Summary
Solid tumors require new blood vessels for growth and metastasis, yet the biology of tumor-specific endothelial cells is poorly understood. We have isolated tumor endothelial cells from mice which spontaneously develop prostate tumors. Clonal populations of tumor endothelial cells expressed hematopoietic and mesenchymal stem cell markers and differentiated to form cartilage and bone-like tissues. Chondrogenic differentiation was accompanied by an up-regulation of cartilage-specific col 2a1 and sox 9, whereas osteocalcin and the metastasis marker osteopontin were up-regulated during osteogenic differentiation. In human and mouse prostate tumors, ectopic vascular calcification was predominately luminal and co-localized with the endothelial marker CD31. Thus, prostate tumor endothelial cells are atypically multi-potent and can undergo a mesenchymal-like transition.
Keywords: endothelial cell, tumor, angiogenesis, stem cell, progenitor, mesenchymal transition, TRAMP, tumor stroma, prostate cancer, metastasis, tumor microenvironment
Significance
Endothelial cells line blood vessels that supply tumors with blood, nutrients, and oxygen during angiogenesis, and act as a conduit for the removal of waste products. However, tumor blood vessels are poorly formed and dysfunctional. We have identified a multi-potent, tumor-specific endothelial cell that undergoes calcification. Ectopic calcification of tumor endothelium may affect normal blood vessel function. For example, loss of the endothelial barrier in calcified tumor blood vessels could enable tumor cell intravasation into the blood stream or impair contractility and blood flow. Vascular calcification in tumors is easily discernible by standard histological techniques and may be useful as a diagnostic tool. Tumor blood vessel calcification adds to the growing list of abnormalities in tumor endothelium.
Introduction
Tumor growth is angiogenesis-dependent and numerous studies have reported the eradication of tumors in mice by targeting the tumor vasculature (Folkman, 2007). Irrespective of these advances, very little is known about the biology of the endothelial cells (EC) which line tumor blood vessels. An assumption of anti-angiogenesis therapy is that tumor endothelial cells (TEC) are normal and derived from nearby, pre-existing vessels. However, there are several key differences between normal and tumor endothelium. Tumor vessels are tortuous, leaky, and lack normal hierarchical organization (Baluk et al., 2005). Isolated TEC show increased drug resistance (Bussolati et al., 2003) and express distinct cell surface markers (Seaman et al., 2007; St Croix et al., 2000). Our laboratory has recently reported that TEC have aneuploid karyotypes (Hida et al., 2004) and express ErbB1 (Amin et al., 2006). While the consequence of these abnormalities in TEC is at present unclear, it is possible that abnormal TEC could directly enable tumor growth, facilitate metastasis, and lead to acquired drug resistance to anti-angiogenic therapies.
Most new blood vessels arise by co-option or sprouting of pre-existing EC. However, certain pathological conditions such as ischemia and cancer are known to depend on both EC sprouting and recruitment of circulating or bone marrow-derived endothelial progenitors (EPC). For example, increased numbers of circulating EPC can be detected following hind-limb ischemia (Takahashi et al., 1999) and bone marrow-derived EPC are required for tumor growth in some tumor models (Lyden et al., 2001; Lyden et al., 1999). On the other hand, studies using engraftment of GFP+ bone marrow in tumor-bearing mice are equivocal. For example, the degree of bone marrow involvement may be time and/or tumor-type dependent (Nolan et al., 2007). In some cases, direct incorporation of GFP+ cells into tumor vessels was observed (Davidoff et al., 2001; Duda et al., 2006; Santarelli et al., 2006; Yung et al., 2004). In other cases, GFP+ cells were peri-endothelial, minimally incorporated into tumor vessels, or randomly scattered throughout the tumor (Larrivee et al., 2005; Machein et al., 2003; Rajantie et al., 2004; Udagawa et al., 2006). While these studies formally prove that bone marrow-derived progenitors are present in or around tumor blood vessels, one unanswered question has been whether or not TEC posses true stem or progenitor-like properties such as multi-potency and clonogenic ability.
Generally, stem cell fate is unidirectional and terminal. For example, hematopoietic stem cells (HSC) give rise to all blood cell types of the lymphoid and myeloid lineages (Spangrude et al., 1988) whereas mesenchymal stem cells (MSC) give rise to chondrocytes, osteoblasts, myoblasts, adipocytes, and neurons (Prockop, 1997). However, an unexpected finding in cancer stem cell research is that some tumor cells express genes associated with multiple cell types. One of the best known examples are melanoma tumor cells which engage in vasculogenic mimicry by expressing endothelial-specific genes (VE-cadherin) and form fluid-transporting conduits (Hendrix et al., 2001). Thus, reprogramming of stem-like melanoma cells in the tumor microenvironment probably enables their adaptation and survival. It is plausible that other stromal progenitors in the tumor microenvironment could also acquire a multi-potent, mutable phenotype due to reprogramming. Therefore, cellular plasticity may not be limited to putative cancer forming stem cells but could also be common in tumor stromal cells such as EC.
TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) mice develop spontaneous prostate tumors beginning at puberty as androgens drive T-antigen (Tag) expression in prostatic secretory cells (Greenberg et al., 1995). An advantage of autochthonous tumor models such as TRAMP is that tumors are heterogeneous and subjected to selection pressure in the tumor microenvironment as they develop. Thus, tumors in TRAMP mice more closely mimic human cancers compared to xenografts. Angiogenesis in TRAMP mice has been well-characterized in vivo with vascular abnormalities beginning at the PIN stage (prostatic intraepithelial neoplasia), which intensifies in poorly differentiated tumors (Ozawa et al., 2005). However, isolation and culture of EC from TRAMP mice or any other autocthonous cancer model has not yet been described.
In the present study, we isolated TEC from spontaneously-growing prostate tumors in TRAMP mice. Clonal populations of TEC could be differentiated to form bone and cartilage–like tissues. These results suggest that TEC possess a stem/progenitor cell property which distinguishes them from EC throughout the normal vasculature, but undergo atypical trans-differentiation, possibly as a consequence of an osteogenic tumor microenvironment.
Results
TEC express markers of bona fide endothelium
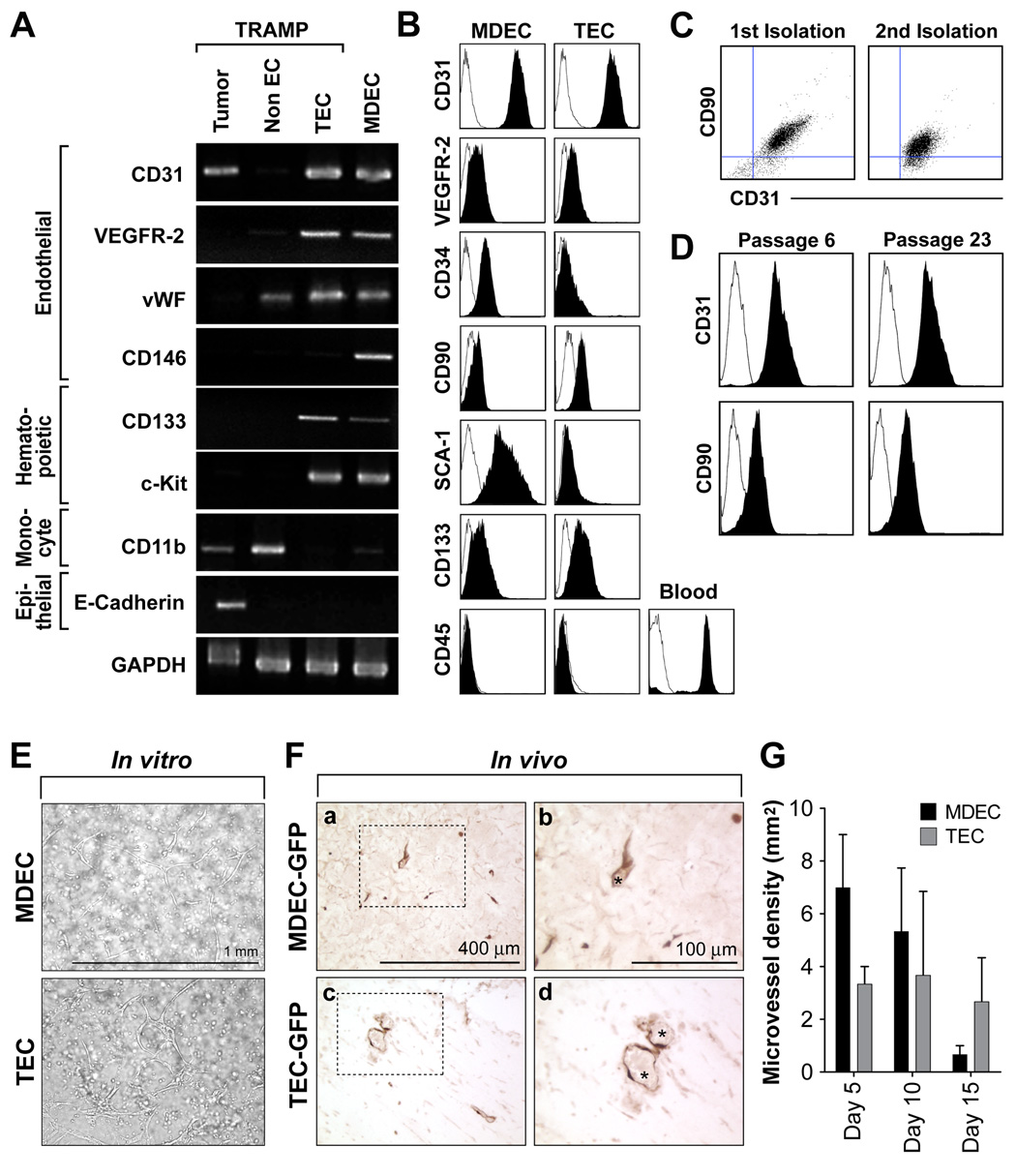
Due to the small size of normal mouse prostate, it has been challenging to obtain enough tissue to isolate normal mouse prostate EC. Thus, mouse dermal endothelial cells (MDEC) from C57BL/6 mice isolated identically to TEC were used as a normal counterpart. In both TEC and MDEC, there was enriched expression for CD31, VEGFR-2, and vWF by semi-quantitative RT-PCR (figure 1A). But CD146, a marker of mature or circulating EC (Bertolini et al., 2006), was expressed in MDEC but not TEC. On the other hand, the stem cell marker CD133 was between 2–3 fold higher in TEC compared to MDEC. Both ECs expressed the stem/hematopoietic marker c-kit, but were negative for the monocyte marker CD11b and the epithelial marker E-cadherin. The isolated TEC and MDEC had a cobblestone morphology and by immunostaining, were uniformly positive for the endothelial markers CD31 and VE-cadherin but negative for the pericyte/mesenchymal marker α SMA (figure S1). The expression of additional markers including CD31, VE-cadherin, VEGFR-2, PDGFR-β, SCA-1, CD34, and ID3 were evaluated by real-time PCR or western blotting and are included as supplemental data (figure S1).
Figure 1. TEC express markers of and function as bona fide endothelium.
(A) Isolated TEC and MDEC (both passage 6) were analyzed for marker expression by RT-PCR. The un-fractioned tumor and the CD31− non-EC fractions from the purification step were also included on the gel. (B) FACS demonstrating uniform staining for the selected markers in MDEC and TEC. (C) FACS analysis of CD31+/CD90+ TEC from two separate isolations. Three to four tumors were pooled in each example and the EC were isolated and expanded in culture as described. (D) The CD31+/CD90+ phenotype was stable and was observable after prolonged culture. (E) Both MDEC and TEC formed tube-like structures in a three-dimensional matrigel culture system in vitro. (F) GFP-tagged MDEC and TEC formed functional blood vessels when injected in vivo. Matrigel plugs from day 10 were stained with anti-GFP antibodies (a–d). The staining shows the luminal position of the labeled EC (dark brown staining). The boxed regions are shown at higher magnification at right. Visible erythrocytes within the vessels are marked with an asterisk (*). (G) Microvessel density over time (n= 3 mice per group). Error bars are +/− SEM.
TEC express the mesenchymal marker CD90 and form blood vessels when engrafted into nude mice
Using flow cytometry, MDEC and TEC expressed comparable levels of CD31 and VEGFR-2 as single peaks on the histogram (figure 1B). However, the EC/HSC marker CD34 was low to absent in TEC compared to MDEC, in good accord with the real-time PCR analysis (figure S1). On the other hand, the MSC marker CD90 was lower in MDEC, but was clearly expressed in TEC. The HSC/MSC marker SCA-1 was not expressed in TEC, but was present in MDEC, consistent with SCA-1 expression in vascular endothelium (van de Rijn et al., 1989). Indeed, SCA-1−/CD31+ EC were rare in TRAMP prostate tumors in vivo and constituted only one percent of the total EC population (figure S2). In good agreement with the RT-PCR analysis, CD133 was about two fold higher in TEC relative to MDEC. Both MDEC and TEC were CD45 negative, indicating absence of hematopoietic precursors. We found that the CD90+/CD31+ phenotype was consistent and stable in TEC from two separate isolations, and was maintained after prolonged periods in culture (figure 1C and 1D). To demonstrate EC functionality, TEC-GFP were prepared with pMX-GFP retroviral infection as previously described (Kitamura et al., 1995). MDEC and TEC formed tube-like structures when cultured within a matrigel plug in vitro and were positioned at the luminal surface of erythrocyte-filled blood vessels when injected in vivo (figure 1E, 1F, and 1G). Taken together, MDEC and TEC expressed the expected mature EC markers and functioned as bona fide endothelium, but the expression of HSC and MSC markers in TEC were atypical.
TEC form mesenchymal-like foci in culture and demonstrate alkaline phosphatase activity when cultured in osteogenic medium
Preliminary experiments indicated that MDEC and TEC were not functional hematopoietic progenitors based on the absence of colony forming units in methylcellulose (data not shown). TEC had an MSC-like profile (CD90+/CD34−) and formed foci in culture, reminiscent of MSC (figure 2A). However, the morphology of TEC foci was distinct from MSC. TEC were cuboidal and did not overlap at their margins in contrast to spindle-shaped, overlapping MSC (figure 2B). We next tested whether TEC could differentiate into mesenchymal lineages such as osteocytes, or adipocytes using selective media. In adipogenic media, about 50% of BM-MSC differentiated into lipid-storing adipocytes based on Oil-Red-O staining (figure 2C and 2D). On the other hand, neither TEC nor MDEC showed any evidence of Oil-Red-O staining when cultured under the same conditions. In osteogenic medium, about 95% of BM-MSC showed areas of alkaline phosphatase (ALP) activity and about 10% of TEC were ALP positive, indicating osteogenic differentiation. MDEC were completely ALP negative. In TEC, ALP activity co-localized with the endothelial marker CD31 in these areas of differentiation (data not shown). These results suggested that TEC might undergo a mesenchymal differentiation prompting us to further investigate this possibility.
Figure 2. TEC form mesenchymal-like foci in vitro and demonstrate alkaline phosphatase activity when cultured in osteogenic medium.
(A) Foci in TEC and BM-MSC were counted and averaged from two, 10 cm2 culture dishes. Error bars are +/− SEM. (B) The morphology of TEC foci was cuboidal (a,b) with no overlap between adjacent cells, while BM-MSC were spindle-shaped with overlapping borders (c,d). The boxed regions are shown in higher magnification at right. (C) BM-MSC underwent adipogenic differentiation (a) and up-regulated ALP activity (b) after culture in adipogenic or osteogenic medium, respectively. TEC did not undergo adipogenic differentiation (c) but did show an up-regulation of ALP activity in osteogenic medium (d). MDEC did not differentiate under any test conditions (e,f). In ‘c, e, and f’ the nuclei were counterstained with hematoxylin. (D) The percentage of positive cells from 10 fields were quantified and plotted on the graph. Error bars are +/− SEM.
TEC undergo mineralization after prolonged culture in osteogenic medium
The Von Kossa reaction detects calcification following the precipitation of silver phosphate at sites of high concentrations of inorganic phosphate (particularly calcium phosphate formed by osteoblasts). No Von Kossa staining was present in MDEC or TEC after two weeks in osteogenic medium (figure 3A). However, after three weeks in differentiation medium, Von Kossa staining was detected in TEC but not MDEC. In good accord with these results, late markers of osteogenic differentiation, osteopontin and osteocalcin, were up-regulated between three and six fold in TEC only after three weeks in osteogenic medium (figure 3B and 3C). These results were consistent with a TEC osteogenic differentiation in vitro.
Figure 3. TEC undergo mineralization after prolonged culture in osteogenic medium.
(A) MDEC were negative for Von Kossa staining in control and osteogenic medium after a two (a,c) or three-week incubation (b,d). TEC were also Von Kossa negative after the two-week incubation (e,g). But intense Von Kossa staining, indicating calcification, was present in TEC after three weeks (f,h). The nuclei were counterstained with hematoxylin. (B,C) Late markers of osteogenic differentiation, osteopontin and osteocalcin were up-regulated about 3-fold in TEC after three weeks in osteogenic medium.
Chondrogenic differentiation of TEC
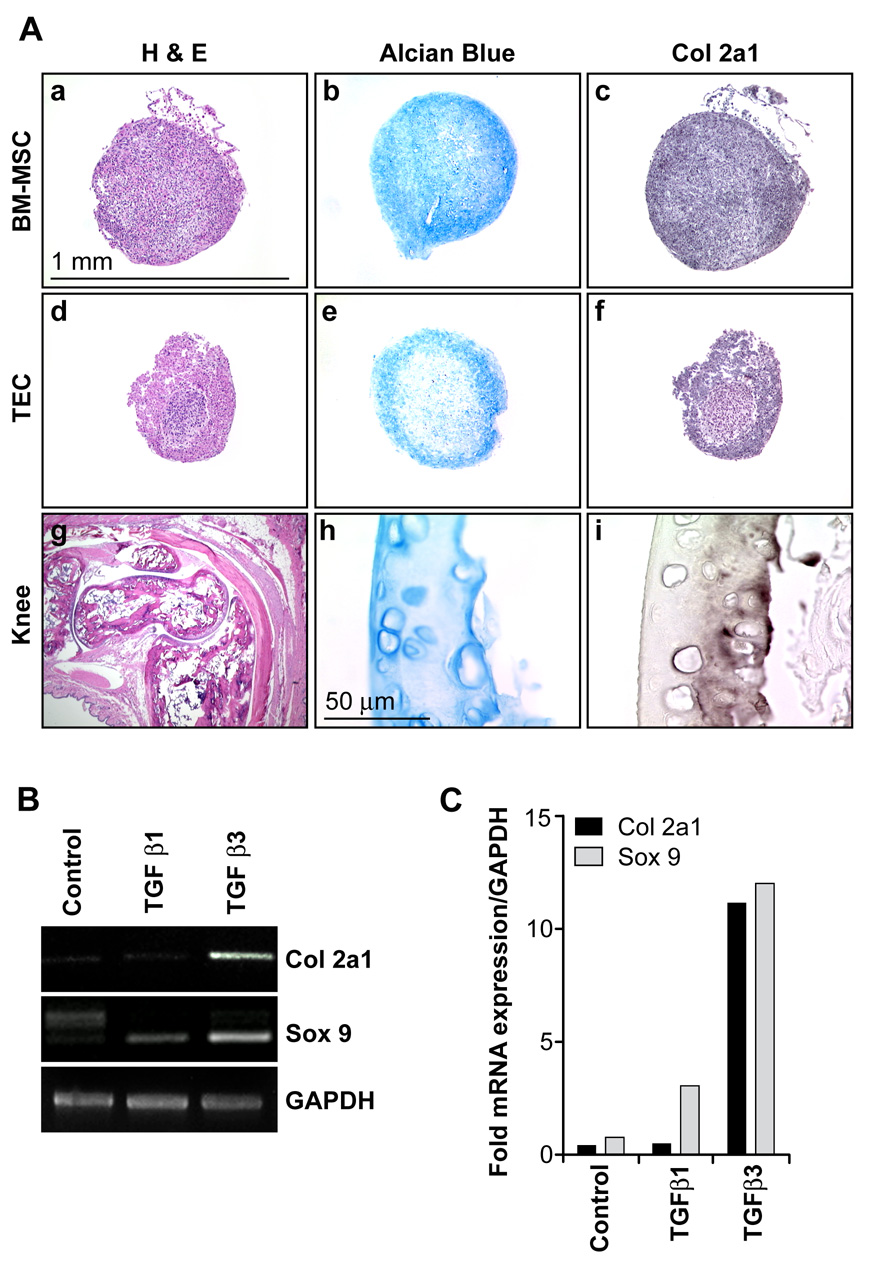
We further tested for TEC multipotency by incubating cells in chondrogenic medium containing TGFβ for up to two weeks (Pittenger et al., 1999). TEC and BM-MSC formed visible pellets after approximately 3–4 days in culture while MDEC never formed visible cell pellets under these conditions. Chondrogenic differentiation was confirmed by positive staining for alcian blue and col 2a1 in the sectioned pellets (figure 4A). Normal mouse leg served as a control where articular cartilage around the knee stained specifically for alcian blue and col 2a1. Exchanging TGFβ1 for TGFβ3 resulted in no visible pellet in TEC. Accordingly, TGFβ3 resulted in a marked up-regulation of mRNAs for chondrocyte-specific col 2a1 (11-fold) and the master chondrogenic transcription factor, sox 9 (12-fold) (figure 4B and 4C).
Figure 4. Chondrogenic differentiation of TEC.
(A) BM-MSC and TEC formed a visible pellet within 3–4 days which was maintained for the two week experiment. Formalin-fixed, paraffin-embedded pellets were sectioned and stained with H&E (a,d), alcian blue (b,e), or col 2a1 antibodies (c,f). An H & E-stained section of mouse leg is also shown (g). Articular cartilage around the mouse knee stained specifically with alcian blue (h) and col 2a1 (i). The one mm scale bar applies to panels ‘a–g’, the 50 µM scale bar to panels ‘h–i’. (B,C) Markers of chondrogenic differentiation, col 2a1 and sox 9, were up-regulated in TEC about 12-fold in the presence of TGFβ3. No pellet was formed in TEC when TGFβ1 was substituted.
TEC can be grown as single cell clones
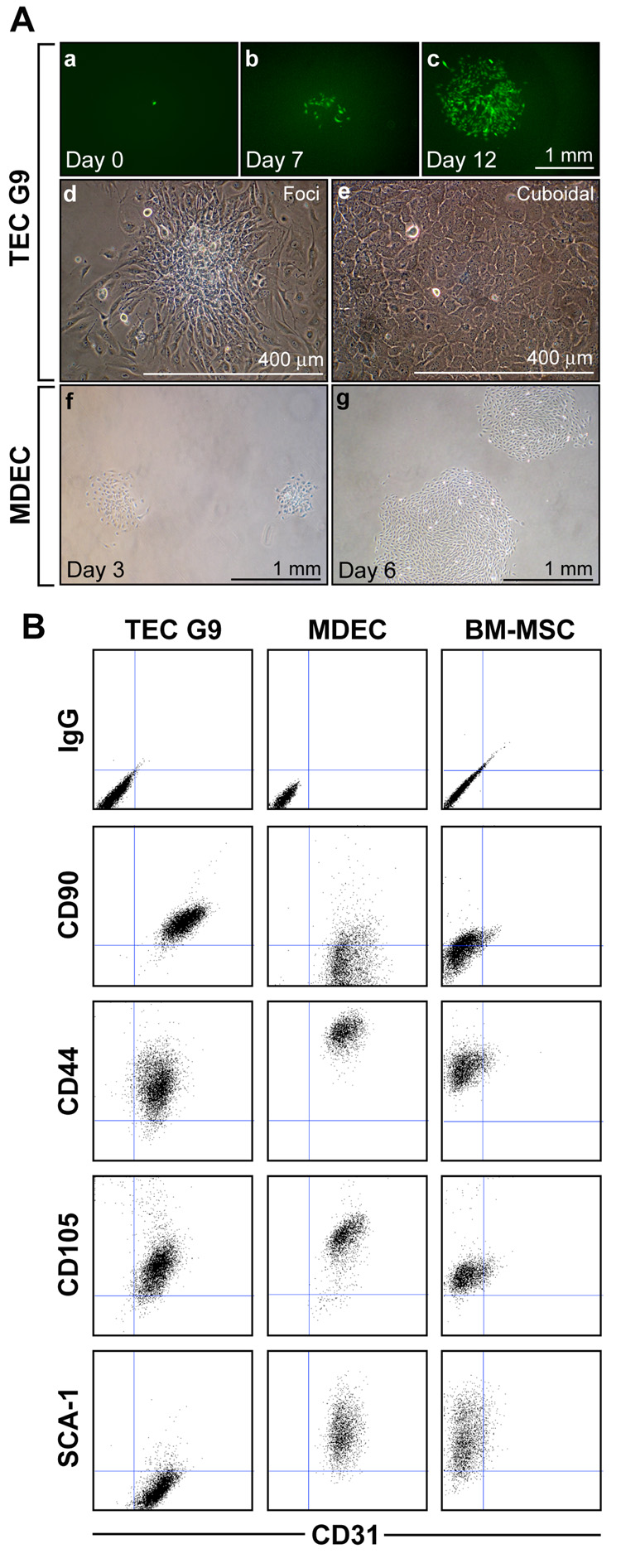
To rule out the possibility of contamination with mesenchymal precursors, we prepared single cell clones of TEC. Limiting dilution assays revealed single cells in individual wells that only occasionally (<0.5%) formed proliferating colonies. A single, highly-proliferative clone of TEC obtained by limiting dilution (clone G9) formed mesenchymal-like foci and flattened, cuboidal-shaped cells when confluent (figure 5A). We were unable to obtain single cell clones in MDEC by limiting dilution; however, clones of MDEC could be readily obtained using cloning rings on colonies of sparsely-plated cells. In TEC clone G9, CD31 double staining showed co-expression with MSC markers CD90, CD44, and CD105, but not SCA-1 (figure 5B). In contrast, an isolated colony of MDEC were double positive for CD31, CD44, CD105, and SCA-1 but low to absent for CD90. BM-MSC were positive for all MSC markers, but did not express CD31 as expected.
Figure 5. TEC can be grown as single cell clones.
(A) TEC infected with retroviral GFP were plated as single cells in 96-well plates. While most single cells never proliferated, occasional colonies could be obtained from single cells (a) that proliferated into clonal populations seven days (b) and 12 days (c) after seeding. TEC clone G9 obtained by limiting dilution formed foci (d) and highly proliferative, cuboidal-shaped cells (e). Single cell clones could not be obtained in MDEC by limiting dilution. But sparsely plated MDEC readily formed colonies which could be selected using cloning rings. MDEC three (f) and six (g) days after initial plating, but before selection using cloning rings. (B) Flow cytometry was carried out on live cells using the indicated antibodies. Consistent with the parental TEC, clone G9 co-expressed CD90 and CD31. Additional mesenchymal markers, CD44 and CD105 were also present while SCA-1 was absent in TEC. A colony of MDEC selected using cloning rings expressed CD31 in addition to CD44, CD105, and SCA-1 but CD90 was low to absent. BM-MSC expressed all markers with the exception of CD31.
Single cell clones of TEC undergo osteogenic and chondrogenic differentiation
Similar to the parental TEC population, TEC G9 underwent mineralization when cultured in osteogenic medium indicated by the blackish-brown Von Kossa staining (figure 6A). TEC G9 also formed a visible pellet in chondrogenic differentiation medium which, when sectioned, stained positive for alcian blue. However, TEC G9 failed to undergo adipogenic differentiation in contrast to BM-MSC, where intracellular lipids were stained with Oil-Red-O. As expected, RT-PCR analysis showed that MDEC failed to up-regulate lineage-specific markers in all differentiation media (figure 6B). Consistent with TEC’s failure to undergo adipogenic differentiation, PPARγ2 was almost undetectable after culture in adipogenic medium, in contrast to BM-MSC. On the other hand, both TEC G9 and BM-MSC up-regulated col 2a1 and osteopontin mRNAs in chondrogenic and osteogenic differentiation medium, respectively. CD31 was co-expressed in TEC and MDEC under all conditions, but was qualitatively diminished in TEC cultured in differentiation media. An additional clone (TEC A2) obtained by limiting dilution had a CD90+/CD31+ phenotype (data not shown) and could be induced to express col 2a1 and osteopontin, but not the adipogenic marker PPARγ2 (figure 6C). Taken together, these results suggest that TEC are clonogenic and can undergo atypical multi-lineage differentiation, but do not undergo adipogenic differentiation characteristic of bona fide BM-MSC.
Figure 6. Single cell clones of TEC undergo osteogenic and chondrogenic differentiation.
(A) After three weeks in osteogenic medium, clone G9 was fixed and stained with Von Kossa solution. Brownish/black staining in G9 (a) and BM-MSC (b) was evident, indicating calcification. Chondrogenic differentiation was also observed in clone G9 (c) and BM-MSC (d), indicated by a visible pellet after two weeks in chondrogenic medium which stained with alcian blue. In adipogenic medium, no Oil-Red-O positive cells were observed in clone G9 (e), in contrast to BM-MSC (f). (B) Semi- quantitative RT-PCR analysis for differentiation markers in each cell type cultured in the indicated differentiation medium. The asterisk (*) indicates that 5 % serum was included in the chondrogenic medium because MDEC did not survive in the serum-free conditions. (C) Col 2a1 and osteopontin, but not PPARγ2 were inducible in an additional TEC clone (A2) cultured in differentiation media.
In vivo calcification of prostate tumor cells and vascular cells
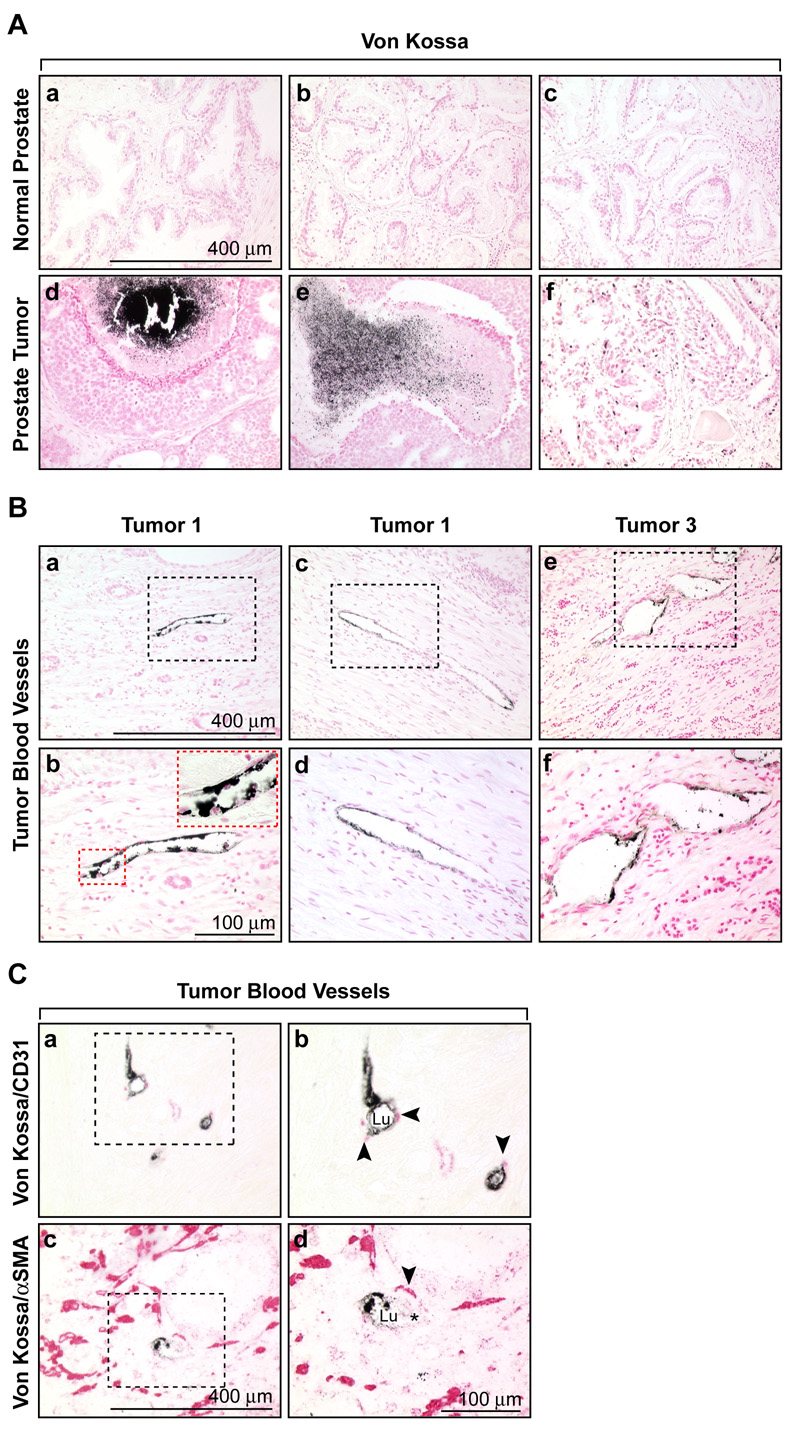
To determine if calcification was a feature of vascular cells in clinical cancers, we used human prostate tissue arrays with tumors of various grades. Low levels of Von Kossa staining were detected in one of eight matched tissue control prostates, but 61 % of prostate tumor specimens showed varying degrees of Von Kossa staining (figure 7A and table I). Eleven percent of these specimens were characterized by densely black, localized granules, characteristic for Von Kossa, whereas between 22 and 28 %showed less robust staining, or individually stained cells throughout the tumor. Some Von Kossa staining was near necrotic regions in these tumors. While no Von Kossa staining was detected in blood vessels from normal prostate tissues, 11 % of tumor specimens showed vascular staining which was localized to the lumen or within the blood vessel wall (figure 7B and figure S3). Von Kossa staining could be localized with the endothelial marker CD31, and the pericyte marker α SMA, confirming the vascular-specific pattern (figure 7C). of a total of 368 CD31+ tumor blood vessels counted, 13 vessels (~4 %) were also stained with Von Kossa, confirming the endothelial sites of calcification. All of these data have been summarized in table I. In TRAMP mice, the chondrocytic marker, col 2a1 and Von Kossa staining were also detected in prostate tumors and tumor blood vessels (figure S4). Thus, tumor endothelial cells in clinical prostate cancers and TRAMP mice are characterized by an atypical trans-differentiation into mesenchymal lineages.
Figure 7. In vivo calcification of prostate tumor cells and vascular cells.
(A) Representative images of Von Kossa staining in normal prostate (a,b,c) and prostate tumors (d,e,f). Calcification in tumors was often associated with highly necrotic regions. (B) Von Kossa staining in tumor blood vessels from three different tumors showing the luminal localization (a,c,e). Boxed regions are shown in higher magnification below (b,d,f). The inset in panel ‘b’ shows a higher magnification of the boxed region. Nuclei in ‘A’ and ‘B’ were counterstained with nuclear fast red. (C) Co-localization of the endothelial marker, CD31 (a,b) and the pericyte marker, α SMA (c,d) with Von Kossa staining in tumor blood vessels. The boxed regions are shown in higher magnification at right. CD31 and α SMA were detected using an alkaline phosphatase-conjugated secondary antibody and appear red in the figure. No counterstain was used. Lu = lumen and the asterisk (*) marks a visible erythrocyte within the vessel lumen in ‘d’.
Table.
| Normal prostate# | Prostate tumor† | ||
|---|---|---|---|
| Number of specimens with Von Kossa staining | 1/8¶ | Low | 10 (28 %) |
| Medium | 8 (22 %) | ||
| High | 4 (11 %) | ||
| Total | 22 (61 %) | ||
| Number of specimens with Von Kossa staining in blood vessels | 0/8 | 4/36 (11 %) | |
| Total number of CD31+/Von Kossa+ blood vessels | ND | 13/368 (~4 %) | |
Matched adjacent tissue (n=8)
Tumor cores of various grades (n=36)
One normal prostate had low levels of diffuse Von Kossa staining, but not in blood vessels
ND = none detected
Discussion
This study describes the isolation and characterization of TEC from spontaneously-growing prostate tumors in TRAMP mice. Similar to normal EC, TEC had a cobblestone or cuboidal morphology and expressed markers such as CD31, VE-cadherin, and VEGFR-2. In contrast, TEC did not express SCA-1, CD34, or CD146, markers common to most vascular EC, but did express CD90. CD90, also known as Thy-1, is a marker not typically found on quiescent endothelium (Lee et al., 1998) but instead is expressed by mesenchymal progenitors. Interestingly, Thy-1 was also found to be highly expressed in human tumor-derived EC in the seminal study by St. Croix et al. (St Croix et al., 2000). TEC were also clonogenic and differentiated into bone and cartilage-like tissues when cultured in selective media. TEC were not tumor cells masquerading as EC nor were they contaminated with tumor cells indicated by absence of T-antigen expression in vivo and in vitro (figure S5). However, absence of T-antigen expression in TEC cannot preclude the possibility that tumor epithelial cell transformation in the prostate does not result in epigenetic alterations in the associated stromal cells as documented in human breast carcinoma (Hu et al., 2005). Taken together, several lines of evidence suggest that TEC can undergo trans-differentiation into mesenchymal lineages: i) single cell clones of TEC underwent differentiation into bone and cartilage ii) potential contamination with bona fide MSC was precluded because TEC could only differentiate into bone and cartilage but not adipocytes iii) TEC expressed EC markers such as CD31 and VE-cadherin which are absent on MSC iv) CD31 protein and bone-related differentiation markers (ALP) were present in the same cells.
What is the origin of TEC? The majority of endothelial cells in tumor blood vessels probably arise by sprouting from nearby, pre-existing EC. However, the turn-over of EC in vivo is extremely slow (approximately one cell division every 1000 days) but increases dramatically during active angiogenesis. Hierarchies of resident EPC with different clonogenic and proliferative abilities reside throughout the vasculature, even into adult life (Ingram et al., 2005; Ingram et al., 2004). Resident EPC may form the immediate angiogenic response to tissue injury by circumventing systemic bone-marrow involvement (O'Neill et al., 2005). Similarly, tissue resident progenitors may form the immediate response to tumor angiogenesis. For example, multipotent CD34− tissue resident progenitors with potential to differentiate into mesodermal and mesenchymal lineages (Jiang et al., 2002; Reyes et al., 2001) and incorporate into tumor blood vessels have been recently described (Bussolati et al., 2005). However, the identification and relative contribution of tissue resident progenitors to tumor vasculature has been difficult to address, probably due to the rarity of these cells in adult tissue.
On the other hand, bone marrow-derived and circulating EPC play a definitive role in tumor growth in some tumor models where they may mediate a postulated metastatic switch (Gao et al., 2008). Furthermore, the ID1+/− ID3−/− knockout mouse which has delayed tumor growth and poorly vascularized tumors fails to incorporate bone marrow-derived progenitors into tumor blood vessels (Lyden et al., 2001; Lyden et al., 1999). While our isolated TEC strikingly over-expressed ID3 in culture, TEC did not express the HSC markers SCA-1 or CD34. Together, this was an unusual expression profile for an EC, but was not entirely indicative of a bone marrow-derived progenitor. Instead, the complete absence of SCA-1 expression in TEC and the rarity of CD31+/SCA-1− EC in prostate tumors in vivo could indicate a possible tissue-resident, rather than a bone marrow-derived origin.
While it appears that TEC are progenitor or stem-like cells, normal EC do not usually undergo a mesenchymal transition - an exception are the rare cardiac valve EC in developing cardiac cushions (Paruchuri et al., 2006) and embryonic EC in the dorsal aorta (DeRuiter et al., 1997). It is interesting that a common ancestor for EC and pericytes has been postulated (Yamashita et al., 2000) given that pericytes are not terminally differentiated cells and can differentiate into fibroblasts, osteoblasts, chondrocytes, and adipocytes (Doherty et al., 1998; Farrington-Rock et al., 2004). Furthermore, MSC themselves may give rise to endothelium under pathobiological conditions (Wang et al., 2005). One possibility is that some TEC arise from mesenchymal progenitors, and undergo a mesenchymal-like differentiation due to factors in the tumor microenvironment. By culturing the isolated TEC in defined differentiation media, we were able to recapitulate this phenomenon in vitro. Thus, tumor stromal cells, like cancer stem cells, may be capable of multi-potent differentiation into atypical lineages (Hendrix et al., 2007; Topczewska et al., 2006).
Ectopic bone and cartilage, osteoclast-like cells, and calcifying vascular cells have been described in diseased blood vessel wall (Tintut et al., 2003; Watson et al., 1994). For example, vascular calcifications are common in the medial layer around atherosclerotic plaques. Angiogenesis is abundant in these areas of lesional calcification, with newly formed vessels proliferating around the calcified deposits (Johnson et al., 2006). The interactions between BMPs, VEGF, pericytes, and resident osteoprogenitor cells are thought to account for cardiac valve and arterial calcification (Collett and Canfield, 2005). Notably, in most vascular diseases, calcification is typically observed in the extracellular matrix surrounding smooth muscle cells in the arterial wall, whereas in human prostate tumors, we observed calcification mainly at the luminal side of capillaries. Therefore, at least in prostate tumors, TEC appear to undergo an atypical mesenchymal transition to form bone-like tissues. Interestingly, TEC cultured in osteogenic medium in vitro transitioned from cobblestone into spindle-shaped cells. It is possible that loss of cell-to-cell contact in calcified tumor blood vessels in vivo could impair blood flow or enable tumor cell intravasation into the bloodstream facilitating metastasis.
An osteogenic tumor microenvironment in prostate cancer has been hypothesized to facilitate metastasis to bone (Chung, 2003; Edlund et al., 2004). For example, the expression of bone-specific proteins in prostate tumor cells may enable their survival once they reach the bone microenvironment (Koeneman et al., 1999). Here, we describe the expression of typically bone-restricted markers such as ALP, osteocalcin, and osteopontin in TEC. Osteopontin is widely used as a biomarker for advanced disease and is a known mediator of bone metastasis (Kang et al., 2003; Nemoto et al., 2001; Wai and Kuo, 2007). Osteopontin contains an RGD motif which mediates cell-to-cell interactions through integrins facilitating anchorage-independent growth and invasion (Allan et al., 2006). Thus, osteopontin expression in TEC could be chemotactic for tumor cells, enabling their interaction with blood vessels, allowing for intravasation. Similarly, serum osteocalcin is elevated in patients with bone-metastatic prostate cancer, where it is primarily a biomarker of bone deposition (Hegele et al., 2007). Sox 9, a transcriptional regulator of col 2a1 during chondrogenesis, was inducible in TEC, and is expressed in prostate cancer cells where it regulates androgen receptor expression (Wang et al., 2007). The chondrocytic marker col 2a1, never described as a marker of tumor blood vessels, was also present in prostate tumor specimens and tumor endothelium, but not in normal prostate. Thus, factors normally expressed in bone, cartilage, or prostate tumor cells are inducible in tumor but not normal EC. Osteogenic factors in the tumor microenvironment may induce the osteogenic and chondrogenic differentiation of tissue-resident or bone marrow-derived progenitors in the tumor stroma.
In conclusion, it has been long assumed that TEC were identical to normal EC. For example, HUVEC are a common surrogate for studying the efficacy of anti-angiogenic drugs in vitro. However, morphological (McDonald and Choyke, 2003), pathophysiological (Hagendoorn et al., 2006), cytogenetic (Hida et al., 2004; Streubel et al., 2004), epigenetic (Grover et al., 2006), gene expression (St Croix et al., 2000), and here, atypical multi-potent plasticity have all been demonstrated in TEC. It will now be important to determine if the mutable properties of prostate TEC might enable tumor cell metastasis, perhaps through loss-of-barrier function, or may be a diagnostic tool, and whether other tumors such as breast and lung cancers undergo calcification in vascular cells.
Experimental Procedures
Cells and media
TEC and MDEC were cultured in low glucose DMEM containing antibiotic/antimycotic, 10 % FBS, 10 % Nu Serum IV (BD PharMingen, San Diego, CA), 3 ng/mL bFGF, 50 ng/mL VEGF (both bFGF and VEGF were a kind gift from the BRB Preclinical Repository), and 100 mg/L porcine heparin (Sigma-Aldrich, St. Louis, MO). BM-MSC were cultured in the same medium, except 20 % FBS was added. All cells were used between 12 and 15 passages.
Mice
TRAMP mice (C57BL/6) were housed in compliance with Boston Children’s Hospital guidelines, and all animal-related protocols were approved by the Institutional Animal Care and Use Committee. Mice were genotyped at weaning (Transnetyx, Cordova, TN).
TEC isolation
Large prostate tumors typically arose by 30–40 weeks in TRAMP mice. Tumors were minced with fine scissors into pieces <1 mm in size. Minced tumors were then transferred to HBSS containing 1 mg/mL collagenase Type I (Worthington, Freehold, NJ) and 100 µg/mL DNAse (Worthington) and placed in a 37 C water bath. The sample was alternately vortexed and disrupted by trituration every 10 minutes over a 30–45 period until the mixture could be freely pipetted. Next, tumor digests were filtered with a 100 µM cell strainer and centrifuged at 1,200 rpm for 15 minutes. The cell pellet was then resuspended in 1X Pharmlyse B (BD Pharmingen, San Diego, CA), and left at room temperature for 10 minutes. After the 10 minute incubation, the sample was centrifuged at 1,200 rpm for five minutes, and the cell pellet was resuspended in 10 mL of MACS buffer (degassed PBS containing 2 mM EDTA and 0.5 % BSA) and filtered again through a 70 µM cell strainer. Cells were centrifuged as before and approximately 250 µL of packed cells were resuspended in 500 µL of MACS buffer. Ten µg of rat anti-mouse CD31 antibody (BD Pharmingen) were added to the resuspended cells which were incubated at room temperature for 10 minutes. After the 10 minute incubation, cells were centrifuged as above and washed twice with MACS buffer. The cells were resuspended in 320 µL of MACS buffer and then 80 µL of goat anti-rat magnetic beads were added (Miltenyi Biotec, Auburn, CA). The mixture was left at room temperature for another 10 minutes, and then filtered through a 100 µM cell strainer before being passed over a magnetic column (Miltenyi Biotech). After washing the column with 8–10 mL of MACS buffer, the bound cells were eluted. The eluted cells were centrifuged and washed three times with culture medium before plating into a T-75 flask coated with 10 µg/mL of human fibronectin (Biomedical Technologies, Stoughton, MA). After 4–6 weeks, cells were washed with phosphate buffered saline (PBS) and detached using three mL of Accutase (Innovative Cell Technologies, San Diego, CA). Cells were centrifuged, washed once with MACS buffer, and resuspended in 200 µL of MACS buffer containing 10 µg of rat anti-mouse ICAM-2 antibodies (BD Pharmingen). The purification procedure was repeated identically as above. The purified endothelial cells eluted from the magnetic column were washed twice with culture medium and then seeded into a single well of a 6-well or 24-well plate. The use of cloning rings was often necessary as a final method for obtaining pure endothelial cell cultures.
Differentiation
For adipogenic differentiation, cells were seeded at a density of 50,000 cells/well in 12-well plates. The next day, the media was removed and replaced with DMEM containing 1 % antibiotic/antimycotic, 10 % FBS, 5 µg/mL insulin, 1 µM dexamthasone, 0.5 µM isobutylmethylxanthine, and 60 µM indomethacin (all reagents purchased from Sigma-Aldrich). After two weeks, cells were washed, fixed in formalin, and stained with Oil-Red-O to detect lipid. For osteogenic differentiation, cells were plated into 2-well chamber slides in medium containing DMEM, 10 % FBS, 1 % antibiotic/antimycotic, 1 µM dexamthasone, 10 mM β-glycerophosphate, and 100 µM ascorbic acid-2-phosphate. After one to three weeks, cells were washed, fixed in formalin and stained for ALP or Von Kossa solution (Diagnostic Biosystems, Pleasanton, CA). For chondrogenic differentiation, cells were detached with Accutase, washed, adjusted to 1×106 cells/mL and centrifuged. The media was carefully aspirated and replaced with DMEM (high glucose) containing 1 % antibiotic/antimycotic, 10 µL/mL insulin-transferrin-selenium, 1 µM dexamethasone, 20 ng/mL TGFβ1 or TGFβ3 (R and D Systems, Minneapolis, MN), and 100 µM ascorbic acid-2-phosphate. In all experiments, control media was DMEM containing 10 % FBS with 1 % antibiotic/antimycotic.
Fluorescence Activated Cell Sorting
Cell were detached with Accutase, washed, and resuspended in PBS/0.1 % BSA containing the indicated conjugated antibodies (all purchased from BD Pharmingen with the exception of the CD90, CD105, and CD44 antibodies which were purchased from eBioscience, San Diego, CA). Cells were analyzed using a BD FACSCalibur System (BD Bioscience, San Diego, CA).
RT-PCR
Total cellular RNA was extracted using the RNeasy kit according to the manufacturer’s directions (Qiagen USA, Valencia, CA). Five µg of RNA was subjected to reverse transcription by standard methods. Two µL of cDNA was then used in a 50 µL PCR reaction containing 1 µM of each primer, 1 µL of dNTPs (10 mM stock), 5 µL of 10X PCR buffer with MgCl2 (Invitrogen, Carlsbad, CA), and 0.4 µL of Taq DNA polymerase (Roche, Basel Switzerland).
Immunofluorescence
Cells were seeded at a density of 10,000 cells/well in fibronectin coated 8-well chamber slides. Confluent cells were washed twice with PBS, and then fixed with ice cold 100 % methanol at −20 C for 20 minutes. The fixed cells were rinsed briefly with PBS and then blocked for one hour at room temperature with PBS containing 5 % BSA. After blocking, antibodies were added overnight at 4 C in a humidified chamber. The next day, cells were rinsed with PBS, and then blocked again for 30 minutes at room temperature. Secondary antibodies were added and the cells were incubated an additional hour at room temperature protected from light. Finally, cells were washed with PBS and then mounted using Gel Mount (Biomeda, Foster City, CA) containing 0.4 µg/mL 4’, 6-diamidino-2-phenylindole (DAPI).
Matrigel plug assay
Two million TEC and MDEC were washed, and re-suspended in 100 µL of phenol red-free matrigel. For the in vitro tube forming assay, 50 µL of this suspension were pipetted into single wells of a 24-well plate and then placed at 37 C for 20 minutes. Each well was then filled with one mL of EC growth media. For the in vivo experiment, 2 × 106 TEC or MDEC were re-suspended in matrigel, and injected into the lateral flank of eight week old nu/nu mice. Implants were removed on the indicated days and fixed in formalin over night.
Tissue microarrays
Human prostate adenocarcinoma tissue arrays are commercially available and were purchased from Biomax US (Rockville, MD). The array had 48 cores in duplicate with tissue-matched controls. Under the guidelines of the Clinical Investigation Policy and Procedure Manual at Children’s Hospital, Boston, commercially available specimens which cannot be linked to the subjects are exempt from IRB review.”
Supplementary Material
Figure S1: Additional TEC characterization. (A) Phase microscopy images of TEC (a) MDEC (b) and mesenchymal-like, non EC (c). The one mm scale bar applies to all panels. By immunofluorescence, the isolated EC uniformly expressed CD31 and VE-cadherin, but not α SMA (d-i). (B) Western blot of pericyte/mesenchymal markers in TEC and mouse dermal fibroblasts (MDF). (C) Western blot for ID3 in TEC versus MDEC. MDEC transiently transfected with an ID3 expression construct were included as a positive control. (D) By real-time PCR, MDEC and TEC expressed mRNAs for endothelial-specific CD31, VE-cadherin, and VEGFR-2, but not the mural/mesenchymal marker PDGFR-β TEC are low to absent for SCA-1 and CD34. The mouse MS-1 endothelial cell line served as a positive control.
Figure S2. SCA-1−/CD31+ TEC are rare in TRAMP prostate tumors. (A) Genito-urinary tract from a 30 week-old TRAMP mouse (a). A six micron section of the dorso-lateral lobe showing that the majority of CD31+ tumor blood vessels were also SCA-1+ (yellow merge). The arrows point to two blood vessels co-expressing both markers (b). Lower magnification of a TRAMP prostate tumor showing extensive SCA-1+ staining in the tumor blood vessels, fibroblastic-like stromal cells, and tumor epithelial ductules (c). The same field is shown in (d) and merged with the CD31 signal in (e). Only occasional CD31+/SCA-1− cells can be observed (green cells, white arrowheads). (B) Flow cytometry of two TRAMP prostate tumors. CD45+ hematopoietic progenitors were excluded by out-gating. The percentage of single and double positive cells were plotted at right. Error bars are +/− SEM.
Figure S3. Von Kossa staining in human prostate tumor blood vessels. The panels (A-L) are additional images of tumor blood vessels stained with Von Kossa. An asterisk (*) marks erythrocytes where visible. Lu = Lumen and Gl = gland. The nuclei were counterstained with nuclear fast red.
Figure S4. Von Kossa and col 2a1 staining in TRAMP prostate tumors. Normal prostate stained with Von Kossa (A) and col 2a1 antibodies (B). Both Von Kossa and col 2a1 were detected in the tumor stroma (C,D) and tumor blood vessels (E,F). For all Von Kossa staining, nuclei were counterstained with fast red. For all col 2a1 staining, nuclei were lightly counterstained with hematoxylin. The boxed region in ‘F’ is a higher magnification of a tumor blood vessel. Lu = lumen and the asterisk (*) marks visible erythrocytes. The arrow heads in ‘F’ point to additional blood vessels. Col 2a1 was detected with an alkaline phosphatase-conjugated secondary antibody (red). Mouse leg was included as a positive control for Von Kossa (G) and col 2a1 (H).
Figure S5: TEC do not express Tag in vivo or in vitro. (A) In each field, the same dorsal-lateral lobe is shown stained with Tag antibodies or counterstained with DAPI. Prostates from non-transgenic mice were negative for Tag. Only diffuse background staining was evident, probably due to non-specific cross-reactivity of the mouse monoclonal Tag antibodies with mouse tissue (a–b). The nuclei of luminal epithelial cells from a TRAMP prostate tumor stained strongly for Tag (c–d). In contrast, tumor- associated endothelial cells in TRAMP prostate tumors were negative for Tag. The arrows point to three endothelial nuclei which are CD31 positive but Tag negative (e–f). (B) Western blotting of tumors or isolated TEC (passage 10) confirmed the absence of both epithelial cytokeratin (CK8) and Tag expression.
Acknowledgments
This work was supported by NIH grants CA37392 and CA45548. ACD wishes to thank Leonora DeBella and the American Cancer Society for supporting his research with a post doctoral fellowship. We thank Kristin Johnson for her excellent assistance with the figures. We thank Dr. Peter Hauschka for critically reviewing this manuscript and Dr. Juan Melero-Martin for assistance with the immunohistochemistry and the EPC. We dedicate this work in memory of Dr. Judah Folkman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan AL, George R, Vantyghem SA, Lee MW, Hodgson NC, Engel CJ, Holliday RL, Girvan DP, Scott LA, Postenka CO, et al. Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer. Am J Pathol. 2006;169:233–246. doi: 10.2353/ajpath.2006.051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. Faseb J. 2003;17:1159–1161. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- Chung LW. Prostate carcinoma bone-stroma interaction and its biologic and therapeutic implications. Cancer. 2003;97:772–778. doi: 10.1002/cncr.11140. [DOI] [PubMed] [Google Scholar]
- Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Ng CY, Brown P, Leary MA, Spurbeck WW, Zhou J, Horwitz E, Vanin EF, Nienhuis AW. Bone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice. Clin Cancer Res. 2001;7:2870–2879. [PubMed] [Google Scholar]
- DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80:444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- Duda DG, Cohen KS, Kozin SV, Perentes JY, Fukumura D, Scadden DT, Jain RK. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood. 2006;107:2774–2776. doi: 10.1182/blood-2005-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J Cell Biochem. 2004;91:686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2007;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;110:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover AC, Tangrea MA, Woodson KG, Wallis BS, Hanson JC, Chuaqui RF, Gillespie JW, Erickson HS, Bonner RF, Pohida TJ, et al. Tumor-associated endothelial cells display GSTP1 and RARbeta2 promoter methylation in human prostate cancer. J Transl Med. 2006;4:13. doi: 10.1186/1479-5876-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagendoorn J, Tong R, Fukumura D, Lin Q, Lobo J, Padera TP, Xu L, Kucherlapati R, Jain RK. Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res. 2006;66:3360–3364. doi: 10.1158/0008-5472.CAN-05-2655. [DOI] [PubMed] [Google Scholar]
- Hegele A, Wahl HG, Varga Z, Sevinc S, Koliva L, Schrader AJ, Hofmann R, Olbert P. Biochemical markers of bone turnover in patients with localized and metastasized prostate cancer. BJU Int. 2007;99:330–334. doi: 10.1111/j.1464-410X.2006.06604.x. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci U S A. 2001;98:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Onishi M, Kinoshita S, Shibuya A, Miyajima A, Nolan GP. Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci U S A. 1995;92:9146–9150. doi: 10.1073/pnas.92.20.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Larrivee B, Niessen K, Pollet I, Corbel SY, Long M, Rossi FM, Olive PL, Karsan A. Minimal contribution of marrow-derived endothelial precursors to tumor vasculature. J Immunol. 2005;175:2890–2899. doi: 10.4049/jimmunol.175.5.2890. [DOI] [PubMed] [Google Scholar]
- Lee WS, Jain MK, Arkonac BM, Zhang D, Shaw SY, Kashiki S, Maemura K, Lee SL, Hollenberg NK, Lee ME, Haber E. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res. 1998;82:845–851. doi: 10.1161/01.res.82.8.845. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Machein MR, Renninger S, de Lima-Hahn E, Plate KH. Minor contribution of bone marrow-derived endothelial progenitors to the vascularization of murine gliomas. Brain Pathol. 2003;13:582–597. doi: 10.1111/j.1750-3639.2003.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- Nemoto H, Rittling SR, Yoshitake H, Furuya K, Amagasa T, Tsuji K, Nifuji A, Denhardt DT, Noda M. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J Bone Miner Res. 2001;16:652–659. doi: 10.1359/jbmr.2001.16.4.652. [DOI] [PubMed] [Google Scholar]
- Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, Mittal V. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill TJt, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- Ozawa MG, Yao VJ, Chanthery YH, Troncoso P, Uemura A, Varner AS, Kasman IM, Pasqualini R, Arap W, McDonald DM. Angiogenesis with pericyte abnormalities in a transgenic model of prostate carcinoma. Cancer. 2005;104:2104–2115. doi: 10.1002/cncr.21436. [DOI] [PubMed] [Google Scholar]
- Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006;99:861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- Santarelli JG, Udani V, Yung YC, Cheshier S, Wagers A, Brekken RA, Weissman I, Tse V. Incorporation of bone marrow-derived Flk-1-expressing CD34+ cells in the endothelium of tumor vessels in the mouse brain. Neurosurgery. 2006;59:374–382. doi: 10.1227/01.NEU.0000222658.66878.CC. discussion 374–382. [DOI] [PubMed] [Google Scholar]
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Streubel B, Chott A, Huber D, Exner M, Jager U, Wagner O, Schwarzinger I. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med. 2004;351:250–259. doi: 10.1056/NEJMoa033153. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Udagawa T, Puder M, Wood M, Schaefer BC, D'Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. Faseb J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989;86:4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2007 doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Wang H, McKnight NC, Zhang T, Lu ML, Balk SP, Yuan X. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res. 2007;67:528–536. doi: 10.1158/0008-5472.CAN-06-1672. [DOI] [PubMed] [Google Scholar]
- Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Yung YC, Cheshier S, Santarelli JG, Huang Z, Wagers A, Weissman I, Tse V. Incorporation of naive bone marrow derived cells into the vascular architecture of brain tumor. Microcirculation. 2004;11:699–708. doi: 10.1080/10739680490521005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Additional TEC characterization. (A) Phase microscopy images of TEC (a) MDEC (b) and mesenchymal-like, non EC (c). The one mm scale bar applies to all panels. By immunofluorescence, the isolated EC uniformly expressed CD31 and VE-cadherin, but not α SMA (d-i). (B) Western blot of pericyte/mesenchymal markers in TEC and mouse dermal fibroblasts (MDF). (C) Western blot for ID3 in TEC versus MDEC. MDEC transiently transfected with an ID3 expression construct were included as a positive control. (D) By real-time PCR, MDEC and TEC expressed mRNAs for endothelial-specific CD31, VE-cadherin, and VEGFR-2, but not the mural/mesenchymal marker PDGFR-β TEC are low to absent for SCA-1 and CD34. The mouse MS-1 endothelial cell line served as a positive control.
Figure S2. SCA-1−/CD31+ TEC are rare in TRAMP prostate tumors. (A) Genito-urinary tract from a 30 week-old TRAMP mouse (a). A six micron section of the dorso-lateral lobe showing that the majority of CD31+ tumor blood vessels were also SCA-1+ (yellow merge). The arrows point to two blood vessels co-expressing both markers (b). Lower magnification of a TRAMP prostate tumor showing extensive SCA-1+ staining in the tumor blood vessels, fibroblastic-like stromal cells, and tumor epithelial ductules (c). The same field is shown in (d) and merged with the CD31 signal in (e). Only occasional CD31+/SCA-1− cells can be observed (green cells, white arrowheads). (B) Flow cytometry of two TRAMP prostate tumors. CD45+ hematopoietic progenitors were excluded by out-gating. The percentage of single and double positive cells were plotted at right. Error bars are +/− SEM.
Figure S3. Von Kossa staining in human prostate tumor blood vessels. The panels (A-L) are additional images of tumor blood vessels stained with Von Kossa. An asterisk (*) marks erythrocytes where visible. Lu = Lumen and Gl = gland. The nuclei were counterstained with nuclear fast red.
Figure S4. Von Kossa and col 2a1 staining in TRAMP prostate tumors. Normal prostate stained with Von Kossa (A) and col 2a1 antibodies (B). Both Von Kossa and col 2a1 were detected in the tumor stroma (C,D) and tumor blood vessels (E,F). For all Von Kossa staining, nuclei were counterstained with fast red. For all col 2a1 staining, nuclei were lightly counterstained with hematoxylin. The boxed region in ‘F’ is a higher magnification of a tumor blood vessel. Lu = lumen and the asterisk (*) marks visible erythrocytes. The arrow heads in ‘F’ point to additional blood vessels. Col 2a1 was detected with an alkaline phosphatase-conjugated secondary antibody (red). Mouse leg was included as a positive control for Von Kossa (G) and col 2a1 (H).
Figure S5: TEC do not express Tag in vivo or in vitro. (A) In each field, the same dorsal-lateral lobe is shown stained with Tag antibodies or counterstained with DAPI. Prostates from non-transgenic mice were negative for Tag. Only diffuse background staining was evident, probably due to non-specific cross-reactivity of the mouse monoclonal Tag antibodies with mouse tissue (a–b). The nuclei of luminal epithelial cells from a TRAMP prostate tumor stained strongly for Tag (c–d). In contrast, tumor- associated endothelial cells in TRAMP prostate tumors were negative for Tag. The arrows point to three endothelial nuclei which are CD31 positive but Tag negative (e–f). (B) Western blotting of tumors or isolated TEC (passage 10) confirmed the absence of both epithelial cytokeratin (CK8) and Tag expression.