Abstract
Depolarization of the mitochondrial inner membrane potential (ΔΨm) associated with oxidative stress is thought to be a critical factor in cardiac dysfunction and cell injury following ischemia-reperfusion or exposure to cardiotoxic agents. In isolated cardiomyocytes, mitochondrially-generated reactive oxygen species (ROS) can readily trigger cell-wide collapse or oscillations of ΔΨm but is it not known whether these phenomena scale to the level of the whole heart. Here we utilize two-photon laser scanning fluorescence microscopy to track ΔΨm, ROS, and reduced glutathione (GSH) levels in intact perfused guinea-pig hearts subjected to ischemia-reperfusion or GSH depletion with the thiol oxidizing agent diamide. Exposure to oxidative stress by either method provoked heterogeneous ΔΨm depolarization and occasional oscillation in clusters of myocytes in the epicardium in association with increased mitochondrial ROS production. Furthermore, the whole heart oxidative stress dramatically increased the sensitivity of seemingly quiescent cells to ΔΨm depolarization induced by a localized laser flash. These effects were directly correlated with depletion of the intracellular GSH pool. Unexpectedly, hearts perfused with nominally Ca2+-free solution or those switched from 0.5 mM Ca2+ to nominally Ca2+-free solution also displayed heterogeneous ΔΨm depolarization and oscillation, in parallel with net oxidation of the GSH pool. The findings demonstrate that metabolic heterogeneity initiated by mitochondrial ROS-induced ROS release is present in the intact heart, and that the redox state of the glutathione pool is a key determinant of loss of ΔΨm.
Keywords: monochlorobimane, reactive oxygen species, mitochondrial membrane potential
Introduction
Mitochondria are key participants in life and death decisions for cells and this is particularly evident for ischemia-reperfusion in the heart. Emerging evidence has indicated that loss of mitochondrial function contributes to contractile failure, arrhythmias, and apoptotic or necrotic cell death[1–3].
Mitochondria are important physiological sources of reactive oxygen species (ROS). During oxidative phosphorylation, up to 2% of oxygen utilization goes to H2O2 production[4]. The detrimental effects of ROS include oxidation of nucleotides[5], calcium overload[6], ΔΨm depolarization and oscillations[7], and ROS-induced ROS release[8, 9]. The “free radical theory of aging”[10] incorporates many of these concepts and, more specifically, ROS production is the centerpiece of the redox mechanisms of mitochondrial aging[11]. More acutely, during normoxic reperfusion after ischemia, an increased rate of ROS production potentiates ischemic injury[12, 13].
In isolated cardiomyocytes, either an increase in ROS[7] or depletion of the antioxidant pool[14] can trigger self-sustained oscillations in ΔΨm in the mitochondrial network when a critical threshold is reached (so called mitochondrial criticality)[9]. This behavior can also be simulated using a computational model of ROS-induced oscillation in a single mitochondrion[15]. Importantly, mitochondrial oscillations were shown to be closely linked with changes in cellular electrical excitability, as a consequence of uncoupled mitochondrial ATP consumption, which drives the activation of ATP-sensitive K+ (KATP) channels in the sarcolemma[7, 16]. Through these mechanisms, we postulated that mitochondrial ΔΨm instability could introduce dispersion of action potential repolarization in the myocardium, increasing the susceptibility to fatal ventricular arrhythmias[17]. Support for this idea was obtained in intact hearts, in which post-ischemic arrhythmias could be blocked by a mitochondrial benzodiazepine receptor ligand[17, 18] that prevents loss of ΔΨm under oxidative stress[7, 19].
Despite the intriguing parallels between the behavior of isolated myocytes and whole heart responses, it remains to be determined if the nonlinear oscillatory phenomena described in single cells can be observed at the level of the whole heart. In the intact myocardial syncytium, cell-cell junctions may modify the cellular response as a result of metabolic, Ca2+, and electrical communication in the tissue. Hence, the present study focused on testing whether conditions known to promote mitochondrial criticality (e.g., GSH depletion, ischemia-reperfusion) can trigger mitochondrial ΔΨm instability in perfused hearts.
Materials and Methods
In accordance with Guide for the Care and Use of Laboratory Animals (NIH, No. 85-23, 1996) and the Johns Hopkins Animal Care and Use Committee, adult guinea pigs (300g) were anesthetized with 260mg pentobarbital and 1000U heparin sodium (i.p.). The hearts were then excised and retrogradely perfused with an oxygenated (100% O2) modified-Tyrode’s solution (138mM NaCl, 4mM KCl, 0.5 mM CaCl2, 1mM MgCl2, 0.33mM NaH2PO4, 10mM glucose, 10mM HEPES pH 7.4) which contained butanedione monoxime (BDM; 20 mM) to suppress contraction[20] in order to enable the tracking of epicardial myocytes over time. Alternatively, for ischemia-reperfusion experiments, the mechanical uncoupler blebbistatin (10μM) was used to arrest contraction, as recently described[21], instead of BDM, in order to address concerns related to possible non-specific effects of the latter compound. The perfusate (7 ml/min) and the tissue chamber were maintained at 37°C.
Whole heart imaging
To minimize pulsatile motion artifacts, the perfusion system was dampened, and hearts were mounted on a custom-built chamber consisting of a heated base, a mounting slot for the perfusion cannula, and a coverslip holder. A glass coverslip (25mm) was gently applied to the surface of the heart. These interventions were necessary to eliminate movement of the microscopic field of view for long duration recordings. A 20× (0.75 N.A.; ischemia-reperfusion experiments) or a 40× (1.0 N.A.; all other experiments) water immersion objective lens was used to image epicardial regions located near (within 5 mm of) the left anterior descending coronary artery (LAD).
Fluorescent probes and imaging conditions
Images were obtained using a BioRad 1024MP two-photon laser-scanning microscope with a 10W Millenia X pumped-Tsunami Ti-sapphire mode-locked laser at 740 nm (80 MHz; <80fs pulses). Fluorescence signals were acquired at focal depths up to a 200μm. 512×512 pixel resolution images were obtained every 3.5 seconds at low laser power (<10mW) to minimize photobleaching.
Tetramethylrhodamine methyl ester (TMRE; loaded by recirculation for 30 min – 1 Hr) at a concentration of 100 nM) was used to measure ΔΨm, as previously described[7]. When stimulated by two-photon laser excitation at 740nm, TMRE emits light with a peak near 580nm (detection bandpass was from 565–645nm). TMRE was easily differentiated from 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-DCF, used to measure ROS formation) which was simultaneously excited at 740nm and emits in the detector bandpass of 500–550nm.
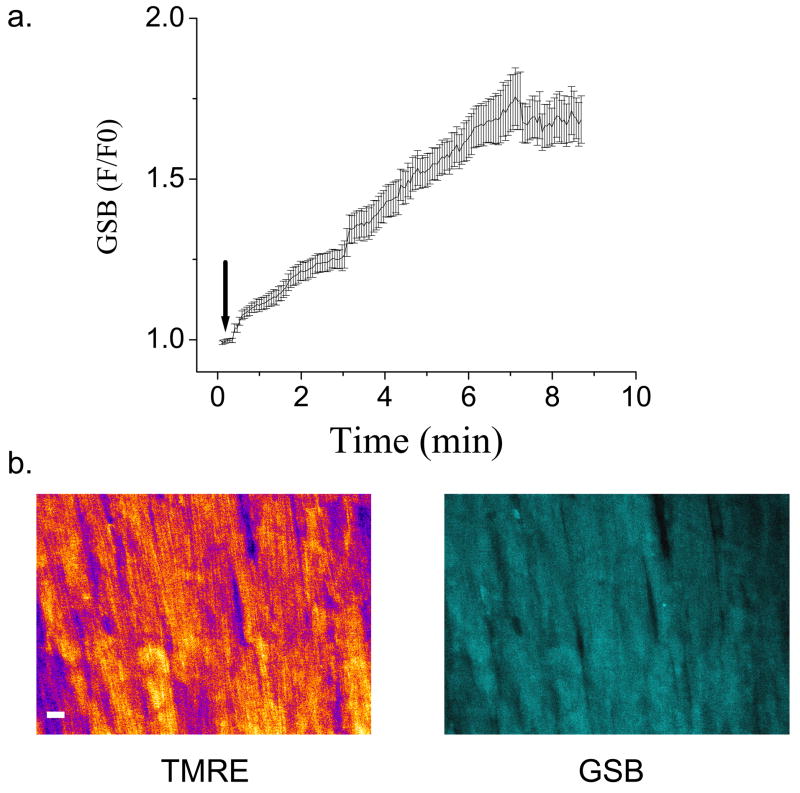
Monochlorobimane (MCB) was used to measure the levels of reduced glutathione (GSH) in the heart or in isolated cells. This compound forms a fluorescent adduct (glutathione S-bimane; GSB) by reaction with GSH, in a reaction catalyzed by endogenous glutathione-S-transferase (GST)[22, 23]. GSB fluorescence was excited at 740nm and its emission (peak at ~480nm) was detected using a shortpass filter with a cutoff at 500nm. GSB could be monitored simultaneously with TMRE; however, since its emission spectrum overlaps with NADH, the latter could not be resolved in the presence of the much brighter GSB signal. In addition, the tail of the GSB emission spectrum partially overlapped with the 500–550nm detection band used for the CM-DCF signal, precluding simultaneous measurement of the CM-DCF. In hearts, after a 20-min stabilization period (to obtain baseline NADH fluorescence signal), monochlorobimane (MCB) 50μM was added to 10ml of perfusate and recirculated (at 1.5ml/min) until a steady-state fluorescence level was achieved. Intracellular accumulation of GSB was observed as an increase in 480nm fluorescence, which was approximately 4 times brighter than the NADH autofluorescence.
Freshly isolated cells were prepared as previously described [7] and superfused with a modified Tyrode’s solution containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 10 HEPES, 1 CaCl2, pH 7.5 (adjusted with NaOH), supplemented with 10mM glucose. The perfusion chamber containing the cardiomyocytes was thermostatically controlled at 37°C with unrestricted access to atmospheric oxygen on the stage of a Nikon E600FN upright microscope.
The concentrations of the probes used in the isolated myocyte experiments were: 100nM TMRM (tetramethylrhodamine methyl ester), 2–5μM CM-H2DCFDA, 4μM MitoSOX and 50μM MCB, loaded into the cells for >20min at 37°C. The response of the MCB fluorescence to changes in GSH was quantified by the addition of exogenous GSH as previously described [14]. In other experiments in isolated cells, we found that MCB concentrations ≤50μM did not affect cell viability, but higher concentrations (>200μM) led to ΔΨm depolarization, GSH depletion and NADH oxidation over time, as a result of depletion of the available GSH pool (see [14]). All fluorescent indicators were purchased from Invitrogen. All other reagents were from Sigma-Aldrich, unless otherwise specified.
Ischemia-reperfusion studies
Global ischemia was initiated by turning off the perfusion pump for 30 min followed by 30 min of reperfusion. In some experiments, regional ischemia was initiated by tightening a ligature placed around the LAD for 40 min, followed by 30 min reperfusion after releasing the occlusion.
Results
Two-photon laser scanning fluorescence microscopy was used to image the epicardium of isolated perfused hearts. This method allowed us to study the behavior of myocytes still connected in the myocardial syncytium with subcellular resolution.
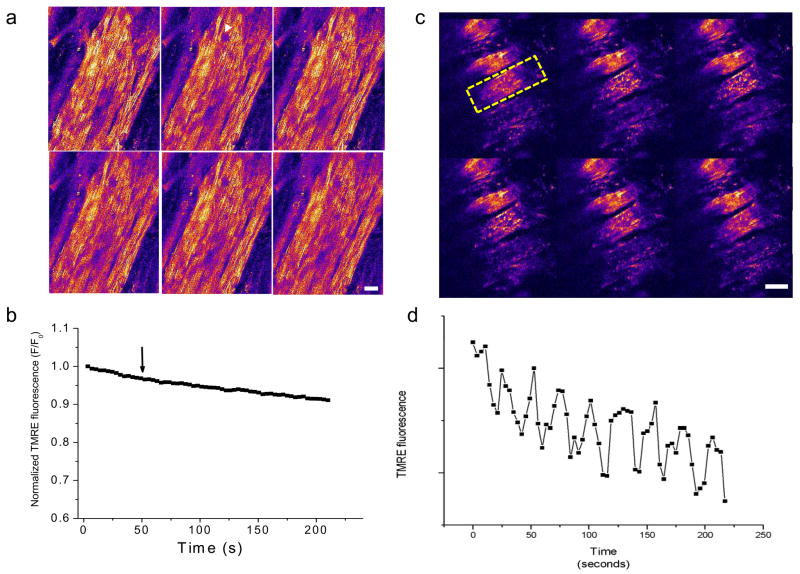
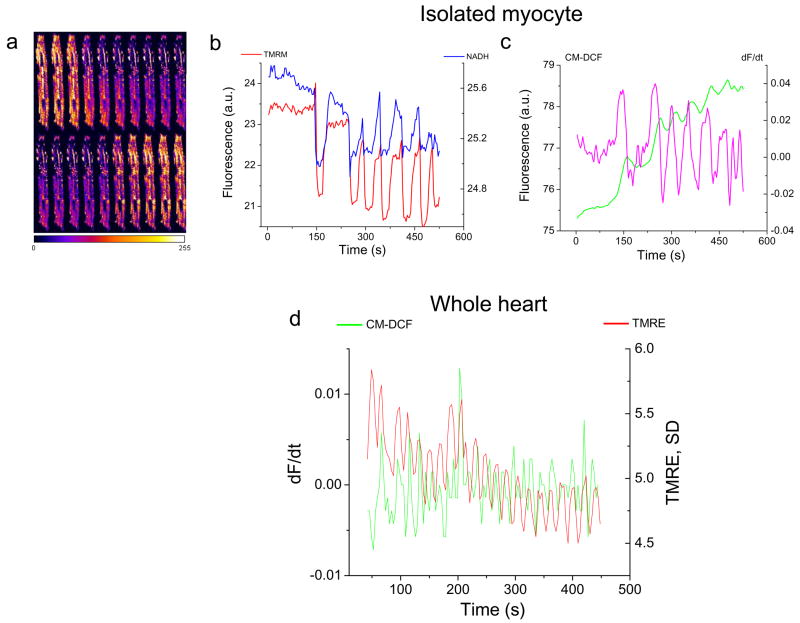
In previous studies[7, 9] we have reported that a localized laser flash, i.e., a high intensity scan of an 8μm × 8μm region of the cell image field, triggers ΔΨm oscillation in the entire mitochondrial network of isolated guinea-pig left ventricular myocytes. Hence, the first experiments in the intact heart were to test whether similar oscillations could be observed. Myocytes in the syncytium were found to be more resistant to laser-flash induced ΔΨm oscillation than in isolated cells when the heart was perfused with buffer containing 0.5 mM Ca2+ (Fig. 1a,b). Unexpectedly, we discovered that hearts perfused with nominally Ca2+-free buffer were much more prone to ΔΨm instabilities, which could be attributed to a difference in the cellular glutathione redox state (see additional experiments below). Under zero-Ca2+ conditions, oscillations in ΔΨm were often spontaneous (Fig. 1c,d and see movie in Supplemental Figure S1) or were readily triggered by a laser flash (see movie showing triggered ΔΨm and NADH oscillation in Supplement Figure S2), occurring either in individual cells or synchronized within clusters of cells. The amplitude and phase relationship of the oscillatory waveform in the whole heart (Figs. 1d and 2d) closely resembled results obtained in isolated cells and the oscillations were self-sustaining for many minutes (Supplemental Figure S1). As in isolated myocytes (Fig. 2a–c), flash-induced ΔΨm oscillations in the intact heart were correlated with bursts of mitochondrial ROS production, indicated by corresponding increases in the rate of oxidation of the CM-DCF fluorescent probe in conjunction with each depolarization cycle (Fig. 2d), consistent with a ROS-induced ROS release mechanism[7, 15].
Figure 1.
Mitochondrial membrane potential oscillations in myocytes of the intact heart perfused with Ca2+-free buffer. a, b) The ΔΨm signal (TMRE fluorescence) was stable over many minutes in hearts perfused with modified Tyrode’s containing 0.5 mM Ca2+ even after a localized laser flash was applied (white arrowhead in panel a, arrow in panel b). c, d) After 2–3 min of normoxic perfusion with Ca2+-free Tyrode’s, ΔΨm became unstable and spontaneous oscillations were observed. Sustained oscillations in ΔΨm were observed for several minutes in the cell indicated by the yellow dashed line in panel c. Both intracellular and intercellular heterogeneity of ΔΨm is evident in the epicardial optical sections. Scale bars in a and b equal 20 microns.
Figure 2.
Comparison of ΔΨm oscillations in isolated cardiomyocytes and intact myocardium. a) A montage of TMRE images during an oscillatory cycle in a single isolated cardiomyocyte. b) The phase relationship of ΔΨm (TMRE, red trace) and NADH (blue trace). c) The time course of CM-DCF fluorescence (green trace) for the same myocyte, and its derivative, which represents the rate of ROS production (dF/dt, magenta trace). d) ΔΨm oscillation and the concomitant bursts of ROS produced during each depolarization in one optical field of the intact heart. The red trace shows the variance of the TMRE signal (a measure of ΔΨm heterogeneity) plotted with the derivative of the ROS signal (CM-DCF, green). In this case, the peaks of the variance plot correspond to ΔΨm depolarization, which correlate with increased ROS production.(a.u: arbitrary fluorescence units)
Cellular heterogeneity of ΔΨm during ischemia and reperfusion
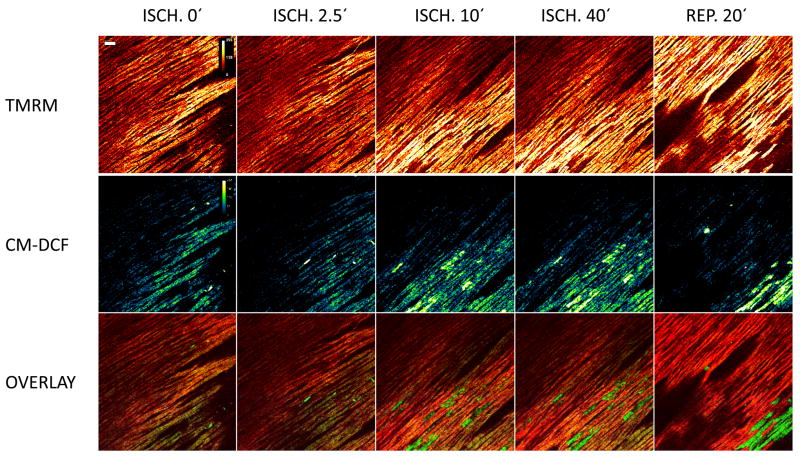
To assess whether pathophysiological stressors in addition to low Ca2+ or local laser flash could trigger ΔΨm instability, hearts were subjected to either global or regional ischemia followed by reperfusion. Striking heterogeneity of mitochondrial polarization was observed within 10–20 min of LAD occlusion, primarily consisting of individual cells whose mitochondria lost ΔΨm (Fig. 3, upper panels). As in isolated myocytes, mitochondrial network depolarization was correlated with very high ROS signals, as reported by CM-DCF fluorescence, recorded simultaneously (Fig. 3, middle panels). After a 40 min ischemia period, the recovery of ΔΨm upon reperfusion was incomplete, and larger clusters of cells undergoing sustained depolarization were observed. Unlike the low Ca2+ condition, no stereotypical oscillations were observed. A similar pattern of depolarization was observed under conditions of simulated ischemia using metabolic inhibition and washout (see Supplemental movie in Figure S3).
Figure 3.
Regional ischemia induces heterogeneity of ΔΨm and ROS signals. Forty minutes of ischemia, initiated by LAD occlusion, precipitated marked cell-to-cell heterogeneity of ΔΨm (upper (panels) that correlated with oxidative stress (middle panels). As indicated in the overlays (lower panels), the highest levels of CM-DCF fluorescence corresponded to cells with completely depolarized mitochondria, which became more pronounced after reperfusion. Scale bar equals 40 microns. Fluorescence intensity is represented by a pseudocolor lookup table between 0 and 255 units, as indicated.
We have previously proposed that “metabolic sinks” induced by ischemia-reperfusion underlie electrical inhomogeneity contributing to an arrhythmogenic substrate, which can be prevented by interventions that block ΔΨm oscillation and stabilize oxidative phosphorylation[17]. The electrical and contractile dysfunction could be prevented by treatment with 4′-chlorodiazepam, but not cyclosporine A[17, 24], consistent with our isolated cell studies implicating a PTP-independent mechanism. In the perfused hearts, the observed mitochondrial depolarization occurring during 30 min ischemia and reperfusion was completely prevented by 4′-chlorodiazepam treatment (Supplemental Figure S4). The present observations support the idea that metabolic sinks develop during ischemia-reperfusion, and that this pattern is related to individual cells experiencing high levels of oxidative stress.
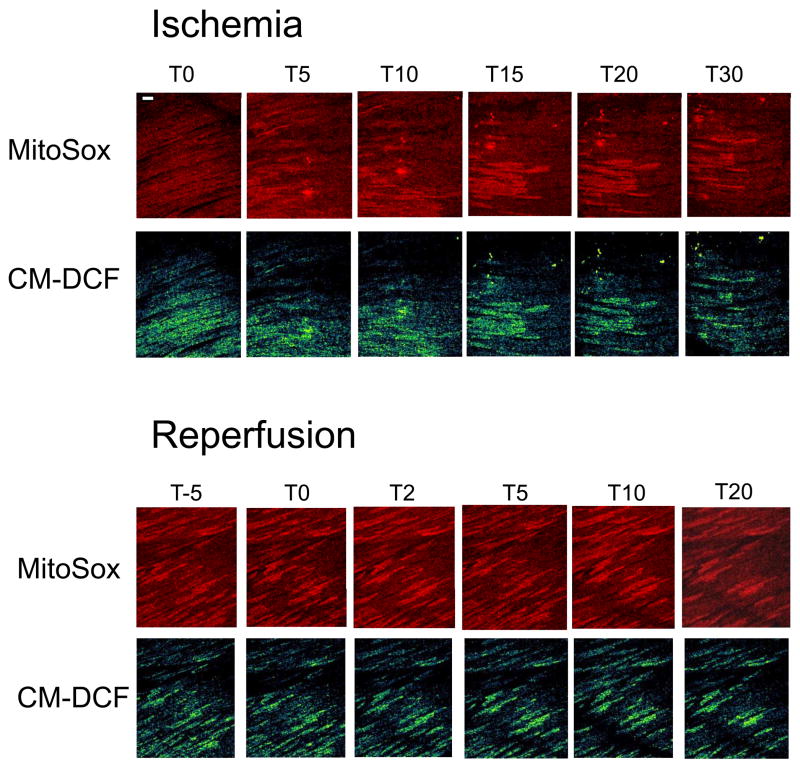
We further investigated which free radical species was contributing to the ischemia-related heterogeneity by loading the superoxide sensor MitoSOX along with CM-DCF in the absence of TMRE. As in the regional ischemia experiment described above, hearts exposed to global ischemia also displayed highly localized increases in oxidative stress in individual cells or clusters of cells (Fig. 4). Increases in CM-DCF fluorescence (indicative of mitochondrial H2O2 production; Fig. 4, lower panels) were correlated with localized increases in MitoSOX fluorescence (indicative of mitochondrial O2.− production; Fig. 4, upper panels), suggesting that the ROS were arising from the electron transport chain.
Figure 4.
Localization of superoxide and H2O2 production in perfused whole heart during ischemia/reperfusion. Double labeling of intact hearts with the mitochondrial superoxide indicator (MitoSOX) and CM-DCF indicate that both ROS contribute to ischemia-reperfusion-related oxidative stress and cellular heterogeneity, consistent with the mitochondrial respiratory chain being the source of the oxidative stress. Scale bar equals 40 microns.
Triggering mitochondrial oscillations in the whole heart: Role of intracellular GSH
In a recent study, we have reported that ΔΨm oscillation and sustained ΔΨm depolarization occur sequentially as a function of the cellular GSH/GSSG redox potential[14]. To test the hypothesis that the increased resistance of myocytes to flash-induced oscillation in the intact heart under normoxic conditions, as compared to isolated cells, is due to a more favorable glutathione redox status, we determined whether ΔΨm oscillations could be triggered in response to depleting the GSH pool with diamide[25], a pro-oxidant thiol reagent, and buthionine sulfoximine (BSO), an inhibitor of GSH synthesis.
GSH levels were monitored by the membrane permeant fluorescent indicator monochlorobimane (MCB)[15, 26]. This probe reports the level of GSH as the fluorescent product glutathione S-bimane (GSB) according to the reversible reaction: MCB + GSH ⇔ GSB, catalyzed by glutathione S-transferase. In initial experiments, we determined that concentrations of MCB up to 50 μM permit measurement of the GSH/GSSG redox status without significantly depleting the intracellular pool of GSH to a level that compromises cell viability (Fig. 5). MCB readily penetrated the cells and was converted to GSB in the intracellular compartment of myocytes in the intact heart. A steady-state fluorescence intensity was attained in less than 10 min of perfusion (Fig. 5a). The GSB fluorescence was homogeneously distributed throughout the cell including the nucleus (Fig. 5b).
Figure 5.
Measurement of reduced glutathione in the intact heart using monochlorobimane. Intact guinea pig hearts loaded with TMRE were perfused at a constant flow with oxygenated Tyrode’s solution for a 20-min stabilization period (to obtain baseline NADH fluorescence signal). Subsequently, 50μM MCB was added (at arrow in panel a) in a total perfusate volume of 10ml and recirculated until a steady state fluorescence was obtained, at which point, MCB-free Tyrode’s perfusion was restored. Intracellular uptake of MCB was observed as an increase in the 480nm fluorescence emission above the NADH baseline. a) The kinetics of GSB production in the intact heart during MCB loading. b) The intracellular distribution of TMRE and GSB in images of the myocardial syncytium. Epicardial images (about 100μm depth) were acquired at 3.5-second intervals. Scale bar in b equals 20 microns.
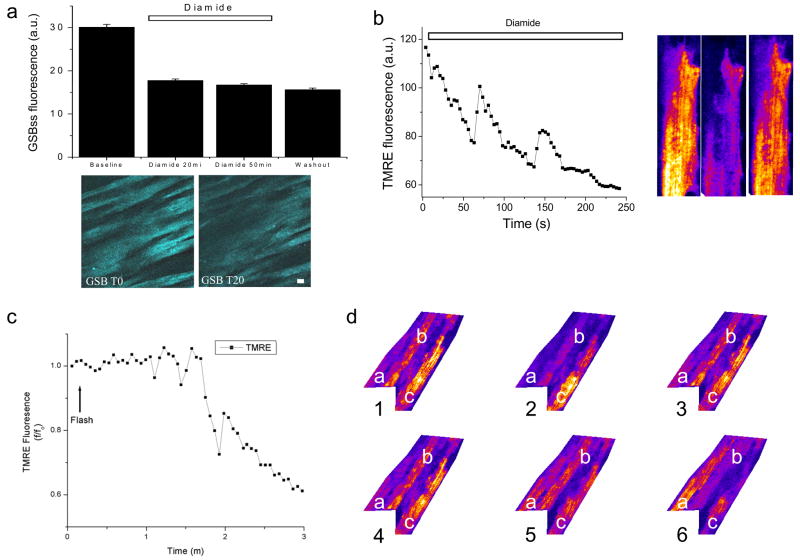
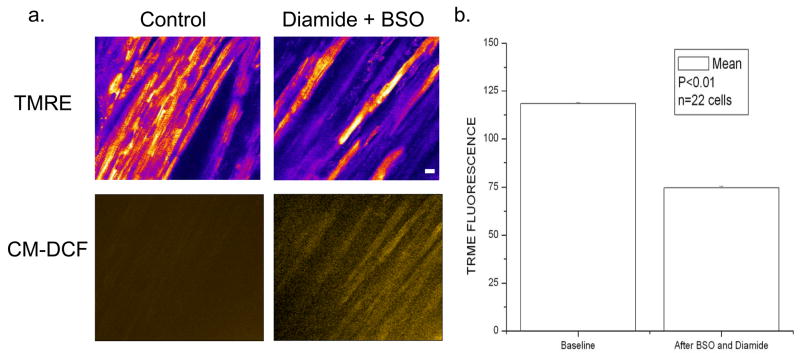
In response to the addition of 1mM diamide plus 1mM BSO, a 40% decrease in GSB fluorescence (Fig. 6a) and a decrease in TMRE fluorescence (Figs. 6b and 7) was observed in the intact heart under normoxic conditions. Oscillations in ΔΨm ensued spontaneously either in the absence (Fig. 6b) or presence of a laser flash (Fig. 6c), under conditions which did not trigger the response in controls (with the same 0.5 mM Ca2+ perfusate). Tracking ΔΨm in neighboring cells after diamide exposure revealed significant desynchronization of the oscillations in the myocardial syncytium – repolarization of an individual cell (e.g., cell “a” in Fig. 6d.) could be observed while an immediate neighbor (cell “b”) underwent ΔΨm oscillation with a third cell depolarizing much later (cell “c”). The decrease in average TMRE fluorescence (Fig. 7b) after diamide (+BSO) was due to the complete collapse of ΔΨm in individual cells, in association with increased oxidation of the ROS probe(Fig. 7a, lower panels). Similar results were obtained when diamide was added in the absence of BSO (results not shown).
Figure 6.
Depletion of reduced glutathione elicits mitochondrial ΔΨm oscillation in the intact heart. During normoxic perfusion, 1mM diamide, a thiol pro-oxidant, and 1mM BSO, an inhibitor of glutathione synthesis, were added to the perfusate to deplete the intracellular GSH pool. a) The average steady-state GSB fluorescence (GSBss) decreased in the presence of diamide and BSO when whole microscopic fields (below) were analyzed. The decreased GSBss levels persisted after washout (W) of the diamide and BSO. After whole-heart treatment with diamide and BSO, oscillations in ΔΨm were observed either spontaneously (b) or after a laser flash (c). d) A series of sequential images (1–6) of 3 representative cells in one optical field of the intact heart. Cell “a” is initially depolarized and repolarizes by image 6. Directly adjacent to cell “a”, cell “b” undergoes two ΔΨm oscillations over the same time period. Cell “c” is polarized in image 1, but partially depolarizes by the end of the sequence.
Figure 7.
ΔΨm loss is correlated with ROS increase in intact perfused hearts treated with BSO and diamide. a) ΔΨm is markedly depolarized (upper panels) in many cells after thiol depletion in intact myocardium in association with increased oxidation of the CM-DCF ROS probe (lower panels). b) The reduction in average TMRE fluorescence after BSO plus diamide application. Scale bar in a equals 20 microns
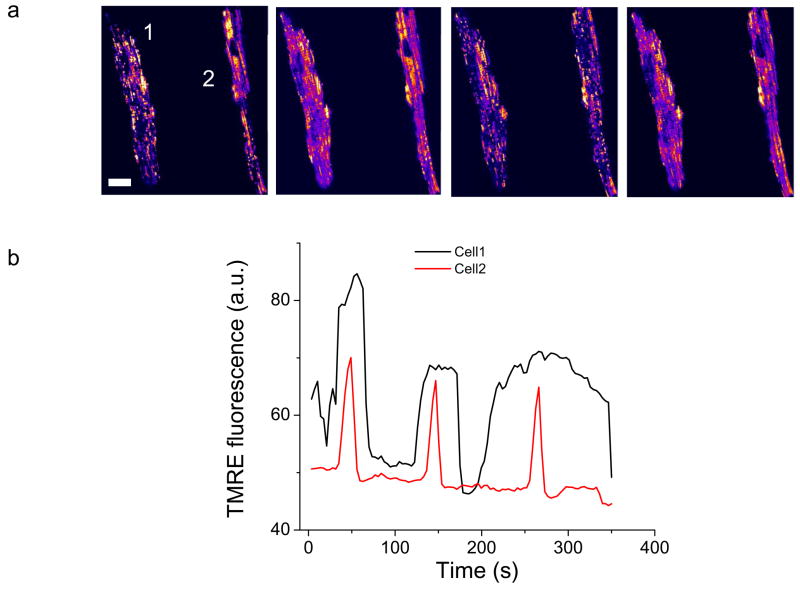
The results obtained for the induction of mitochondrial instability in the intact heart were quite similar to those observed for isolated myocytes treated with diamide, albeit with a different sensitivity to diamide, consistent with the isolation procedure resulting in partial depletion of the GSH pool. Treatment of isolated myocytes with ten times less diamide (0.1 mM) triggered ΔΨm oscillations (in the absence of a flash) after a ~30% decrease of the GSH pool (Fig. 8). Prolonged exposure to diamide led to sustained ΔΨm depolarization and cell death (hypercontracture) in 95% of the cardiomyocytes after 90 min, while higher concentrations of diamide (> 0.5 mM) killed the isolated cells in less than one hour.
Figure 8.
ΔΨm Oscillations induced by diamide in isolated cardiomyocytes. Freshly isolated cardiomyocytes were treated with 0.1mM diamide in Tyrode’s solution containing 1 mM Ca2+ and imaged by two photon laser scanning fluorescence microscopy. a) TMRE images during different phases of the oscillatory cycle. b) Representative time courses of diamide-induced ΔΨm oscillations in the two separate (unsynchronized) cells. Scale bar in a equals 20 microns
Spontaneous ΔΨm oscillations with Ca2+-free perfusion correlates with decreased GSH and increased ROS
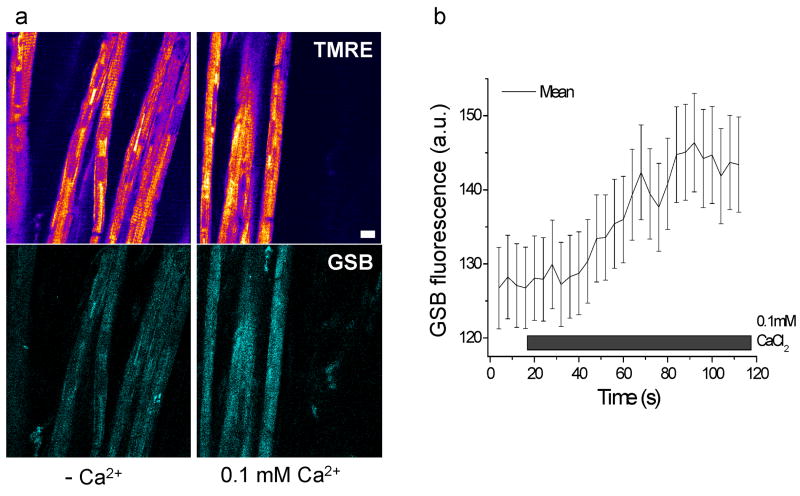
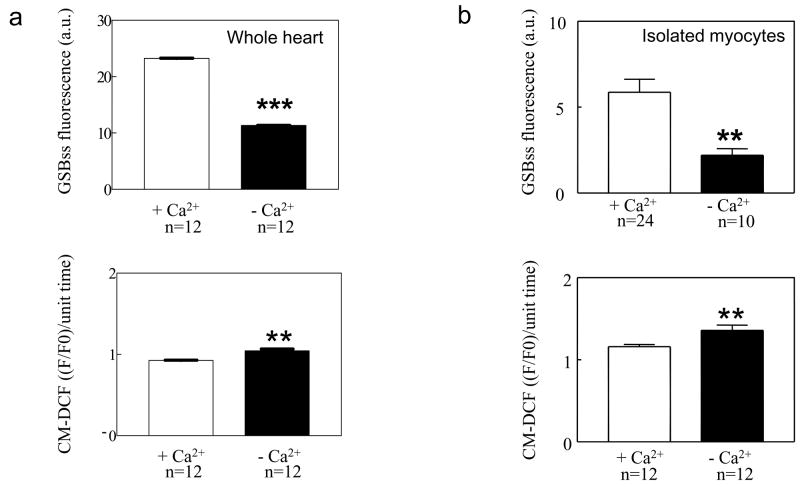
Enlightened by the finding that GSH plays a crucial role in the susceptibility of cells in the intact heart to undergo ΔΨm oscillation, we explored whether GSH levels were altered under nominally Ca2+ free conditions, which also increased mitochondrial instability (Fig. 1). GSB fluorescence was recorded in hearts perfused with Ca2+-free buffer and then Ca2+ was increased to 100μM (Fig. 9a). Increasing perfusate Ca2+ resulted in a significant increase in the mean intracellular GSH signal (Fig. 9b) and tended to suppress ΔΨm oscillations (not shown). Furthermore, nominally Ca2+-free perfused hearts (Fig. 10a) and isolated myocytes (Fig. 10b) had significantly higher CM-DCF fluorescence in concert with lower GSH signals.
Figure 9.
Enhanced GSH levels upon raising perfusate Ca2+ in the intact heart. a) An intact heart loaded with TMRE and MCB was perfused with nominally Ca2+-free buffer under normoxic conditions (left panels). Subsequent perfusion with buffer containing 100μM Ca2+ increased GSB fluorescence (right panels). b) GSB fluorescence increase following increase in Ca2+ in the perfusate. Scale bar in a equals 20 microns
Figure 10.
Summary of the effects of extracellular Ca2+ on GSH and ROS accumulation in isolated myocytes and intact heart. Removal of extracellular Ca2+ (nominally Ca2+ free) decreases GSH (upper panels) and increases ROS levels (lower panels) in intact hearts (a) and isolated cardiomyocytes (b) when compared with their respective controls (0.5mM Ca2+ in intact hearts and 1mM Ca2+ in isolated cardiomyocytes).
Discussion
The main contribution of the present work was to show that although cardiomyocytes in intact normally perfused hearts are more resistant to oxidative stress-induced effects on mitochondrial energetics than isolated myocytes, mitochondrial ΔΨm oscillations and/or depolarization can be triggered by ischemia and reperfusion or glutathione depletion. The findings extend the mechanistic findings concerning the mitochondrial ROS-dependent oscillator described in single cardiac myocytes[7, 9] and computational models[14] to the level of the myocardial syncytium. As such, the new information bridges the gap between cellular studies and whole heart mapping and arrhythmias that we have reported earlier[17].
The loss of mitochondrial function is widely acknowledged as a seminal event in necrotic and apoptotic cell death and is emerging as a key mediator of ischemia-reperfusion injury in the heart. What is still unclear is the precise mechanism responsible for the collapse of ΔΨm under conditions of metabolic stress. Alterations in Ca2+ handling and ROS clearly contribute to mitochondrial dysfunction, and over the past few decades the focus has been on trying to define the conditions leading to a mitochondrial permeability transition, i.e., the formation of a large cyclosporin-sensitive hole in the inner membrane that dissipates the protonmotive force and results in the leakage of large constituents (including apoptosis-inducing agents) up to 1.5 kDa out of the mitochondrial matrix.
On the other hand, recent evidence suggests that in some circumstances even relatively mild forms of stress, such as substrate deprivation[16, 19] or highly localized laser excitation[7] can induce changes in mitochondria that scale to produce emergent patterns of ΔΨm depolarization or oscillation throughout the mitochondrial network of the cardiomyocyte. This process, which we have called “mitochondrial criticality”[1, 9], can occur in the absence of mitochondrial Ca2+ overload. The approach to the critical state involves an autocatalytic positive feedback loop of ROS-induced ROS release, that, depending on the intensity of the stimulus, can activate either the permeability transition pore (PTP), or a lower conductance mitochondrial ion channel that supports oscillation[14]. The latter effect can be reversed and mitochondrial membrane potential stabilized by mitochondrial benzodiazepine ligands or inhibition of mitochondrial ROS production[7, 14].
During ischemia or metabolic inhibition, the mitochondrial ATP synthase can reverse to become a proton pumping ATPase[27]. In the short term, ATP hydrolysis by the FOF1 ATPase can delay the loss of ΔΨm during ischemia as long as the cellular ATP is not severely depleted; however, this futile cycle of ATP consumption can quickly alter the cytosolic ATP/ADP ratio resulting in the rapid activation of sarcolemmal KATP channels with an immediate impact on cell electrophysiology. In fact, the duration of the action potential can be directly correlated with the mitochondrial energy state[7], resulting in striking temporal heterogeneity of repolarization. This dispersion of repolarization in both time and space in the intact myocardium has been proposed as a mechanism underlying the increased propensity towards reentrant arrhythmias in post-ischemic hearts[17], through the creation of “metabolic sinks” in the tissue. Metabolic sinks are postulated to be clusters of cells in the syncytium that have lost ΔΨm and have high levels of KATP current activation, which causes the propagating wave of depolarization to be slowed or blocked due to current dissipation into the region of high background K+ conductance.
The findings of the present study provide direct evidence that several methods of inducing oxidative stress, simulated ischemia-reperfusion, glutathione depletion, or zero Ca2+ perfusion can render the mitochondria of cells in the whole heart prone to depolarization, which can occur spontaneously or be readily triggered by laser excitation of a small fraction of the cell volume. Importantly, it is shown that ΔΨm can oscillate in some cells, indicating a reversible process, and that depolarization can occur both during ischemia, when the PTP is not thought to be activated[28], and at reperfusion, when we have shown that the early electrophysiological and contractile alterations can be reversed on reperfusion by the mitochondrial benzodiazepine ligand 4′-chlorodiazepam[17, 24], but not the PTP inhibitor cyclosporin A. This distinction is critical for identifying the exact mitochondrial target responsible for the ΔΨm depolarization during ischemia and at the onset of reperfusion in order to develop rational strategies for therapeutic intervention. Similar to our previous studies on the electrical and contractile dysfunction during ischemia-reperfusion, in the present study, the loss of mitochondrial function was prevented by pretreatment with 4′-chlorodiazepam.
A recent report by Masumoto-Ida et al [29], which employed two-photon imaging of TMRE-loaded perfused rat hearts during no-flow ischemia and reperfusion, showed sustained depolarization of ΔΨm in individual myocytes occurring primarily on reperfusion. These depolarizations were tentatively attributed to PTP opening; however, the main effect of cyclosporine A was to delay, but not prevent, the average rate of ΔΨm loss in the microscopic fields analyzed, suggesting the possible involvement of other permeability pathways. In that study[29], and in some of our experiments, BDM was present, which might lead to an underestimation of the extent of ΔΨm depolarization during metabolic stress. In addition to the effect of BDM to decrease energy demand, it has also been reported to alter phosphorylation pathways, blunt Ca2+ signals, and protect hearts against ischemia-reperfusion injury[30]. In the present work, we show that similar patterns of mitochondrial ΔΨm loss during ischemia-reperfusion are present in hearts mechanically arrested with the new agent used for optical mapping studies, blebbistatin, which is thought to be more specific in its actions[21]. This change in methodology does not, however, remove the limitation imposed by eliminating contractile work during the protocols.
Also relevant to the delineation of which mitochondrial ion channel may be activated by oxidative stress, we have recently reported that ΔΨm oscillations in single intact and partially-permeabilized cells occur when the GSH/GSSG ratio decreases to an intermediate level, with the sensitivity of ΔΨm to GSH:GSSG depending on the activities of the glutathione transporter, NADPH/NADH transhydrogenase and the NADPH glutathione reductase[14]. While oscillations occurred at intermediate GSH:GSSG, further depletion of the reduced glutathione pool eventually results in the activation of the PTP. We proposed that two-separate sequentially activated mitochondrial ion channels were involved, an inner membrane anion channel (4′-chlorodiazepam inhibited) supporting mitochondrial oscillation and the PTP, responsible for irreversible ΔΨm depolarization. The present findings are consistent with partial depletion of the GSH pool being responsible for ΔΨm oscillation. Moreover, the enhanced sensitivity of isolated cardiomyocytes, as compared to myocytes in the intact heart, to local laser flash is likely to be due to partial depletion of the glutathione pool during cell isolation.
An unexpected finding was that ΔΨm oscillations could often be observed spontaneously simply by perfusion with nominally Ca2+-free buffer. This effect was apparently due to an increase in ROS production and glutathione depletion in the absence of Ca2+. The reason for the increase in oxidative stress remains to be investigated in future studies. It should be noted that low or zero-Ca2+ perfusion would be expected to increase resting cytosolic Na+ as a result of a shift in the reversal potential of the sarcolemmal Na+/Ca2+ exchanger. Based on recent studies, we have proposed that elevated Na+i can compromise mitochondrial Ca2+ uptake and NADH production[31], which, in turn can influence the redox state of the antioxidant systems (e.g., NADPH/NADP+, GSH/GSSG) of the cell[32], potentially providing an explanation for the Ca2+-free perfusion effect.
The utility of the two-photon fluorescence imaging in the intact heart was readily demonstrated by the ability to resolve subcellular details and in the capability to excite multiple fluorophores simultaneously with a single excitation wavelength. This facilitates examination of the correlation between different cell parameters such as ΔΨm, ROS, and GSH levels and provides useful mechanistic information. While in the present study, we have not attempted to establish a wider view of the size and shape of the observed metabolic sinks in the context of the whole sheet of connected epicardial cells, the high resolution views we have obtained show that desynchronization of the mitochondrial energy state and the phase of oscillation can be present even in neighboring cells. More extensive studies will be required to determine under what conditions, and to what extent, the metabolic transitions can propagate through the tissue. In this regard, we have previously reported that redox waves can travel across cell-cell junctions in isolated myocyte pairs[33].
Concluding remarks
In the present work, we show that mitochondrial ΔΨm oscillations can be elicited in intact perfused hearts in response to oxidative stress and partial depletion of antioxidant defenses. These results provide direct evidence supporting the notion that ROS-dependent mitochondrial oscillation can contribute to myocardial heterogeneity of energy metabolism, as previously suggested by studies of isolated cardiomyocytes. Extensive cellular ΔΨm heterogeneity during ischemia and at the onset of reperfusion gives strong support to the idea that spatiotemporal heterogeneity of mitochondrial energetics underlies ischemia and reperfusion-related arrhythmias and contractile dysfunction.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R37-HL54598, R33-HL87345 and P01-HL081427(BO’R) and K08-HL071662-05 (MKS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aon MA, Cortassa S, Akar FG, O’Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta. 2006 Feb;1762(2):232–40. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circulation research. 2003 Aug 22;93(4):292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2007 Sep 27; doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 4.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. The Biochemical journal. 1973 Jul;134(3):707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denq RY, Fridovich I. Formation of endonuclease III-sensitive sites as a consequence of oxygen radical attack on DNA. Free radical biology & medicine. 1989;6(2):123–9. doi: 10.1016/0891-5849(89)90109-3. [DOI] [PubMed] [Google Scholar]
- 6.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997 Jun 27;89(7):1145–53. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 7.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003 Nov 7;278(45):44735–44. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 8.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. The Journal of experimental medicine. 2000 Oct 2;192(7):1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aon MA, Cortassa S, O’Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4447–52. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harman D. The free radical theory of aging: the effect of age on serum mercaptan levels. Journal of gerontology. 1960 Jan;15:38–40. doi: 10.1093/geronj/15.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa T. Mechanism of somatic mitochondrial DNA mutations associated with age and diseases. Biochim Biophys Acta. 1995 May 24;1271(1):177–89. doi: 10.1016/0925-4439(95)00026-z. [DOI] [PubMed] [Google Scholar]
- 12.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–7. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. American journal of physiology. 2002 Feb;282(2):C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 14.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007 Jul 27;282(30):21889–900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortassa S, Aon MA, Winslow RL, O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004 Sep;87(3):2060–73. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265(5174):962–6. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 17.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005 Dec;115(12):3527–35. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O’Rourke B. Effects of 4′-Chlorodiazepam on Cellular Excitation-Contraction Coupling and Ischemia-Rreperfusion Injury in Rabbit Heart. Cardiovasc Res. 2008 Feb 28; doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J Physiol. 2000;529(Pt 1):23–36. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backx PH, Gao WD, Azan-Backx MD, Marban E. Mechanism of force inhibition by 2,3-butanedione monoxime in rat cardiac muscle: roles of [Ca2+]i and cross-bridge kinetics. J Physiol. 1994 May 1;476(3):487–500. doi: 10.1113/jphysiol.1994.sp020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007 May;4(5):619–26. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 22.Cook JA, Pass HI, Russo A, Iype S, Mitchell JB. Use of monochlorobimane for glutathione measurements in hamster and human tumor cell lines. International journal of radiation oncology, biology, physics. 1989 May;16(5):1321–4. doi: 10.1016/0360-3016(89)90307-6. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Li X, Rozanski GJ. Regulation of glutathione in cardiac myocytes. Journal of molecular and cellular cardiology. 2003 Sep;35(9):1145–52. doi: 10.1016/s0022-2828(03)00230-x. [DOI] [PubMed] [Google Scholar]
- 24.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O’Rourke B. Effects of 4′-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res. 2008 Jul 1;79(1):141–9. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosower NS, Kosower EM. Formation of disulfides with diamide. Methods in enzymology. 1987;143:264–70. doi: 10.1016/0076-6879(87)43050-4. [DOI] [PubMed] [Google Scholar]
- 26.Kosower NS, Kosower EM. Thiol labeling with bromobimanes. Methods in enzymology. 1987;143:76–84. doi: 10.1016/0076-6879(87)43015-2. [DOI] [PubMed] [Google Scholar]
- 27.Rouslin W, Broge CW, Grupp IL. ATP depletion and mitochondrial functional loss during ischemia in slow and fast heart-rate hearts. The American journal of physiology. 1990 Dec;259(6 Pt 2):H1759–66. doi: 10.1152/ajpheart.1990.259.6.H1759. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. The Biochemical journal. 1995 Apr 1;307(Pt 1):93–8. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto-Ida M, Akao M, Takeda T, Kato M, Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation. 2006 Oct 3;114(14):1497–503. doi: 10.1161/CIRCULATIONAHA.106.628834. [DOI] [PubMed] [Google Scholar]
- 30.Boban M, Stowe DF, Kampine JP, Goldberg AH, Bosnjak ZJ. Effects of 2,3-butanedione monoxime in isolated hearts: protection during reperfusion after global ischemia. The Journal of thoracic and cardiovascular surgery. 1993 Mar;105(3):532–40. [PubMed] [Google Scholar]
- 31.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circulation research. 2006 Jul 21;99(2):172–82. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Rourke B, Maack C. The role of Na dysregulation in cardiac disease and how it impacts electrophysiology. Drug Discovery Today. 2007 doi: 10.1016/j.ddmod.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romashko DN, Marban E, O’Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci U S A. 1998;95(4):1618–23. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.