Abstract
FGF21 functions as a metabolic regulator. The FGF21 transcript is reported to be abundantly expressed in liver, but little is known about the regulation of FGF21 expression in other tissues. In this study, we show that levels of FGF21 protein expression were similar in skeletal muscle and liver from fasted mice. FGF21 transcript and protein expression were upregulated in gastrocnemius muscle of skeletal muscle-specific Akt1 transgenic mice. Serum concentration of FGF21 was also increased by Akt1 transgene activation. In cultured skeletal muscle cells, FGF21 expression and secretion was regulated by insulin, Akt transduction and LY294002. These data indicate that skeletal muscle is a source of FGF21 and that its expression is regulated by a PI3-kinase/Akt1 signaling pathway-dependent mechanism.
Keywords: FGF21, Akt, metabolism, transcript, transgenic mice
1. Introduction
Fibroblast growth factor-21 (FGF-21) is a member of FGF super family that is reported to be primarily expressed in liver and expressed to a lower extent in thymus [1]. Recent evidence indicates that FGF-21 is an important endogenous regulator for systemic glucose and lipid metabolism. It has been demonstrated that FGF-21 enhances glucose uptake via insulin-independent manner in mouse and human adipocytes in vitro [2]. It has also been shown that transgenic mice that overexpress FGF-21 in liver are resistant to diet-induced obesity. Therapeutic administration of FGF-21 to diabetic rodents (ob/ob and db/db mice) or diabetic rhesus monkeys improved plasma glucose and lipid profile to near normal levels [2,3]. Knockdown of hepatic FGF-21 transcript leads to hyperlipidemia and fatty liver in mice fed a ketogenic diet [4], whereas overexpression of FGF-21 in liver leads to ketone body production [5], suggesting that FGF-21 functions as a regulator of the organism’s adaptation to starvation. In this regard, hepatic FGF-21 gene expression is regulated by PPARα [4-6]. Furthermore, the FGF-21 gene is a direct target of PPARγ [7], and the synergy between FGF21 and PPARγ pathways was demonstrated in 3T3-L1 adipocytes [8].
Akt1 is serine-threonine protein kinase that is activated by various extracellular stimuli through a phosphatidylinositol 3-kinase (PI3-kinase) pathway. Numerous studies have implicated Akt signaling in insulin signaling as well as the control of cellular growth and organ size and cellular hypertrophy [9,10]. Overexpression of Akt in skeletal muscle results in fiber hypertrophy in vitro and in vivo [11-13]. We have recently demonstrated that Akt1-mediated skeletal muscle growth in obese mice leads to a reduction in accumulated white adipose tissue and improvements in metabolic parameters [14]. These findings led us to the hypothesize that activation of Akt1 signaling in skeletal muscle could lead to the secretion of hormonal factors or “myokines” that act on adipose, hepatic or central nervous system tissues to normalize metabolic parameters. Myokines are defined as proteins that are produced and secreted from muscle that have paracrine or endocrine functions [15]. While it has been well-established that muscle secretes factors required for blood vessel recruitment [13,18], the secretion of metabolic regulatory proteins by this tissue has only received recent attention. Interleukin-6 is an example of a myokine that is released into the circulation in response to exercise and modulates the function of remote metabolically important tissues [16]. Recently it was also shown that visfatin, a putative regulator of inflammation and glucose metabolism, is produced at higher levels in skeletal muscle than visceral adipose tissue in chickens [17]. Here, we show that skeletal muscle is a source of secreted FGF-21 and that its expression is regulated by an Akt1 signaling pathway-dependent mechanism.
2. Materials and Methods
2.1 Cell Culture and Adenoviral Infection
C2C12 mouse myoblasts (American Type Culture Collection) were maintained in growth medium (DMEM supplemented with 20% FBS) as described elsewhere [13]. To induce differentiation, cells were shifted to differentiation medium (DMEM supplemented with 2% heat-inactivated horse serum) for 4 days. Cells were then infected with adenoviral constructs encoding constitutively active Akt1 (Adeno-myrAkt1) or β-galactosidase (Adeno-βgal) at a multiplicity of infection (MOI) of 250 for 16 hours. The transfection efficiency was greater than 90% under these conditions [13]. In some experiments, C2C12 myocytes were pretreated with LY294002 (20 μmol/L) or vehicle for 1 hour before stimulation with insulin (10 nmol/L).
2.2 Animals
Generation and phenotypic characterization of skeletal muscle-specific inducible Akt1 transgenic (TG) mice have been previously described elsewhere in detail [14]. In brief, 1256 [3Emut] MCK-rtTA TG mice [19] were crossed with TRE-myrAkt1 TG mice [20] to generate double TG (DTG) mice. For Akt1 transgene expression, DTG mice were induced with doxycycline (DOX, 0.5 mg/ml) in their drinking water. 1256 [3Emut] MCK-rtTA single TG littermates were treated with DOX in the same manner as DTG mice and used as controls. Three weeks after DOX treatment, sera and gastrocnemius muscle from control or transgenic mice were harvested for mRNA and protein analysis. For fasting experiments, food pellets were removed from the cages of ad libitum-fed male C57BL/6 mice for 48 hours. Mice were then anesthetized, gastrocnemius muscles and liver were immediately removed for protein analysis.
2.3 Determination of FGF-21 mRNA
Total RNA was prepared by Qiagen using protocols provided by the manufacturer and cDNA was produced using ThermoScript RT-PCR Systems (Invitrogen). Real-time PCR was performed on iCycler iQ Real-Time PCR Detection System (BIO-RAD). SYBR Green I was used as a double-stranded DNA-specific dye as described previously [21]. Transcript levels were determined relative to the signal from GAPDH, and normalized to the mean value of samples from control. Primers were as follows: 5′-GCTGCTGGAGGACGGTTACA-3′ and 5′-CACAGGTCCCCAGGATGTTG-3′ for mouse FGF-21 and 5′-GCTAAGCAGTTGGTGGTGCA-3′ for mouse GAPDH. Microarray analysis was carried out as described previously [22].
2.4 Western Blots and Immunoprecipitations
C2C12 cell lysates, culture media, and skeletal muscle tissue samples were subjected to SDS-PAGE and Western blot analysis. Antibodies used in Western blots were against: phospho-Akt from Cell Signaling Technology (Beverly, MA); FGF-21 (monoclonal; clone#84-E12; raised against full length human FGF-21 protein); GAPDH [8]. Immunoprecipitation from lysates was performed using the same anti-FGF-21 antibodies. Immune complexes were probed with anti-FGF-21 (polyclonal; clone PR050928A; raised against full length human FGF-21 protein). For immunodetection, goat anti-mouse and anti-rabbit HRP conjugates were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and the ECL detection system from Amersham Biosciences (Piscataway, NJ) was used.
2.5 FGF-21 ELISA
Serum samples were measured for concentrations of FGF-21 using anti-FGF-21 antibodies by sandwich ELISA method. The wells of a 96-well microtiter plate were coated overnight at 4°C with monoclonal anti-FGF-21 antibody at a concentration of 5 μg/mL (0.1 mL/well). All assay steps were carried out in 0.1 mL/well additions with 1 hour incubations at room temperature. After washing and blocking, standards and samples were added to the wells. Polyclonal anti-FGF-21 (1:10000 dilution) was added and detected with a 1:5000 dilution of donkey-anti-rabbit-HRP from Jackson ImmunoResearch (West Grove, PA). Samples were analyzed in duplicate. The standard curve range for the assay was 0.39 to 50 ng/mL.
2.6 Statistical Analyses
All data are presented as mean ± SEM. Statistical comparison of data from two experimental groups were made by using Student’s t test. Comparison of data from multiple groups was made by ANOVA with Fisher’s PLSD test. A level of P<0.05 was accepted as statistically significant.
3. Results
3.1 FGF-21 induction by Akt signaling in skeletal muscle
Microarray analysis was performed on inducible, skeletal muscle-specific Akt1 transgenic mice [14]. This analysis revealed that FGF-21 was upregulated approximately four-fold in gastrocnemius muscle at the 3 week time point following transgene induction (Fig. 1A). Akt-mediated regulation of FGF-21 transcript in the transgenic mice was confirmed by quantitative real-time PCR (QRT-PCR) (Fig. 1B).
Fig. 1.
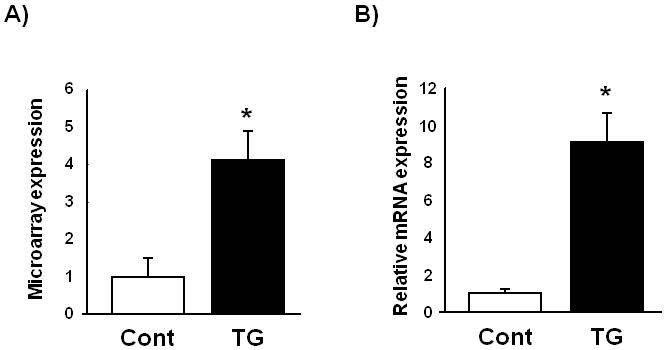
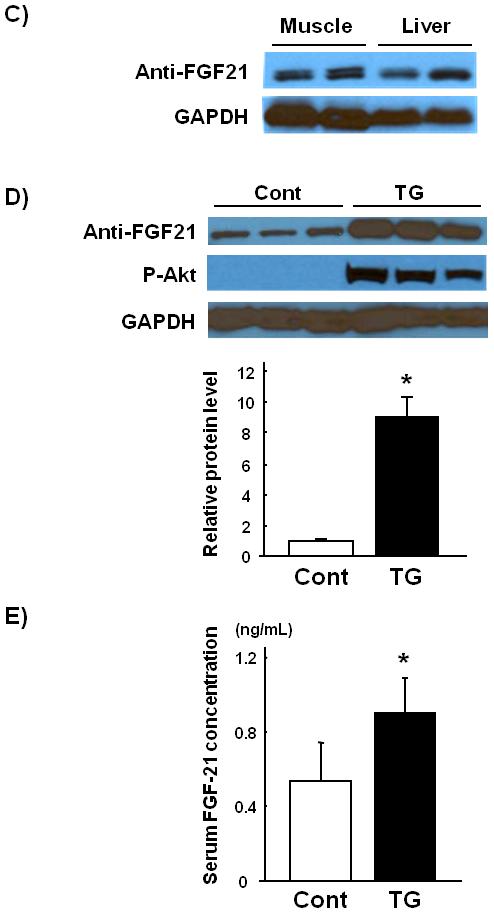
Skeletal muscle FGF-21 transcript and protein levels are upregulated in skeletal muscle-specific inducible Akt1 transgenic mice. (A) Microarray analysis of FGF-21 transcript regulation in gastrocnemius muscle harvested from skeletal muscle-specific inducible Akt1 transgenic mice 3 weeks after Akt1 transgene activation. (B) Relative transcript expression levels of FGF-21 as measured by QRT-PCR in control and transgenic gastrocnemius muscle. (C) Comparable levels of FGF-21 protein expression in gastrocnemius muscle and liver of wild type mice that have been fasted for 48 hours. (D) Representative western blot analysis and quantified relative protein expression levels of FGF-21 in gastrocnemius muscle from control or skeletal muscle-specific Akt1 transgenic mice 3 weeks after transgene induction. (E) Serum FGF-21 levels in control or skeletal muscle-specific inducible Akt1 transgenic mice. Results are presented as mean ± SEM (n=4). *P<0.05 vs. control. Cont; control mice, TG; skeletal muscle-specific inducible Akt1 transgenic mice.
FGF-21 protein expression in skeletal muscle tissue samples were examined by western blot analysis using a monoclonal antibody, clone#84-E12, directed to human FGF-21. Because FGF-21 transcript is dramatically induced by fasting [4,5], we used 48-hours fasted liver tissues as a positive control. Comparison of gastrocnemius muscle and liver from fasted mice revealed similar levels of FGF-21 protein in these tissues (Fig. 1C).
We next examined the effects of Akt1 activation on FGF-21 protein expression using skeletal muscle-specific Akt1 transgenic mice. FGF-21 protein expression was robustly upregulated in gastrocnemius muscle following 3 weeks of Akt1 transgene induction (Fig. 1D). Serum FGF-21 concentration was determined by FGF21-specific ELISA. Serum levels of FGF-21 were also increased by the induction of the Akt1 transgene in skeletal muscle compared with control mice (Fig. 1E). These results indicate that activation of Akt1 signaling in skeletal muscle lead to increase in circulating and tissue-resident FGF-21 levels.
3.2 FGF-21 is upregulated by Akt1 in C2C12 myocytes
To examine changes in gene expression in cultured myocyte in response to Akt1 overexpression, differentiated C2C12 myocytes were transfected with Adeno-βgal or Adeno-myrAkt1 for 16 hours and microarray analysis was performed. FGF-21 transcript was markedly upregulated in C2C12 cells treated with Adeno-myrAkt1 (Fig. 2A). The upregulation of FGF-21 transcript was confirmed by QRT-PCR (Fig. 2B).
Fig. 2.
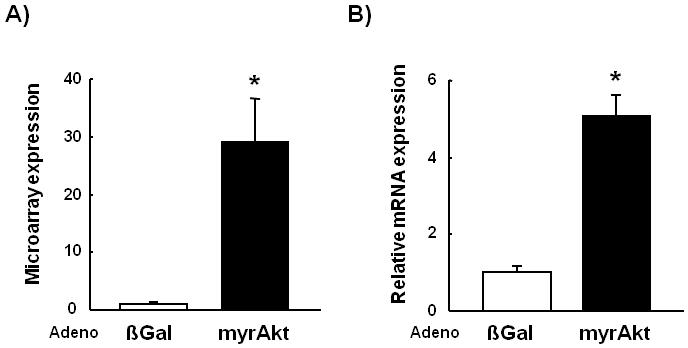
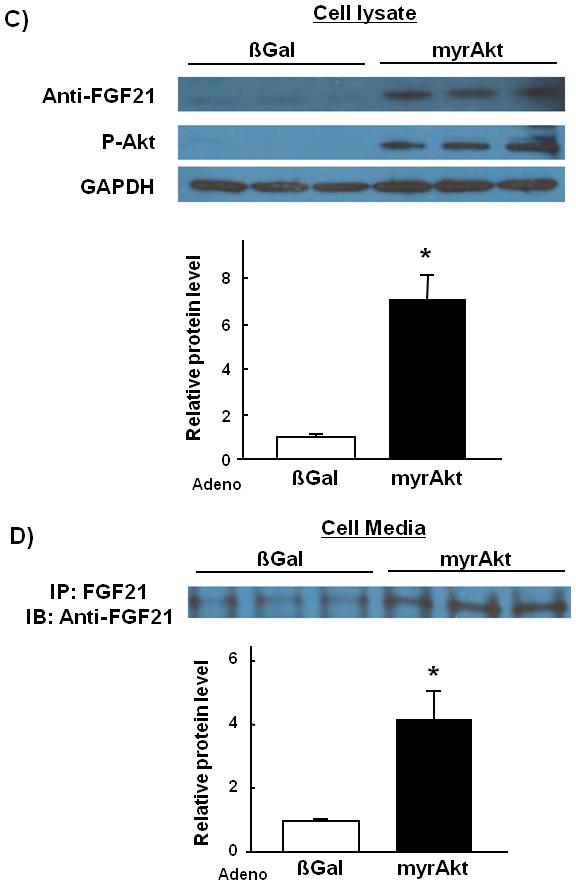
FGF-21 transcript and protein levels are upregulated in cultured C2C12 myocytes by Akt1 overexpression.(A) Microarray analysis of FGF-21 transcript expression in Adeno-myrAkt1-treated C2C12 myocytes cell lysates compared with control. (B) Relative transcript expression levels of FGF-21 as measured by QRT-PCR. (C) Representative western blot analysis and quantified relative protein expression levels of FGF-21 in adeno-βGal or adeno-myrAkt1-treated C2C12 myocyte cell lysates. (D) Representative Western blot analysis and quantified relative protein expression levels of FGF-21 in C2C12 cell culture media. Results are presented as mean ± SEM (n=4). *P<0.05 vs. control.
FGF-21 protein expression levels in culture media and cell lysates were examined by Western blot analysis (Fig. 2C). FGF-21 protein expression was induced 7-fold in Adeno-myrAkt1-treated C2C12 myocytes cell lysates compared with control. FGF-21 protein in culture medium was increased 4-fold by overexpression of constitutively active Akt1 (Fig. 2D).
3.3 FGF-21 expression is upregulated by insulin in a PI3-kinase-dependent manner in cultured C2C12 myocytes
To examine the regulation of FGF-21 expression by insulin, a hormone known to naturally induce AKT activation. C2C12 myocytes were treated with physiological levels of insulin (10 nmol/L) or vehicle in the presence or absence of pre-treatment with the PI3-kinase inhibitor LY294002. FGF-21 expression in the cell lysate and the culture media was significantly increased in response to insulin stimulation (Fig. 3). Pretreatment with LY294002 inhibited both basal and insulin-stimulated FGF-21 expression. These results indicate that FGF-21 protein is produced in cultured myocytes and is a downstream target of PI3-kinase/Akt1 activation.
Fig. 3.
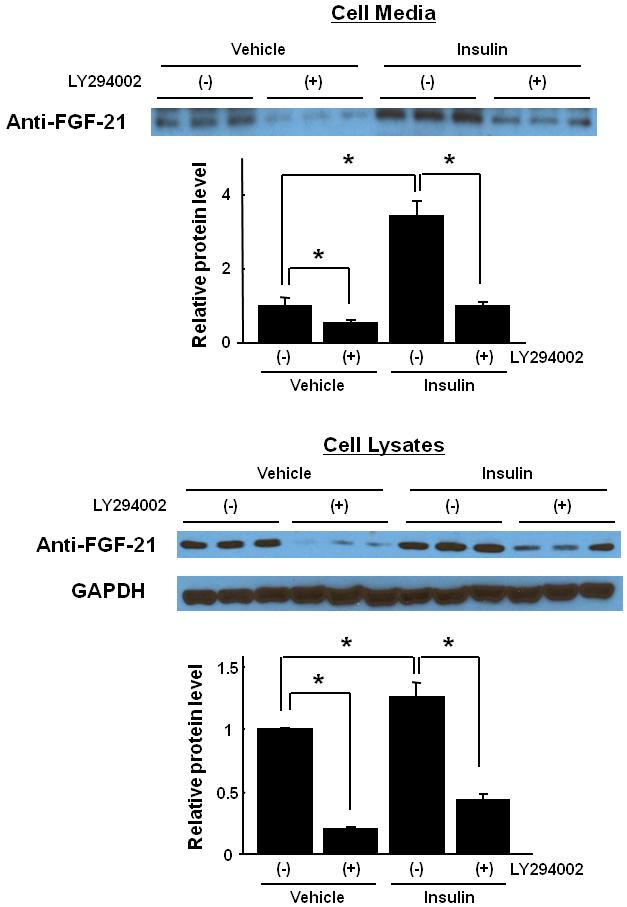
FGF-21 protein expression is upregulated by insulin, and inhibited by PI3-kinase inhibitor in cultured C2C12 myocytes. Representative western blot analysis and quantified relative protein expression levels of FGF-21 in C2C12 myocytes culture media and cell lysates pretreated with LY294002 (20 μmol/L) or vehicle for 1 hour before stimulation with insulin (10 nmol/L) or vehicle.
4. Discussion
Since its identification by Nishimura et al. as a member of FGF super family, it has generally been assumed that FGF21 is predominantly expressed in liver, and a number of recent reports describing the exceptionally dynamic regulation of FGF-21 mRNA expression in liver [4-6] have confirmed these initial observations. Nevertheless, FGF21 has also been detected in pancreas and in cells of various origins, suggesting that the FGF21 expression profile may indeed be broader than initially thought [23].
In the present study, we examined for the first time skeletal muscle expression of FGF-21 transcript and protein using monoclonal antibodies raised against the full length FGF-21. We found that in skeletal muscle FGF-21 protein expression is essentially comparable to that in fasted liver. Because hepatic FGF-21 transcript is upregulated by fasting [4,5], these results indicate that skeletal muscle can be an important source of FGF-21 production.
In this study, we also demonstrated that FGF-21 expression in skeletal muscle is upregulated upon activation of the PI3-kinase/Akt1 signaling pathway. We found that both FGF-21 mRNA and protein were induced by Akt1 overexpression or insulin stimulation in skeletal muscle cells. Conversely, insulin-stimulated FGF21 expression was inhibited by incubation with a PI3-kinase inhibitor. Under these conditions we also detected FGF-21 protein in the media. Furthermore FGF-21 protein levels in the media were increased following transduction with an activated Akt transgene or treatment with insulin, and reduced by treatment with a PI3K inhibitor, indicating that FGF21 can be secreted by skeletal muscle cells in PI3K/AKT-dependent fashion. Overexpression of constitutively active Akt1 in the type IIb fibers of transgenic mice also led to an increase in circulating FGF-21, suggesting that the secretion of this factor from muscle may be physiologically significant. Thus, in addition of being direct target of PPARα in liver in response to fasting [5,6] or ketogenic diet (KD) [4] and PPARγ in adipose[7], FGF21 expression and secretion in skeletal muscle can be under control of PI3K/AKT pathway.
While FGF21 does not appear to be an insulin mimetic or sensitizer [2], the ability of physiological levels of insulin to induce the expression of FGF21 in muscle cells as well as in 3T3-L1 adipocytes (data not shown), and of FGF21 to stimulate insulin production/secretion in pancreatic islets and β-cells [24], raises the intriguing possibility of crosstalk between these metabolic hormones. Indeed, FGF21 levels are significantly elevated in overtly insulinemic ob/ob mice [6]. In healthy animals, however, insulin levels are low during fasting and peak after feeding, whereas FGF21 expression is regulated in the opposite manner [5,6]. Thus, further studies on the interplay between insulin and FGF21 under normal physiological conditions and in diseased states are warranted.
Myogenic Akt signaling is preferentially activated by resistance training, which leads to the growth of type IIb muscle fibers that are referred to as fast/glycolytic [25-27]. We recently reported that Akt1 transgene activation in skeletal muscle of mice leads to type IIb muscle hypertrophy and the upregulation of glycolytic pathways, whereas oxidative pathways are decreased [14]. We also reported that Akt1-induced type IIb fiber growth in obese mice leads to appreciable reductions in fat mass and a normalization of metabolic parameters. These metabolic improvements result, at least in part, from muscle-induced changes in the phenotypic and transcriptional profile of the liver that promote hepatic fatty acid oxidation and ketone body production. Based upon the findings of the current study, we speculate that FGF-21 released from skeletal muscle may be one of the critical factors involved in this inter-tissue communication. Consistent with this hypothesis, the phenotype of myogenic Akt1 transgene activation in obese mice is similar to the effects of FGF21 administration to diabetic rodents and non-human primates [2-5]. Furthermore, FGF-21 induces transcription of enzymes and transporters required for hepatic fatty acid oxidation and ketogenesis [4-6], and the same transcript changes are also observed in the liver of skeletal muscle-specific Akt1 transgenic mice [14].
Here, we provide evidence that FGF-21 is an Akt1-mediated skeletal muscle-derived secreted protein or myokine. These findings support the hypothesis that stimulators of Akt1 signaling in skeletal muscle, such as resistance training exercise, could attenuate obesity-related metabolic disorders through production and secretion of metabolic-regulatory myokines.
5. Acknowledgements
Y.I. was supported by a grant from the American Heart Association Postdoctoral Fellowship Award, Northeast Affiliate. K.W. was supported by National Institutes of Health grants HL77774, HL86785, AG15052 and HL81587. N.O. was supported by grant 0635593T from the American Heart Association.
Abbreviations
- DOX
doxycycline
- FGF21
fibroblast growth factor-21
- KD
ketogenic diet
- PI3-kinase
phosphatidylinositol 3-kinase
- QRT-PCR
quantitative real-time PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203–6. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- [2].Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kharitonenkov A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–81. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- [4].Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- [5].Inagaki T, et al. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [6].Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–40. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- [7].Wang H, Qiang L, Farmer SR. Identification of a domain within PPAR{gamma} regulating expression of a group of genes containing FGF21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol. 2007 doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J Cell Physiol. 2007;210:1–6. doi: 10.1002/jcp.20847. [DOI] [PubMed] [Google Scholar]
- [9].Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- [10].Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–65. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- [11].Bodine SC, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- [12].Lai KM, et al. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takahashi A, et al. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–14. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Izumiya Y, et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–72. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- [16].Pedersen BK, Febbraio M. Muscle-derived interleukin-6--a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun. 2005;19:371–6. doi: 10.1016/j.bbi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [17].Krzysik-Walker SM, Ocon-Grove OM, Maddineni SR, Hendricks GL, 3rd, Ramachandran R. Is visfatin an adipokine or myokine? Evidence for greater visfatin expression in skeletal muscle than visceral fat in chickens. Endocrinology. 2008;149:1543–50. doi: 10.1210/en.2007-1301. [DOI] [PubMed] [Google Scholar]
- [18].Arany Z, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- [19].Grill MA, Bales MA, Fought AN, Rosburg KC, Munger SJ, Antin PB. Tetracycline-inducible system for regulation of skeletal muscle-specific gene expression in transgenic mice. Transgenic Res. 2003;12:33–43. doi: 10.1023/a:1022119005836. [DOI] [PubMed] [Google Scholar]
- [20].Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–46. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- [22].Schiekofer S, Shiojima I, Sato K, Galasso G, Oshima Y, Walsh K. Microarray analysis of Akt1 activation in transgenic mouse hearts reveals transcript expression profiles associated with compensatory hypertrophy and failure. Physiol Genomics. 2006;27:156–70. doi: 10.1152/physiolgenomics.00234.2005. [DOI] [PubMed] [Google Scholar]
- [23].Kharitonenkov A, Shanafelt AB. Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs. 2008;22:37–44. doi: 10.2165/00063030-200822010-00004. [DOI] [PubMed] [Google Scholar]
- [24].Wente W, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–8. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- [25].Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. Faseb J. 2005;19:786–8. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- [26].Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol. 2001;90:1936–42. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- [27].Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol. 2003;553:213–20. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]


