and Kruskal-Wallis test for continuous variables.
Multivariable regression analysis was performed to examine the effect of different levels of renal function on use of evidence-based treatment and mortality. The models adjust for potential confounder factors including age, gender, race, coronary artery disease/ischemic etiology, anemia, cerebrovascular accident/transient ischemic attack, diabetes, hyperlipidemia, hypertension, pulmonary disease and ejection fraction. Generalized estimating equations were used to account for within-hospital clustering, because patients at the same hospital are more likely to be treated similarly relative to patients in other hospitals.
All statistical analyses were performed using SAS software (version 8.2, SAS Institute Inc, Cary, NC).
RESULTS
Baseline Characteristics
The distribution of kidney function among the study patients (N=15,560) who were enrolled from 137 hospitals revealed that a majority had some degree of CKD: moderate (43.9%), severe (14.2%), and kidney failure (6.6%) (Table 1). The average age was 73 years, 50% were female, 74% of the patients were white, and almost one-fifth were African American. Forty-seven percent of patients had an ischemic etiology for their heart failure, 70% had hypertension, and 42% had diabetes mellitus. Of the 12,281 patients (79%) with assessment of left ventricular function, the overall ejection fraction was 35% with 25th and 75th interquartiles of 25 and 53%. Forty-four percent of patients had systolic dysfunction.
Table 1.
Baseline Patient Characteristics by Kidney Function Group
| Total (N=15,560; 100%) | Normal (N=1,317; 8.5%) | Mild (N=4,169; 26.8%) | Moderate (N=6,834; 43.9%) | Severe (N=2,214; 14.2%) | Kidney failure (N=1,026; 6.6%) | P-value | |
|---|---|---|---|---|---|---|---|
| Age | 76 (64, 83) | 63 (52, 78) | 73 (60, 82) | 79 (69, 85) | 78 (70, 84) | 70 (57, 79) | <0.0001 |
| Male | 49.9 | 56.8 | 55.5 | 47.6 | 43.8 | 47.2 | <0.0001 |
| Race | |||||||
| Caucasian | 73.8 | 61.2 | 70.4 | 79.1 | 78 | 58.5 | <0.0001 |
| American African | 17.7 | 29.4 | 21.4 | 13.5 | 13.5 | 25.0 | |
| Asian | 1.8 | 1.5 | 1.5 | 1.6 | 1.9 | 4.0 | |
| Hispanic | 5.9 | 6.9 | 5.7 | 5.1 | 6.0 | 11.5 | |
| Other | 0.8 | 1.0 | 0.9 | 0.7 | 0.6 | 1.1 | |
| Medical history | |||||||
| Anemia | 15.5 | 9.5 | 9.9 | 16.0 | 24.5 | 24.0 | <0.0001 |
| Atrial fibrillation | 30.2 | 21.0 | 29.1 | 34.3 | 31.1 | 17.1 | <0.0001 |
| Coronary artery disease | 47.8 | 38.0 | 42.8 | 51.4 | 53.0 | 44.5 | <0.0001 |
| Cerebrovascular disease | 14.2 | 10.6 | 13.2 | 15.3 | 14.6 | 14.0 | 0.0008 |
| Diabetes | 42.2 | 38.4 | 36.2 | 42.1 | 51.9 | 51.8 | <0.0001 |
| Hyperlipidemia | 33.5 | 26.4 | 31.7 | 35.3 | 37.0 | 29.8 | <0.0001 |
| Hypertension | 70.1 | 63.9 | 68.4 | 70.1 | 72.7 | 79.2 | <0.0001 |
| Pulmonary | 26.2 | 27.3 | 26.5 | 26.7 | 25.8 | 20.9 | <0.0001 |
| Peripheral Vascular Disease | 11.6 | 6.9 | 8.8 | 12.0 | 16.4 | 16.1 | <0.0001 |
| History of Smoking | 14.4 | 28.6 | 18.7 | 10.7 | 8.9 | 15.1 | <0.0001 |
| Ischemic etiology for heart failure | 46.6 | 39.9 | 41.6 | 50.6 | 52.2 | 37.1 | <0.0001 |
| Admission data | |||||||
| Body mass index | 27.5 (23.7, 33.2) | 28.6 (23.9, 36.3) | 27.8 (23.8, 33.6) | 27.4 (23.7, 32.8) | 27.5 (23.7, 32.9) | 26.5 (23.1, 31.3) | <0.0001 |
| Systolic blood pressure | 138 (118, 160) | 139 (120, 160) | 139 (120, 160) | 136 (117, 157) | 134 (113, 158) | 149 (122, 180) | <0.0001 |
| Ejection fraction (%) | 35 (25, 53) | 35 (20, 53) | 35 (24, 51) | 35 (25, 54) | 39 (25, 54) | 43 (30, 55) | <0.0001 |
| Serum Creatinine (mg/dL) | 1.3 (1.0, 1.9) | 0.7 (0.6, 0.8) | 1.0 (0.9, 1.1) | 1.4 (1.3, 1.7) | 2.5 (2.1, 2.9) | 5.8 (4.5, 8.3) | <0.0001 |
| Hemoglobin (g/dL) | 12.1 (10.6, 13.5) | 12.8 (11.2, 14.0) | 12.7 (11.4, 14.1) | 12.0 (10.7, 13.4) | 11.0 (9.9, 12.3) | 11.4 (10.0, 12.6) | <0.0001 |
Participating hospitals in this analysis had the following characteristics: median bed size 330 (interquartile range, 194 to 486), 56.2% academic, 12.7% heart transplant center, 66.1% interventional procedures (performed percutaneous coronary intervention and/or coronary artery bypass graft surgery), and diverse regional distribution (19.8% northeast, 30.1% midwest, 36.2% south, and 13.9% west).
In-hospital Mortality
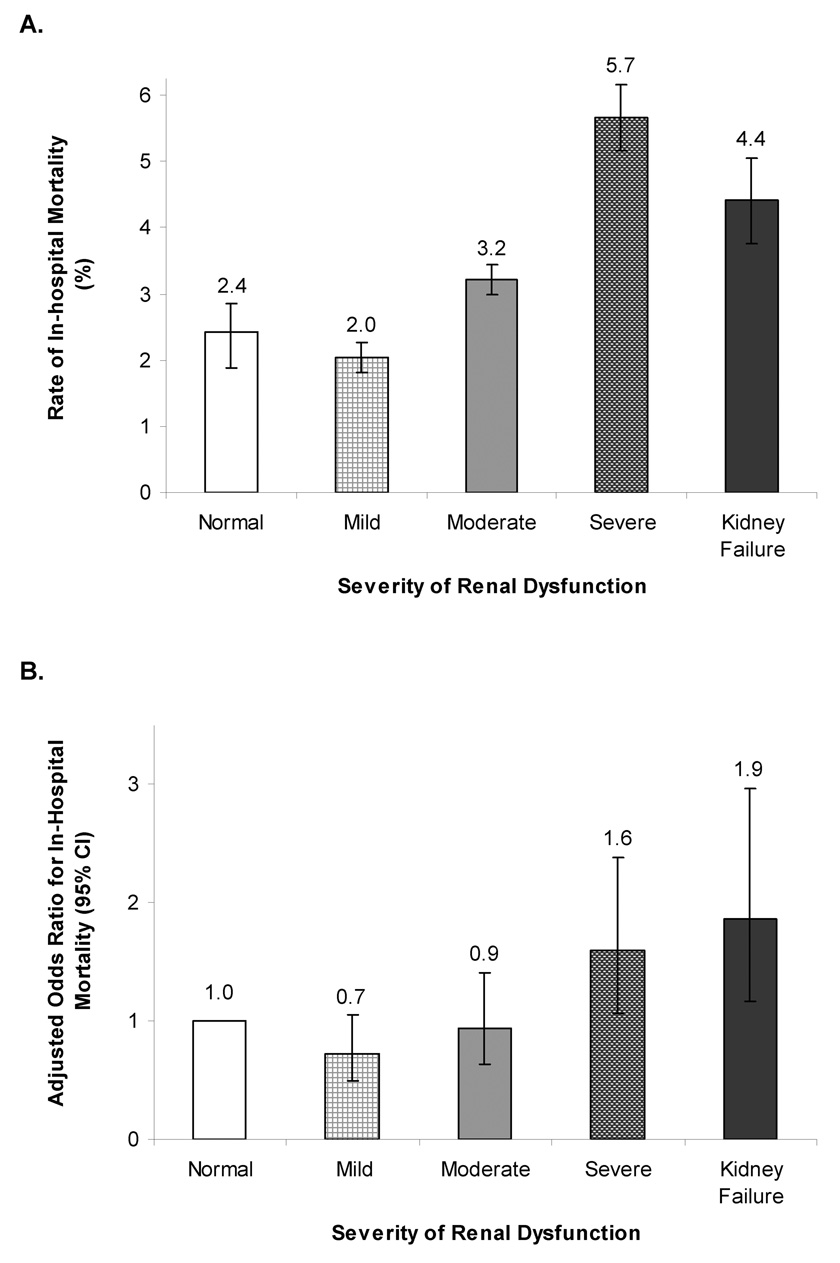
There were 495 in-hospital deaths (3.3%) during the study. Inpatient mortality was higher for patients with more severe CKD (Figure 1a). After multivariable adjustment for patient characteristics, higher mortality rates persisted among patients with severe kidney disease and renal failure (Figure 1b).
Figure 1. Unadjusted and Risk-Adjusted In-hospital Mortality.
Unadjusted rates of in-hospital mortality (A) increased with more severe renal dysfunction and were highest among those with severe renal dysfunction. After multivariable adjustment (B), in-hospital mortality rates increased with more severe renal dysfunction with the highest rates among patients with kidney failure. Variables for risk-adjustment – age, gender, race, body mass index at admission, systolic blood pressure at admission, heart rate at admission, anemia, cerebrovascular disease, diabetes, hyperlipidemia, hypertension, pulmonary disease, peripheral vascular disease, coronary artery disease/ischemic etiology, ejection fraction (%).
Performance Measures
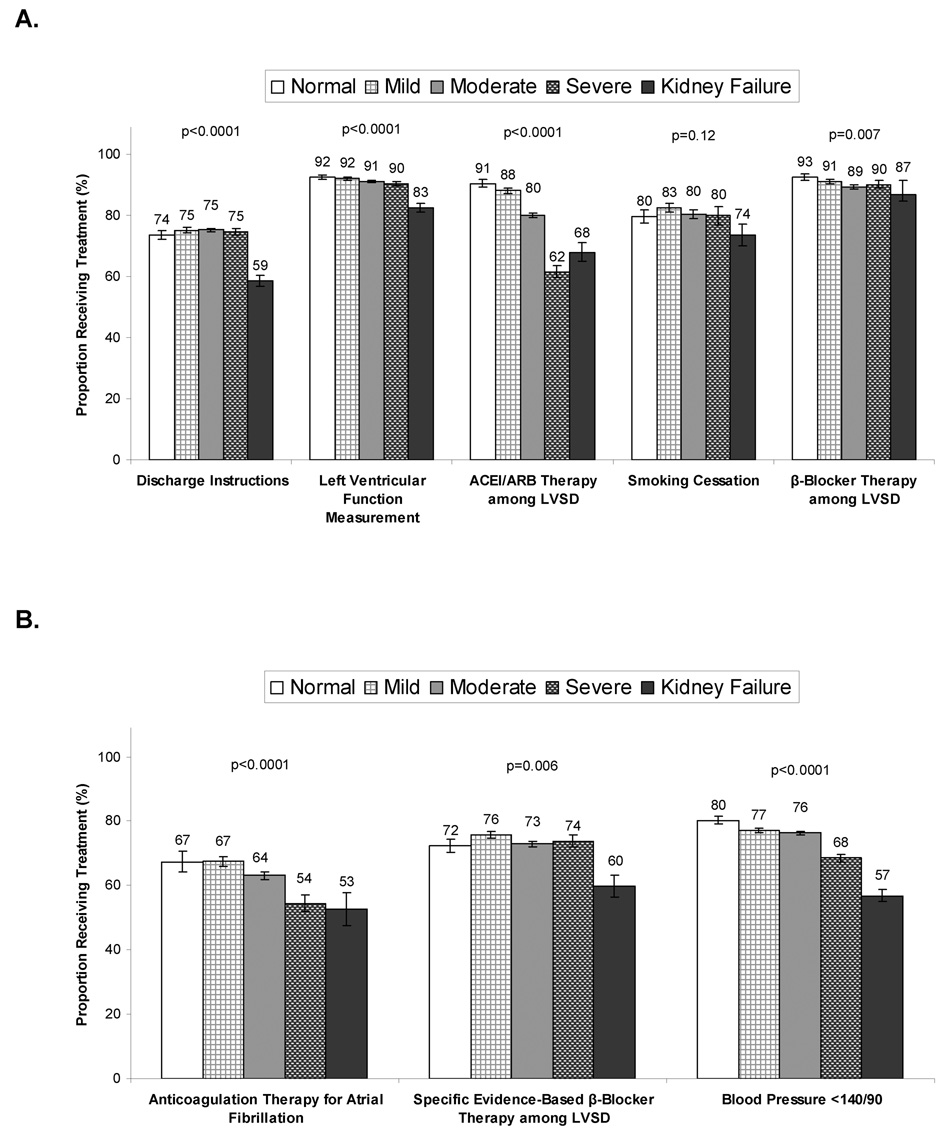
Adherence to each individual performance measure varied by kidney function (Figure 2a). The proportion of eligible patients who received complete discharge instructions was 74% overall, but significantly lower among patients with severe kidney disease (59%, p<0.0001). A greater proportion of eligible patients received evaluation of their left ventricular systolic function (91% overall), but as kidney function declined, smaller proportions of eligible patients were evaluated (p<0.0001 for trend). A similar pattern was observed for the proportion of eligible patients who received ACEI or ARB therapy with fewer patients with advanced kidney disease (62–68%) receiving therapy (p<0.0001 for trend). Overall, a very high proportion of eligible patients received discharge β-blocker therapy, including 90% of patients with severe kidney disease, though patients with renal failure were slightly less likely to receive such therapy (87%; p=0.007 for trend). The proportion of eligible patients who were discharged with smoking cessation counseling varied by kidney function. As kidney function declined, smaller proportions of eligible patients with atrial fibrillation received anticoagulation. Overall, the composite performance scores decreased as kidney function declined (p<0.0001 for trend). Finally, the proportion of eligible patients who received care according to additional quality measures (anticoagulant for patients with atrial fibrillation, evidence-based β-blocker for left ventricular systolic dysfunction, and blood pressure less than 140/90) varied significantly by kidney function group (Figure 2b). We also examined use of alternative vasodilator therapy with hydralazine and nitrates among patients with systolic dysfunction who did not receive ACEI or ARB therapy (N=1,309). Overall rates of use were 13.1%, with use varying based on kidney function (normal 1.5%; mild 2.7%, moderate 13.4%, severe 22.0% and ESRD 12.1%; p<0.0001).
Figure 2. Conformance to Performance and Quality Measures, According to Kidney Function Groups.
Conformance to several performance measures (A) decreased with more severe renal dysfunction, including assessment of left ventricular function, ACEI or ARB therapy, and β-blocker therapy. However, provision of discharge instructions and smoking cessation referral were only significantly lower among those with kidney failure. Conformance to quality measures (B) decreased with more severe renal dysfunction for last blood pressure < 140/90 and anticoagulation with atrial fibrillation, while they were lower only among those with kidney failure for specific evidence-based β-blocker therapy. Error bars represent 95% confidence intervals and P-values are for trends across kidney function groups for each performance and quality measure.
Although many differences were observed in the unadjusted analyses, risk-adjustment demonstrated fewer differences in conformity to performance measures by kidney function group (Table 2). For example, when compared with eligible patients with normal kidney function, only those with kidney failure were significantly less likely to receive discharge instructions (OR 0.52, 95% confidence interval [95% CI] 0.43 to 0.64), assessment of left ventricular function (OR 0.44, 95% CI 0.32 to 0.62), warfarin for atrial fibrillation patients (OR 0.57, 95% CI 0.33 to 0.98), or evidence-based β-blocker (OR=0.63, 95% CI 0.43 to 0.91). Nonetheless, one performance measure (use of ACEI or ARB at discharge among patients with systolic dysfunction), one quality measure (blood pressure control), and the composite performance measure (100% compliance with all of the performance measures) all demonstrated the pattern found throughout the unadjusted analyses – lower conformance to recommended care at lower levels of kidney function.
Table 2.
Risk-Adjusted Performance Measures According to Kidney Function Group
| Adjusted OR (95% C.I.) | ||||
|---|---|---|---|---|
| Mild vs. Normal | Moderate vs. Normal | Severe vs. Normal | Kidney failure vs. Normal | |
| Discharge instructions* | 0.99 (0.88, 1.11) | 1.04 (0.91, 1.18) | 1.01 (0.86, 1.19) | 0.52 (0.43, 0.64) |
| Evaluation of LV systolic function*† | 1.01 (0.81, 1.26) | 1.04 (0.86, 1.26) | 0.95 (0.73, 1.25) | 0.44 (0.32, 0.62) |
| ACEI or ARB for LV systolic dysfunction* | 0.80 (0.61, 1.04) | 0.47 (0.36, 0.62) | 0.19 (0.13, 0.28) | 0.23 (0.14, 0.38) |
| Smoking cessation counseling* | 1.15 (0.88, 1.51) | 1.14 (0.87, 1.50) | 1.10 (0.79, 1.53) | 0.68 (0.34, 1.35) |
| β-blocker for LV systolic dysfunction | 0.94 (0.59, 1.49) | 0.79 (0.49, 1.25) | 0.89 (0.56, 1.43) | 0.57 (0.30, 1.09) |
| Composite performance measure (100% compliance*) | 1.00 (0.89, 1.12) | 0.92 (0.83, 1.03) | 0.79 (0.68, 0.92) | 0.45 (0.36, 0.56) |
| Warfarin for atrial fibrillation | 0.98 (0.67, 1.44) | 0.90 (0.63, 1.29) | 0.65 (0.41, 1.02) | 0.57 (0.33, 0.98) |
| β-blocker (Bisoprolol, Carvedilol, or Metoprolol XL) for LV systolic dysfunction | 1.27 (1.04, 1.55) | 1.12 (0.92, 1.37) | 1.18 (0.91, 1.54) | 0.63 (0.43, 0.91) |
| Discharge blood pressure < 140/90mmHg | 0.77 (0.64, 0.92) | 0.70 (0.58, 0.85) | 0.52 (0.42, 0.63) | 0.48 (0.38, 0.61) |
Variables for risk-adjustment – age, gender, race, body mass index at admission, systolic blood pressure at admission, heart rate at admission, anemia, cerebrovascular disease, diabetes, hyperlipidemia, hypertension, pulmonary disease, peripheral vascular disease, coronary artery disease/ischemic etiology, ejection fraction (%).
Joint Commission and Centers for Medicaid and Medicare Services performance measure.
Evaluation of left ventricular systolic function was not adjusted for ejection fraction.
DISCUSSION
In a large contemporary cohort of patients hospitalized with HF in all regions of the United States, we confirmed that the prevalence of renal dysfunction is extremely high. In addition to higher rates of in-hospital mortality with worsening renal dysfunction, we found that those with the highest long-term risk based on renal function often did not receive effective therapies that are currently included in HF performance and quality measures. In particular, there was lower use of ACEI or ARB therapy and control of blood pressure even though both of these treatments improve HF outcomes among patients with renal disease. Although conformity in performance measure adherence was attributable to other patient characteristics, evidence based medications were significantly less likely to be used among patients with kidney failure.
These findings corroborate a growing body of literature that has highlighted greater underuse of medications for cardiovascular disease as kidney function declines.5,16 Underuse of recommended therapies is a problem that is has long been recognized among patients with heart failure.17 Paradoxical under-utilization of evidence-based therapies has also been described in other high-risk and under-represented patient subgroups such as the elderly, blacks, women, and patients with peripheral arterial disease, diabetes mellitus, or multiple chronic conditions. Overall, underuse of appropriate therapies is responsible for the majority of the quality gap throughout all of healthcare.18
A paradoxical decline in use of evidence-based therapies among patient at high risk for poor outcomes in the setting of HF and renal dysfunction is likely to be largely explained by 3 major reasons. First, the evidence upon which guideline-based treatment recommendations have been made came from randomized trials that largely excluded patients with significant renal dysfunction. Second, patients with renal dysfunction may be more likely to suffer adverse effects of several medications, some of which may belife-threatening (e.g., hyperkalemia). Third, several recommended HF medications may reduce systolic blood pressure in patients with values already compromised due to systolic dysfunction. Together, physicians seem less likely to prescribe these medications in the absence of strong evidence of efficacy in patients with renal dysfunction when they may also have a greater risk of significant side-effects and complications.
However, there is a growing body of evidence that demonstrates that the benefits of several medications used in the treatment of HF are likely to be of equal or greater benefit to those with renal dysfunction when compared to those without. In a recent study by Berger et al,19 in-hospital use of ACEI or ARB therapy was associated with a substantial reduction in 30-day mortality (OR 0.45, 95% CI 0.28–0.59) while discharge ACEI or ARB therapy was associated with a significant reduction in 1-year mortality (OR 0.72, 95% CI 0.58–0.91) that is similar to the effect found in patients without CKD.20 In a separate study, McAlister et al21 found similar effects on 1-year mortality for ACEI and β-blocker therapy among patients with creatinine clearance below and above 60 mL/min. However, patients in the present study with more severe renal dysfunction had lower rates of HF with left ventricular systolic dysfunction that may have been related to poor volume control and less likely to be impacted by ACEI or ARB therapy. In addition, limited data exists regarding the benefit of ACEI or ARB therapy among patients with ESRD who may receive little or no benefit. Unfortunately, in the present study we were unable to determine the longer-term impact of conformity to the treatment guidelines because post-discharge follow-up was not available.
Reasons for decreased conformity with non-pharmacologic therapies remain unclear. Despite strong recommendations to provide discharge instructions for patients hospitalized with HF, provide smoking cessation counseling, and assess left ventricular systolic function, these management strategies are used less often as kidney function declines. Although some associations are partially explained by other patient characteristics, patients with severe kidney disease are far less likely to receive discharge instructions and assessment of left ventricular systolic function. Previously among patients with non-ST segment elevation acute coronary syndromes, we have also observed these patterns of decreased use of medications and non-pharmacologic therapies (smoking cessation counseling, dietary counseling, cardiac rehabilitation referral).16
The most recently published report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends that healthcare providers lower both systolic and diastolic blood pressure in order to reduce HF.22 In this study, blood pressure control at discharge decreased with greater severity of renal dysfunction despite high grade evidence for control of hypertension in the long-term management of HF.3 Although diuretic-based antihypertensive therapy prevents HF in many patients, ACEI or ARB and β-blockers are equally effective.22 Here, the use of β-blockers decreased very little with increased severity if renal dysfunction, however, there were much greater decreases in the use of ACEI or ARB therapy. But we also found modest use of hydralazine and nitrates among patients with renal dysfunction and systolic dysfunction who were not receiving ACEI or ARB therapy. This finding suggested that intolerance to ACEI or ARB therapy may lead to use of alternative vasodilator therapy with hydralazine and nitrates. However, even among patients with severe kidney disease the rate of hydralazine and nitrate use was only 22%. The extent to which greater use of ACEI or ARB therapy in particular would improve blood pressure control cannot be ascertained from our study, however, it is likely to be an effective strategy in improving long-term outcomes in patients with HF who do not have significant contraindications or side-effects precluding their use.
Because effective therapies for patients with HF are dramatically underused,5 several quality improvement efforts have been launched.7 The GWTG-HF continuous quality improvement program has potential to attenuate and potentially reverse these patterns of underuse. While we found patterns of medication underuse similar to that reported in other smaller cohorts,5 the treatment rates were much higher in absolute terms for patients with heart failure and CKD (62% ACEI or ARB and 90% β-blocker treatment rates with severe CKD).
The high prevalence of renal dysfunction among patients with HF has been well described. In a prospective cohort of 754 patients with heart failure, McAlister et al23 found that 16% had a creatinine clearance ≤30 mL/min while 40% had clearances between 30–59 mL/min. In a larger, more recent study, Heywood et al24 found very similar prevalence rates among patients hospitalized with HF: 13.1% had an estimated GFR between 15–29 mL/min/1.73m2 while 43.5% had an estimated GFR between 30–60 mL/min/1.73m2 (nearly identical to the prevalence rates found in our study: 14.2% and 43.9%, respectively). The present study also confirms prior studies that demonstrated that renal dysfunction was a powerful independent prognostic factor among patients with HF. Severe renal dysfunction was associated with a 60% increase in in-hospital mortality while kidney failure nearly doubled the odds of in-hospital mortality. Although these in-hospital mortality rates were similar to those found in the study by Heywood et al,24 we observed slightly lower mortality rates among those with advanced kidney dysfunction (5.7% vs. 7.6% for estimated GFR between 15–29 mL/min/1.73m2; 4.4% and vs. 6.5% for estimated GFR <15 mL/min/1.73m2). It is unclear whether these differences may be due to differing patient characteristics (e.g., higher systolic blood pressures among patients with advanced CKD in the study by Heywood et al.) or treatments received (e.g., much lower rates of treatment with ACEI or ARBs in the study by Heywood et al.). Finally, the demographic and clinical characteristics of our study cohort were similar to those from a fee-for-service Medicare cohort, highlighting the representativeness of our results to most hospitalized patients with HF.
The findings in our study must be considered in light of several limitations. First, data were collected based on the medical record and depend on the accuracy and completeness of clinical documentation. Contraindications and intolerance were recorded as they were found to be documented in the medical record, but a proportion of patients reported to be eligible for treatment who were not treated may have had contraindications or intolerance that were indeed present but not documented. These findings may not apply to hospitals that differ in patient characteristics or care patterns from GWTG-HF hospitals. Treatment guidelines may not have been applicable to some patients with severe renal dysfunction and HF who were very ill and were in the process of being discharged to hospice for terminal care. Although the MDRD formula is a recommended method for estimating renal function it should be applied when renal function is stable, and this may not be the case for many patients hospitalized with HF, potentially limiting its accuracy in this population. Although this study was able to identify the increasing risks for adverse in-hospital outcomes with the severity of renal dysfunction, we did not attempt to identify the mechanisms by which renal function may influence mortality. Next, our primary outcome was limited to in-hospital outcomes. Although the link between process measures during hospitalization and various outcomes may be valid, we were not able to directly explore the process-outcome relationship because it would be implausible to attribute in-hospital mortality events to processes of care at discharge. Thus, the full implications of suboptimal processes of care among patients with HF and CKD remain unclear.
CONCLUSIONS
In a large contemporary cohort of patients hospitalized with HF, we found that the prevalence of renal dysfunction is extremely high. Despite higher rates of in-hospital mortality with severity of renal dysfunction, we also found an inverse relationship between the degree of renal dysfunction and conformity with current HF performance and quality measures, including pharmacologic and non-pharmacologic management strategies. Further efforts are needed to improve the care of patients with HF and kidney disease.
Acknowledgment
This study was funded by the American Heart Association with support from an unrestricted educational grant from GlaxoSmithKline, Inc. Dr. Patel is the recipient of grant K23 DK075929-01 from the National Institutes of Diabetes & Digestive & Kidney Diseases. Dr. Hernandez is supported by an American Heart Association Pharmaceutical Roundtable grant 0675060N. Dr Peterson is the recipient of grant R01 AG025312-01A1 from the National Institute on Aging. Dr Fonarow holds the Eliot Corday Chair in Cardiovascular Medicine and Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the American Heart Association Scientific Sessions 2006, Chicago, IL, November 12–15, 2006
REFERENCES
- 1.Association AH. Heart Disease and Stroke Statistics - 2007 Update. Dallas, TX: American Heart Association; 2007. [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 4.Prevalence of chronic kidney disease and associated risk factors--United States, 1999–2004. MMWR. 2007;56:161–165. [PubMed] [Google Scholar]
- 5.Ezekowitz J, McAlister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich PA, Fonarow GC. Are registry hospitals different? A comparison of patients admitted to hospitals of a commercial heart failure registry with those from national and community cohorts. Am Heart J. 2006;152:935–939. doi: 10.1016/j.ahj.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Bonow RO, Bennett S, Casey DE, Jr, et al. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol. 2005;46:1144–1178. doi: 10.1016/j.jacc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Abraham WT, Albert NM, et al. Influence of a Performance-Improvement Initiative on Quality of Care for Patients Hospitalized With Heart Failure: Results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) Arch Intern Med. 2007;167:1493–1502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 12.Joint Commission on Accreditation of Healthcare Organizations. [Accessed July 14, 2007];Specifications Manual for National Hospital Quality Measures. 2006 http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm.
- 13.Centers for Medicare and Medicaid Services. [Accessed July 30, 2007];Overview of Specifications for Hospital Compare measures. 2006 http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalOverviewOfSpecs200512.pdf.
- 14.Centers for Medicare and Medicaid Services. [Accessed July 30, 2007];The Hospital Quality Alliance (HQA) Ten Measure "Starter Set". 2005 http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalStarterSet200512.pdf.
- 15.National Kidney Foundation Kidney Disease Outcome Quality Initiative Advisory B. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39:S1–S246. [PubMed] [Google Scholar]
- 16.Han JH, Chandra A, Mulgund J, et al. Chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. Am J Med. 2006;119:248–254. doi: 10.1016/j.amjmed.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Tu JV, Juurlink DN, et al. Risk-Treatment Mismatch in the Pharmacotherapy of Heart Failure. JAMA. 2005;294:1240–1247. doi: 10.1001/jama.294.10.1240. [DOI] [PubMed] [Google Scholar]
- 18.McGlynn EA, Asch SM, Adams J, et al. The Quality of Health Care Delivered to Adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 19.Berger AK, Duval S, Manske C, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease. Am Heart J. 2007;153:1064–1073. doi: 10.1016/j.ahj.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 20.The SOLVD Investigattors. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 21.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal Insufficiency and Heart Failure: Prognostic and Therapeutic Implications From a Prospective Cohort Study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 24.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High Prevalence of Renal Dysfunction and Its Impact on Outcome in 118,465 Patients Hospitalized With Acute Decompensated Heart Failure: A Report From the ADHERE Database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]