Abstract
Purpose:
RTOG 95-17 is a prospective Phase II cooperative group trial of APBI alone using multicatheter brachytherapy following lumpectomy in select early stage breast cancers. Tumor control and survival outcomes are reported.
Materials and Methods:
Eligibility criteria included stage I/II breast carcinoma confirmed to be <3cm, unifocal, invasive non-lobular histology with 0-3 positive axillary nodes without extracapsular extension. APBI treatment was delivered with either Low Dose Rate (LDR) (45 Gy in 3.5-5 days) or High Dose Rate (HDR) (34 Gy in 10 BID fractions over 5 days). Endpoints evaluated included in-breast control, regional control, mastectomy-free rate, mastectomy-free survival, disease-free survival and overall survival. The study was designed to analyze the HDR and LDR groups separately and without comparison.
Results:
Between 1997 and 2000, 100 patients were accrued and 99 were eligible; 66 treated with HDR and 33 treated with LDR. Eighty seven patients had T1 lesions and 12 had T2 lesions. Seventy nine were pathologically N0 and 20 were N1. Median follow-up in the HDR group is 6.14 years with the 5-year estimates of in-breast, regional and contralateral failure rates of 3%, 5% and 2%, respectively. The LDR group experienced similar results with a median follow-up of 6.22 years. The 5-year estimates of in-breast, regional and contralateral failure rates of 6%, 0% and 6%, respectively.
Conclusion:
Patients treated with multicatheter partial breast brachytherapy on this trial experienced excellent in-breast control rates and overall outcome that compare to reports from APBI studies with similar extended follow-up.
Keywords: Brachytherapy, breast cancer, breast cancer trials, radiation therapy for breast cancer, accelerated partial breast irradiation
Introduction
All of the initial studies evaluating accelerated partial breast irradiation (APBI) that included conservative patient selection criteria and appropriate quality assurance measures were performed with multicatheter brachytherapy.1, 2 These early studies have provided the clinical basis upon which many patients have been treated and new ideas have been generated. As a result of perceived difficulty in constructing an effective multicatheter implant and the obvious degree of associated breast trauma, alternative dose delivery methods were sought as soon as early reports of treatment outcome proved APBI to be a potential treatment alternative to the standard 6-7 weeks of whole breast irradiation. Through the subsequent years, the increasing interest in accelerated partial breast irradiation shown by both physicians and patients has been joined by an expansion of technology and treatment delivery concepts in this area of breast cancer treatment.3-12 These new ideas share the common goal of maintaining local control rates while reducing the overall treatment time. Each with its own unique potential advantages and merit, these ideas have built upon the basic concepts established with the multicatheter implant experience. Lacking in numbers of treated patients and/or follow-up, adoption of these newer approaches into wide spread clinical use has been slow with many awaiting extended follow-up. With limited long term data available, it will continue to be important to return to the original multicatheter trials, report updated treatment outcome data and assure that the founding principles of APBI remain supported as follow-up intervals increase. The Radiation Therapy Oncology Group protocol 95-17 is one of the original multicatheter trials investigating the use of APBI as the sole method of adjuvant radiotherapy following lumpectomy in early stage breast cancer.13 This multi-institutional phase II trial applied conservative patient eligibility criteria, included guidelines for brachytherapy delivery and included a thorough quality assurance program. Now with greater than five year follow-up of the 99 patients treated on this trial, we report the long term local and regional tumor control and survival outcomes.
Materials and Methods
Eligibility Criteria
The intent of the patient eligibility criteria was to maximize the likelihood that any residual microscopic disease would be confined to the immediate vicinity of the lumpectomy cavity. Eligibility criteria included unicentric breast lesions that were stage T1 or T2 (≤3cm) and pathologically identified as infiltrating non-lobular carcinoma that had been resected with a pathologically negative margin (tumor not extending to the inked surgical margin). Axillary lymph node evaluation, in this era prior to widespread use of sentinel lymph node mapping, required at least six lymph nodes to be removed and patients with N0 or N1 were eligible as long as no greater than 3 lymph nodes were positive with metastatic disease and there was no evidence of extracapsular extension identified. All ages were eligible. Ineligibility criteria included evidence of extensive intraductal component, any lobular component (invasive or in situ) or history of a collagen vascular disease.
Target definition and dose delivery
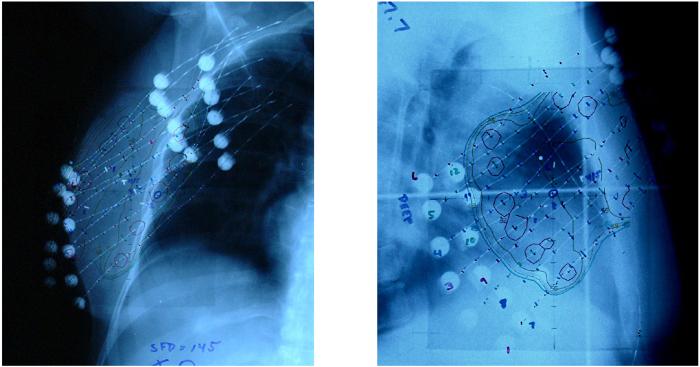
Ideally, 6 surgical clips were placed at the time of lumpectomy to delineate the extent of the resultant surgical cavity. Alternatively, if surgical clips were not placed, gold seed markers were later allowed to be placed post-operatively with image guidance thus delineating the lumpectomy cavity. Once eligibility criteria were met and trial registration complete, brachytherapy catheters were placed and treatment planning performed. Orthogonal film, 2-dimensional treatment planning was the basis of dosimetric planning for all cases, figure 1. The cavity was delineated through surgical clip identification on both orthogonal films and digitized into the 2-dimensional brachytherapy treatment planning system. The treatment target was then identified and was defined as a 2 cm margin peripheral to the cavity and 1 cm anteriorly and posteriorly. Representative axial, coronal and sagittal views were used to optimize target coverage and dose homogeneity. Catheter and seed/dwell position location was then digitized to facilitate dosimetric planning and a 2-dimensional dosimetric plan created, figure 2. Several dosimetric parameters were utilized to assure proper dose delivery including peripheral dose, prescribed dose, 90% of the prescribed dose, mean central dose and 150% of the mean central dose. If high dose rate brachytherapy was used, the prescription dose was 3.4 Gy bid for 5 days and a total resultant dose to the periphery of the target of 34 Gy over 5 treatment days. If Low Dose Rate brachytherapy was used, then the patient was admitted to the hospital for a 3.5 – 5 day time period over which 45 Gy was delivered. Systemic disease management was determined by the treating physicians and the initiation of any chemotherapy was not permitted to start for a minimum of 2 weeks following the completion of the brachytherapy treatment.
Figure 1.
Orthogonal film based implant evaluation. Surgical clips identified for cavity delineation and dummy sources were placed in catheters for subsequent position digitization.
Figure 2.
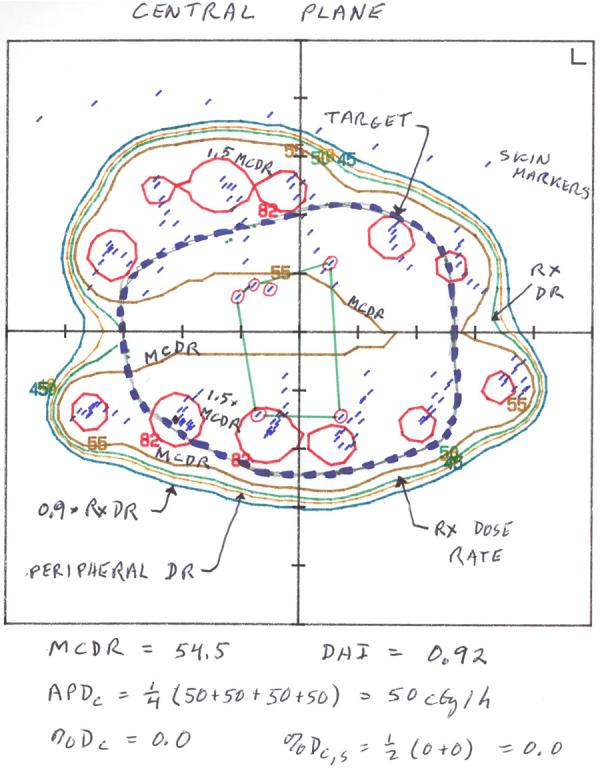
Central plane view of 2-dimensional brachytherapy implant dosimetric plan
Quality assurance and rapid review
An important aspect of this protocol was the thorough quality assurance program employed. Each participating institution was required to undergo a credentialing process which reviewed the necessary skills and knowledge for proper multicatheter treatment planning and dosimetric submission process. Completion of the institutional credentialing process was necessary prior to patient enrollment. Subsequently, the implant dosimetry on every case was required to be reviewed by the principal investigator prior to the initiation of dose delivery. All dosimetric information was reviewed through a twenty-four hour rapid turn-around review process that provided confirmation that the implant was ideally constructed and the dose to be delivered as per protocol. If deviation was identified, recommendations were made and corrections performed prior to the initiation of dose delivery thus assuring protocol compliant treatment for all patients.
Endpoint definitions
Patients were followed routinely after treatment by the radiation oncologist and surgeon in an alternating fashion. The disease status, toxicity and cosmetic outcome were documented at regular intervals. Toxicity has been reported in a previous publication.14 All local, regional and distant disease failures were documented as well as any new primary disease or mastectomy performed for any reason. This information allowed evaluation of several endpoints including in-breast control, regional control, mastectomy-free rate, mastectomy-free survival, disease-free survival and overall survival. Any disease recurrence within the treated breast was defined as a local failure and the location within the breast was further described as within the volume (of treatment) and/or outside the volume (of treatment). If recurrent disease was documented in the ipsilateral axillary, infraclavicular, supraclavicular or internal mammary lymph node regions then the recurrence was registered as a regional failure. Second primary failure was defined as any new primary disease including any contralateral breast cancers. Failure for the disease-free survival endpoint includes local, regional, distant or contralateral failure, as well as death from any cause.
Statistics
The protocol was designed to collect, analyze and present outcomes for HDR and LDR brachytherapy techniques separately and was neither designed to compare differences between the two methods or to collectively present the outcome of the entire cohort of 99 patients. The method of brachytherapy dose delivery was the decision of the treating radiation oncologist. However, if the assumption can be made that the two treatment approaches are equivalent then the conclusions drawn from this analysis can be strengthened through a larger combined study group. We believe this assumption to be valid and therefore have presented the data both individually, HDR and LDR, as well as combined.
Results
RTOG protocol 95-17 opened in May of 1997 until March of 2000. Over this time period, eleven institutions participated by enrolling a total of 100 patients, only one of which was excluded from analysis as a result of undergoing a sentinel lymph node procedure as opposed to the required level I/II axillary dissection. (Table 1) Review of the pretreatment characteristics found that in general this was a conservatively selected cohort of patients, although it should be noted that 20% were node positive and 21% were under that age of 50. The majority of the enrolled patients were ≥ 50 years old, postmenopausal and presented with T1, N0 disease that was frequently estrogen receptor and/or progesterone positive. (Table 2) From the total cohort of 99 patients, only 21% were <50 and 14% known to be premenopausal. The number of patients that presented with tumor sizes ≤ 2 cm is 88% and 80% of patients were without evidence of axillary nodal disease. The majority of patients received some form of adjuvant systemic treatment as a part of their treatment course. Within the HDR treated group 32 (49%) received adjuvant tamoxifen, 8 (12%) received chemotherapy and 8 (12%) received both. In the LDR treated group 8 (24%) received tamoxifen, 7 (21%) chemotherapy and 7 (21%) both.
Table 1.
Patient Accrual by Submitting Institution
| Virginia Commonwealth University, MCVH | 31 |
| Ochsner Clinic CCOP | 20 |
| Univ. Colorado Health Sciences Center | 12 |
| Medical College of Wisconsin | 10 |
| Mt Sinai Comprehensive Cancer Center | 9 |
| Beaumont CCOP | 8 |
| Roger Maris Cancer Center | 5 |
| Wake Forest University Baptist Medical | 2 |
| Tulane University Medical Center | 1 |
| Cleveland Clinic Foundation | 1 |
| University of Texas-MD Anderson Cancer | 1 |
Table 2.
Pretreatment Characteristics
| HDR (n=66) | LDR (n=33) | Combined HDR & LDR (n=99) |
||
|---|---|---|---|---|
| Median (min, max) |
62 (33, 83) |
62 (40, 80) |
62 (33, 83) |
|
| Age | <50 | 14 (21%) | 7 (22%) | 21 (21%) |
| 50-59 | 15 (23%) | 9 (27%) | 24 (24%) | |
| 60-69 | 13 (20%) | 8 (24%) | 21 (21%) | |
| ≥70 | 24 (36%) | 9 (27%) | 33 (33%) | |
| 80 | 1 ( 2%) | 1 ( 3%) | 2 (2%) | |
| Karnofsky Score | 90 | 10 (15%) | 9 (27%) | 19 (19%) |
| 100 | 54 (81%) | 22 (67%) | 76 (77%) | |
| Unknown | 1 ( 2%) | 1 ( 3%) | 2 (2%) | |
| T-stage | T1 | 58 (88%) | 29 (88%) | 87 (88%) |
| T2 | 8 (12%) | 4 (12%) | 12 (12%) | |
| N-stage | N0 | 53(80%) | 26(79%) | 79 (80%) |
| N1 | 13(20%) | 7(21%) | 20 (20%) | |
| Estrogen | Negative | 10(15%) | 11(33%) | 21 (21%) |
| Receptor Status | Positive | 53(80%) | 21(64%) | 74 (75%) |
| Unknown | 3(5%) | 1(3%) | 4 (4%) | |
| Progesterone | Negative | 14(21%) | 13(39%) | 27 (27%) |
| Receptor Status | Positive | 49(74%) | 19(58%) | 68 (69%) |
| Unknown | 3(5%) | 1(3%) | 4 (4%) | |
| Pre-menopausal | 10 (15%) | 4 (12%) | 14 (14%) | |
| Menopausal Status | Peri-menopausal | 2 (3%) | 2 (6%) | 4 (4%) |
| Post-menopausal | 52 (79%) | 27 (82%) | 79 (80%) | |
| Unknown | 2 ( 3%) | 0 | 2 (2%) | |
Median follow-up for the entire group of 99 patients is 7 years. In the group treated with HDR brachytherapy the median follow-up was 6.55 years (min.93, max 8.97) and for the group of patients treated with LDR brachytherapy, 7.09 years (min. 3.01, max. 9.24). The estimated 5yr local failure rate (in-breast failure) is 4% for the 99 patient cohort. (Table 3) Upon evaluation of the location of failure within the breast, it is found that there is a lower rate of failure outside the volume of treatment (elsewhere failure), 2% 5yr estimate, as compared to failures within volume (true recurrence/marginal miss), 3% 5 yr estimate. Details indicate that of those that failed within the treated breast, 4 patients failed within the volume, 1 patient failed outside the volume and one had an in-breast failure documented as both. Patient and tumor characteristics for each of the 6 patients who developed a local failure are listed in Table 4. A total of 4 regional (nodal) failures were documented, 3% 5yr estimate, with the patient and tumor characteristics for each of these 4 patients listed in Table 5. In breast local failure and regional failure events were infrequent and relationships and trends to patient and tumor characteristics were not apparent. Contralateral and distant 5 yr estimated failure rates were low as expected, see Table 3. Nine patients proceeded to mastectomy, six for recurrent disease and three for soft tissue toxicity/cosmetic failure. The 5 yr estimated survival rates; mastectomy free, disease free and overall, are comparable to that expected for the level of disease encountered in this patient cohort.
Table 3.
Failure pattern endpoint
| HDR (n=66) | LDR (n=33) | Combined Group (n=99) |
||||
|---|---|---|---|---|---|---|
| Failures | 5-Year Estimate |
Failures | 5-Year Estimate |
Failures | 5-Year Estimate |
|
| Local failure | 2 | 3% | 4 | 6% | 6 | 4% |
| Within volume | 2 | 3% | 3 | 3% | 5 | 3% |
| Outside volume | 1 | 2% | 1 | 3% | 2 | 2% |
| Regional failure | 3 | 5% | 1 | 0% | 4 | 3% |
| Local-regional failure | 4 | 6% | 4 | 3% | 8 | 5% |
| Contralateral failure | 1 | 2% | 2 | 6% | 3 | 3% |
| Distant failure | 9 | 5% | 1 | 3% | 10 | 4% |
| Second primary failure | 4 | 6% | 3 | 6% | 7 | 6% |
| Mastectomy failure | 3 | 5% | 6 | 13% | 9 | 8% |
| Mastectomy free survival | 12 | 88% | 8 | 85% | 20 | 87% |
| Disease free survival | 14 | 86% | 7 | 88% | 21 | 87% |
| Overall survival | 9 | 92% | 3 | 94% | 12 | 93% |
HDR – High Dose Rate Brachytherapy
LDR – Low Dose Rate Brachytherapy
Table 4.
Patient and Tumor Characteristics for Patients Documented to have Local Failure
| Pt# | Method of treatment |
Age | Systemic therapy |
ER status | PR status | T-stage | N-stage | Time to failure (Years) |
|---|---|---|---|---|---|---|---|---|
| 1 | HDR | 41 | CTX | Neg | Neg | T1 | N1 | 1.96 |
| 2 | HDR | 72 | None | Neg | Neg | T1 | N0 | 2.23 |
| 3 | LDR | 51 | None | Pos | Pos | T1 | N0 | 5.68 |
| 4 | LDR | 43 | None | Not done | Not done | T1 | N0 | 4.30 |
| 5 | LDR | 40 | None | Pos | Pos | T1 | N0 | 6.14 |
| 6 | LDR | 44 | TAM | Neg | Neg | T2 | N0 | 3.86 |
HDR – high dose rate brachytherapy
LDR – low dose rate brachytherapy
TAM – tamoxifen
CTX - chemotherapy
Table 5.
Patient and Tumor Characteristics for Patients Documented to have Regional Failure
| Pt# | Method of treatment |
Age | Systemic Therapy |
ER status | PR status | T-stage | N-stage | Time to failure (Years) |
|---|---|---|---|---|---|---|---|---|
| 1 | HDR | 55 | TAM/CTX | Neg | Neg | T1 | N0 | 1.5 |
| 2 | HDR | 77 | None | Pos | Neg | T1 | N0 | 4.18 |
| 3 | LDR | 40 | None | Pos | Pos | T1 | N0 | 6.82 |
| 4 | HDR | 41 | CTX | Neg | Neg | T1 | N1 | 2.83 |
HDR – high dose rate brachytherapy
LDR – low dose rate brachytherapy
TAM – tamoxifen
CTX - chemotherapy
Discussion
At its inception, APBI found support through extrapolation from contemporary pathologic studies and in-breast failure patterns.1 This data suggests that in selected patients, the location within the breast that an in-breast failure is most likely to occur is in the immediate area of the lumpectomy cavity. Furthermore, the risk of failing elsewhere in the breast (or in a location remote from the initial lumpectomy) was only anticipated to be 1-3% and expected to occur whether whole breast irradiation was delivered or not. 15-17 This finding led to the proposal that the impact of whole breast irradiation on the reduction of in-breast failures is primarily at the site of lumpectomy and the immediately surrounding breast tissue. This also suggested that the whole breast irradiation was unable to defend against new primary disease arising elsewhere in the breast and thus suggested that partial breast irradiation would yield equivalent results as compared to whole breast irradiation.18
Presently, the continued support for APBI can be drawn from several phase I-II studies now reported with extended follow-up. The majority of these reports are single institutional experiences. The results from this RTOG 95-17 study represent the most mature multi-institutional data studying APBI where appropriate selection criteria and quality assurance parameters were applied. With a median follow-up of 6.7 years, the reported overall in-breast failure rate of 4% is comparable to many WBI series.
The design of RTOG protocol 95-17 preceded image-based catheter placement techniques, CT-based implant evaluation, CT-based 3D brachytherapy treatment planning, a digital quality assurance submission process and the development of in-breast dose delivery treatment devices. In the short time since this protocol closed, we have technologically moved far beyond the catheter placement techniques and orthogonal film based dosimetry that was used in this trial. As a result of the multicatheter methodology applied within the early APBI protocols, it was anticipated that successful treatment outcome would require a higher degree of technical skill and experience to assure success. To better understand whether the multicatheter technique could be propagated to multiple centers, a multi-institutional study was necessary to evaluate how well the approach could be used across various practices. All eleven participating institutions, illustrated in table 1, were successful in treatment delivery according to protocol. The 3 tiered quality assurance program and case review process employed (Pre-approval credentialing of institutions, rapid review of each case prior to tx and retrospective review of each case including an independent calculation of delivered dose) provided a successful vehicle to assure consistent treatment delivery for all cases. This has enabled outcome data assessment across the patient cohort as well as lead to the suggestion that multi-catheter brachytherapy could be widely adopted if desired. Details of the quality assurance program are the subject of a forthcoming publication. Since the closure of this protocol, advances have lead to improved target identification, dose delivery techniques and better definition of dose homogeneity boundaries. These advances should logically lead to improved in-breast control and reduced toxicity as compare to the outcome seen with the original treatment approaches and the brachytherapy technique used within this protocol.
Although >5 year follow-up data has been presented at national and international meetings over the past year, few institutions have published their long term results. Formal publications are anticipated but, at this time, only three institutions have contributed data with extended follow-up equalling >5 years. These three single institutions have reported long term results from PBI treated with a multicatheter brachytherapy technique, utilized a quality assurance program and applied conservative selection criteria.19-21 Despite slight variability in selection criteria, the majority of women treated within these trials were >50 years old, presented with small invasive ductal tumors, resected with negative margins, node negative and positive estrogen receptors (Table 6). The original pilot trial of 50 patients treated at the Ochsner Clinic was reported in 2000 by King, et al.19 Median follow-up at the time of publication was 75 months and in-breast failure rate was excellent at 2%. Three patients failed in the regional nodes and therefore the loco-regional failure rate was 8%. The National Institute of Oncology in Budapest Hungary also published results, Polgar, et al, on their initial experience of 45 patients treated on an institutional phase I/II trial.20 These results were published in 2004 with a median follow-up of 81 months. The 5 and 7 year actuarial in-breast failure rate was 4.4% and 9.0%, respectively – this difference was noted to not be statistically different. All of these failures were registered as elsewhere failures. Recently, Vicini, et al, updated their multicatheter APBI treatment experience of 199 patients with 96 month median follow-up.21 In-breast actuarial 5 year failure rates remain stable at 1.8% with 10 year actuarial in-breast failure rates to slightly increase to 3.6%. Overall they are reporting six in-breast failures to date with 3 listed as a true-recurrence marginal miss and 3 listed as elsewhere failures. However, this was determined by the location of failure within the breast. When Loss of Heterozygosity (LOH) testing was used to determine the potential of a clonogenic relationship with the original primary lesion, only one of the six in-breast failures was determined to be a new primary, thus reducing the elsewhere failure rate, by LOH testing, to be only .8%. The remaining 5 cases were labeled as a local recurrence of the initial primary lesion.
Table 6.
Patient Selection Criteria for experience reported with >5 year follow-up
| Institution | # Cases |
Age (median) |
Size (Median) |
Margin | ER+ | LN− |
|---|---|---|---|---|---|---|
| Ochsner Clinic19 | 51 | 63 (mean) |
14 mm (mean) |
Neg | 64% * (21%unk) |
71% |
| NIO, Hungary20 Phase I/II Trial |
45 | 56 | 12mm | Neg | 84% | 80% (17%cNO) |
| William Beaumont Hosp 21 | 199 | 65 | 11 mm | Neg | 86% | 88% |
| RTOG 95-17 | ||||||
| HDR | 66 | 62 | 88% T1 | Neg | 80% | 80% |
| LDR | 33 | 62 | 88% T1 | 64% | 79% | |
reported in abstract update of 163 patients, San Antonio Breast meeting, 2005
Conclusions
This report of extended follow-up results for RTOG 95-17 reveals excellent results. With a median follow-up of 7 years, the five year actuarial in-breast failure rate is reported at only 4%, HDR brachytherapy 3% and LDR 6%. The reported overall survival rate for the entire cohort is expectedly high at 93% and individually 92% and 94% for HDR and LDR, respectively. As stated, the majority of patients received systemic management as hormonal therapy, chemotherapy or both. The small patient numbers in this study limits the ability to assess the impact of systemic management on reducing failure rates and patterns, but some degree of contributory affect would be expected. The importance of these results is accentuated with the knowledge that this RTOG study is a multi-institutional study representing varying experience and skill level with this treatment technique. Despite this, the local control and survival outcome are excellent and compare directly with single institutions results reporting with similar extended follow-up. This report of RTOG protocol 95-17 continues to support the concept of APBI. The inclusion of patients with 1-3 positive nodes without extracapsular extension and younger women remains controversial as appropriate selection criteria for APBI. Twenty percent of the patients treated on this trial were node positive and 21% were less than 50 years old without any obvious adverse outcome. Although this is a limited experience, this should encourage enrollment of younger patients and women of all ages with 1-3 positive nodes on the accruing phase III trial so that a definitive answer can be obtained. However, in general, the patient group treated within the guidelines of this protocol conforms to the conservative selective criteria seen in all three of the trials published with similar extended follow-up.19-21 As a result, unless treating as per a protocol dictating otherwise, an appropriately conservative approach should be considered with patient selection and dose delivery when treating with APBI until additional long term outcome reports are available.
Acknowledgments
Supported by NCI grants U24 CA 81647, U10 CA21661, U10 CA37422.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the American Society for Therapeutic Radiology and Oncology, Philadelphia, PA, 2006
Author Disclosure: D.W. Arthur, none; K. Winter, None; R.R. Kuske, Nucletron, Company/Organization,F. Consultant/Advisory Board; J. Bolton, None; R. Rabinovitch, None; J. White, None; W.F. Hanson, None; R.M. Wilenzick, None; B. McCormick, None.
References
- 1.Arthur DW, Vicini FA. Accelerated partial breast irradiation as a part of breast conservation therapy. J Clin Oncol. 2005;23:1726–1735. doi: 10.1200/JCO.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 2.Vicini FA, Kestin LL, Goldstein NS, et al. Impact of young age on outcome in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Clin Oncol. 2000;18:296–306. doi: 10.1200/JCO.2000.18.2.296. [DOI] [PubMed] [Google Scholar]
- 3.Keisch M, Vicini F, Kuske RR, et al. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;55:289–293. doi: 10.1016/s0360-3016(02)04277-3. [DOI] [PubMed] [Google Scholar]
- 4.Dickler A, Kirk MC, Chu J, et al. The MammoSite breast brachytherapy applicator: a review of technique and outcomes. Brachytherapy. 2005;4:130–136. doi: 10.1016/j.brachy.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Vicini FA, Remouchamps V, Wallace M, et al. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;57:1247–1253. doi: 10.1016/s0360-3016(03)01573-6. [DOI] [PubMed] [Google Scholar]
- 6.Formenti SC, Truong MT, Goldberg JD, et al. Prone accelerated partial breast irradiation after breast-conserving surgery: preliminary clinical results and dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;60:493–504. doi: 10.1016/j.ijrobp.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 7.Taghian AG, Kozak KR, Doppke KP, et al. Initial dosimetric experience using simple three-dimensional conformal external-beam accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2006;64:1092–1099. doi: 10.1016/j.ijrobp.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Taghian AG, Kozak KR, Katz A, et al. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int J Radiat Oncol Biol Phys. 2006;65:1404–1410. doi: 10.1016/j.ijrobp.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Gatti G, Luini A, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery. Arch Surg. 2003;138:1253–1256. doi: 10.1001/archsurg.138.11.1253. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya JS, Tobias JS, Baum M, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol. 2004;5:165–173. doi: 10.1016/S1470-2045(04)01412-3. [DOI] [PubMed] [Google Scholar]
- 11.Pignol JP, Keller B, Rakovitch E, et al. First report of a permanent breast 103Pd seed implant as adjuvant radiation treatment for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2006;64:176–181. doi: 10.1016/j.ijrobp.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 13.Kuske RR, Bolton JS. A phase I/II trial to evaluae brachytherapy as the sole method of radiation therapy for stage I and II breast carcinoma. Radiation Therapy Oncology Group Publication no. 1055. 1995 [Google Scholar]
- 14.Kuske RR, Winter K, Arthur D, et al. A phase I/II trial of brachytherapy alone following lumpectomy for select breast cancer: Toxicity analysis of radiation therapy oncology group 95-17. Int J Radiat Oncol Biol Phys. 2002:54. doi: 10.1016/j.ijrobp.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 16.Clark RM, McCulloch PB, Levine MN, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst. 1992;84:683–689. doi: 10.1093/jnci/84.9.683. [DOI] [PubMed] [Google Scholar]
- 17.Liljegren G, Holmberg L, Bergh J, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326–2333. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 18.Morrow M. Rational local therapy for breast cancer. N Engl J Med. 2002;347:1270–1271. doi: 10.1056/NEJMe020112. [DOI] [PubMed] [Google Scholar]
- 19.King TA, Bolton JS, Kuske RR, et al. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer. Am J Surg. 2000;180:299–304. doi: 10.1016/s0002-9610(00)00454-2. [DOI] [PubMed] [Google Scholar]
- 20.Polgar C, Major T, Fodor J, et al. High-dose-rate brachytherapy alone versus whole breast radiotherapy with or without tumor bed boost after breast-conserving surgery: seven-year results of a comparative study. Int J Radiat Oncol Biol Phys. 2004;60:1173–1181. doi: 10.1016/j.ijrobp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Vicini FA, Antonucci JV, Wallace M, et al. Long-term efficacy and patterns of failure after accelerated partial breast irradiation: A molecular assay-based clonality evaluation. Int J Radiat Oncol Biol Phys. 2007 doi: 10.1016/j.ijrobp.2006.12.007. [DOI] [PubMed] [Google Scholar]