Abstract
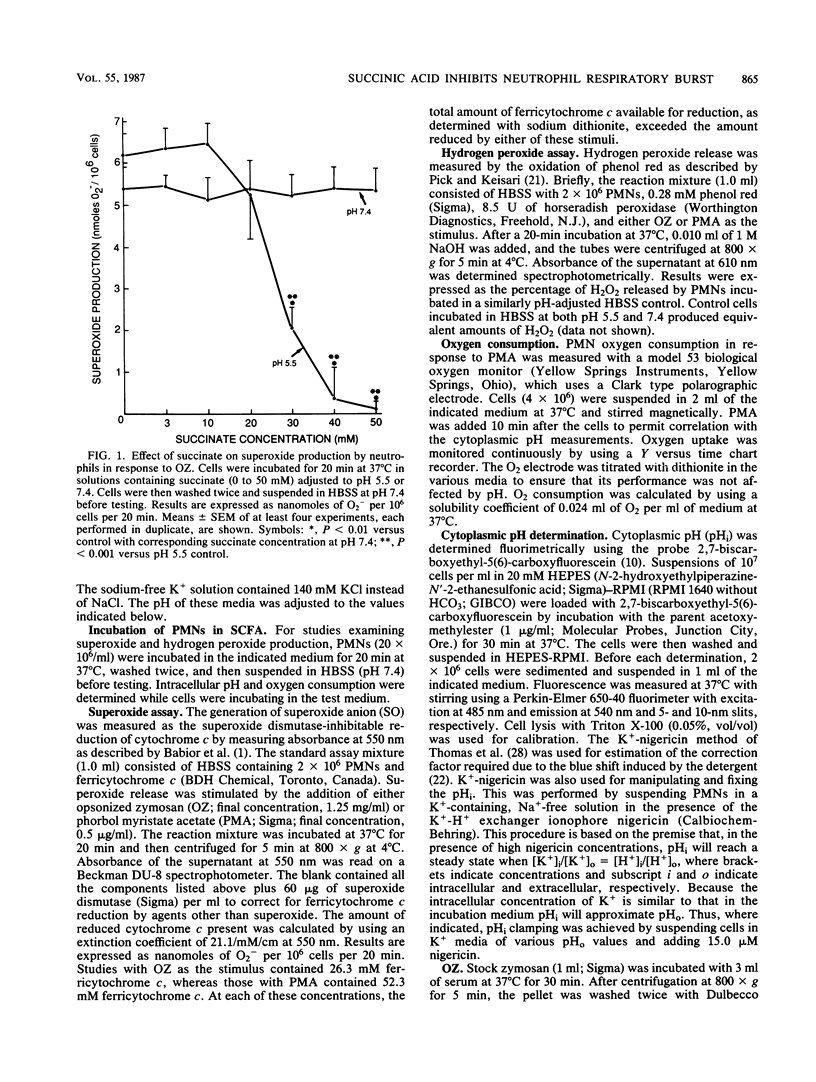
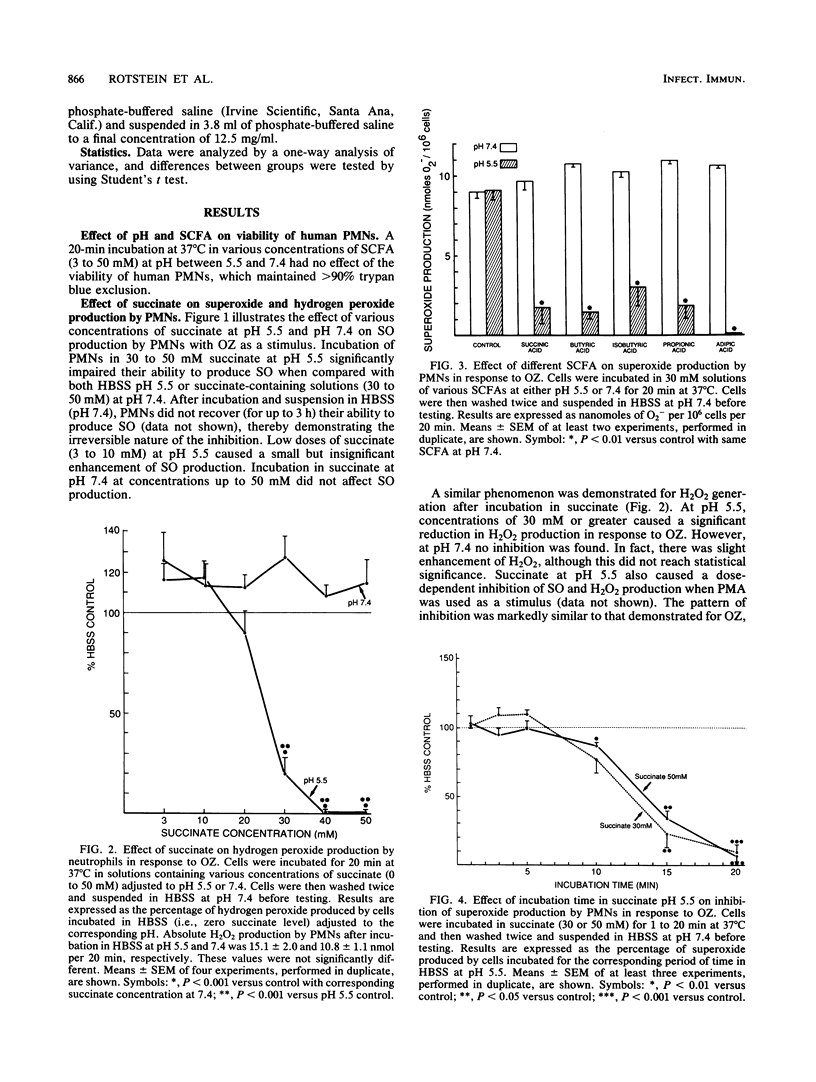
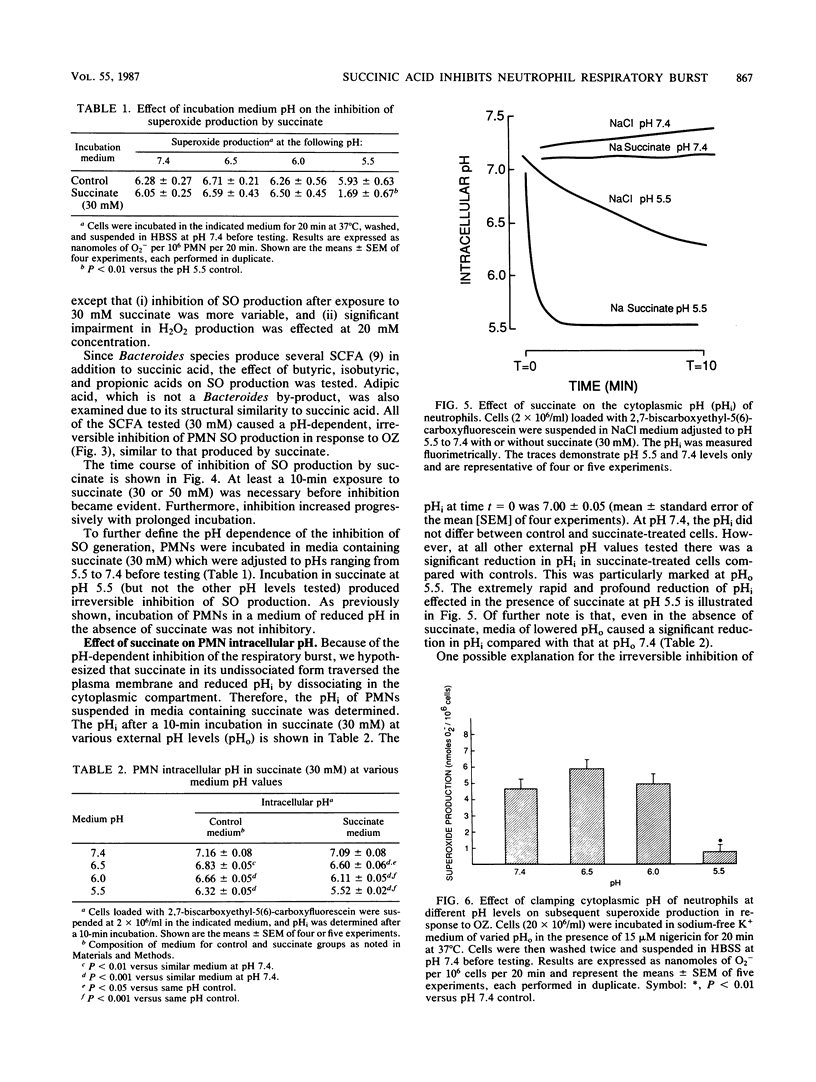
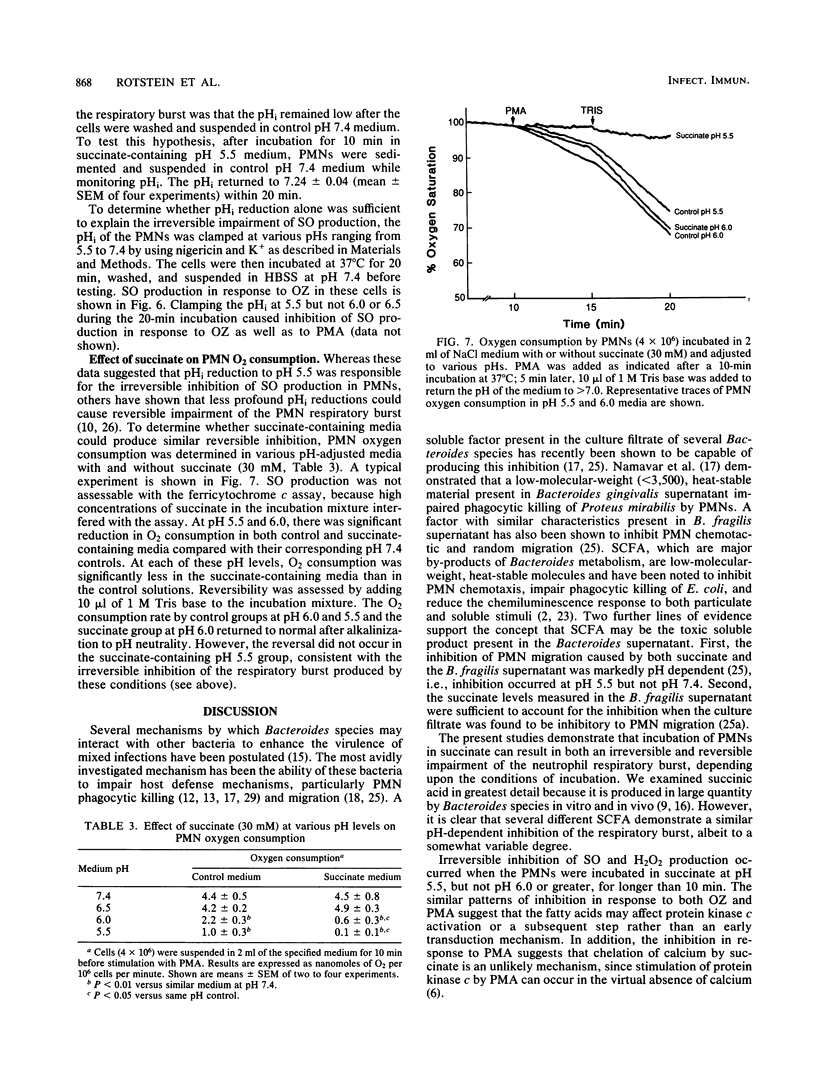
Short-chain fatty acids, particularly succinic acid, are major metabolic by-products of Bacteroides species. To determine their role as potential virulence factors in infections containing Bacteroides species, short-chain fatty acids were examined for their effect on the neutrophil respiratory burst. Succinate (30 to 50 mM) irreversibly impaired superoxide and hydrogen peroxide production in response to opsonized zymosan and phorbol myristate acetate when neutrophils were treated at pH 5.5 but not pH 6.0 or greater. Several other short-chain fatty acids tested produced similar inhibition. Reversible inhibition of oxygen consumption was found when neutrophils were incubated in succinate-containing medium (pH 6.0) as well as control medium (pH 6.0 and 5.5). Neutrophil cytoplasmic pH was measured by fluorimetric techniques to determine whether the inhibition was mediated via a reduction in intracellular pH. The intracellular pH of cells in control medium (pH 6.5 or less) was significantly reduced compared with pH 7.4. The addition of succinate (30 mM) to these media caused a further significant reduction in cytoplasmic pH at each pH level. The reduction in intracellular pH was sufficient to account for both the irreversible and reversible impairment of the neutrophil respiratory burst. Thus, short-chain fatty acids appear to exert their inhibition, at least in part, by reducing intracellular pH. These data also demonstrate the potential for interactions between Bacteroides species and their microenvironment to increase the virulence of an infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I., Hunter V., Walker R. I. Synergistic effect of bacteroides, Clostridium, Fusobacterium, anaerobic cocci, and aerobic bacteria on mortality and induction of subcutaneous abscesses in mice. J Infect Dis. 1984 Jun;149(6):924–928. doi: 10.1093/infdis/149.6.924. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Rashad A. L., Mazza J. A., Hammond D. beta-Lactamase activity in human pus. J Infect Dis. 1980 Oct;142(4):594–601. doi: 10.1093/infdis/142.4.594. [DOI] [PubMed] [Google Scholar]
- Connolly J. C., McLean C., Tabaqchali S. The effect of capsular polysaccharide and lipopolysaccharide of Bacteroides fragilis on polymorph function and serum killing. J Med Microbiol. 1984 Jun;17(3):259–271. doi: 10.1099/00222615-17-3-259. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. A rapid one-step procedure for purification of mononuclear and polymorphonuclear leukocytes from human blood using a modification of the Hypaque-Ficoll technique. J Immunol Methods. 1978;24(3-4):389–393. doi: 10.1016/0022-1759(78)90143-6. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Mayhew J. W., Bartlett J. G., Thadepalli H., Onderdonk A. B. Rapid diagnosis of anaerobic infections by direct gas-liquid chromatography of clinical speciments. J Clin Invest. 1976 Feb;57(2):478–484. doi: 10.1172/JCI108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am J Physiol. 1986 Jul;251(1 Pt 1):C55–C65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Jones G. R., Gemmell C. G. Impairment by Bacteroides species of opsonisation and phagocytosis of enterobacteria. J Med Microbiol. 1982 Aug;15(3):351–361. doi: 10.1099/00222615-15-3-351. [DOI] [PubMed] [Google Scholar]
- Kelly M. J. The quantitative and histological demonstration of pathogenic synergy between Escherichia coli and Bacteroides fragilis in guinea-pig wounds. J Med Microbiol. 1978 Nov;11(4):513–523. doi: 10.1099/00222615-11-4-513. [DOI] [PubMed] [Google Scholar]
- Mackowiak P. A. Microbial synergism in human infections (second of two parts). N Engl J Med. 1978 Jan 12;298(2):83–87. doi: 10.1056/NEJM197801122980206. [DOI] [PubMed] [Google Scholar]
- Mayhew J. W., Onderdonk A. B., Gorbach S. L. Effects of time and growth media on short-chain fatty acid production by Bacteroides fragilis. Appl Microbiol. 1975 Apr;29(4):472–475. doi: 10.1128/am.29.4.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namavar F., Verweij-Van Vught A. M., Vel W. A., Bal M., MacLaren D. M. Polymorphonuclear leukocyte chemotaxis by mixed anaerobic and aerobic bacteria. J Med Microbiol. 1984 Oct;18(2):167–172. doi: 10.1099/00222615-18-2-167. [DOI] [PubMed] [Google Scholar]
- Namavar F., Verweij A. M., Bal M., van Steenbergen T. J., de Graaff J., MacLaren D. M. Effect of anaerobic bacteria on killing of Proteus mirabilis by human polymorphonuclear leukocytes. Infect Immun. 1983 Jun;40(3):930–935. doi: 10.1128/iai.40.3.930-935.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmith P. E., Grinstein S. Impairment of Na+/H+ exchange underlies inhibitory effects of Na+-free media on leukocyte function. FEBS Lett. 1986 Jun 23;202(1):79–85. doi: 10.1016/0014-5793(86)80653-6. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Fiegel V. D., Nelson R. D., Simmons R. L. Succinic acid, a metabolic by-product of Bacteroides species, inhibits polymorphonuclear leukocyte function. Infect Immun. 1985 May;48(2):402–408. doi: 10.1128/iai.48.2.402-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Simmons R. L. Lethal microbial synergism in intra-abdominal infections. Escherichia coli and Bacteroides fragilis. Arch Surg. 1985 Feb;120(2):146–151. doi: 10.1001/archsurg.1985.01390260016003. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Sorenson J. J., Fiegel V. D., Nelson R. D., Simmons R. L. A Bacteroides by-product inhibits human polymorphonuclear leukocyte function. Arch Surg. 1986 Jan;121(1):82–88. doi: 10.1001/archsurg.1986.01400010096012. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Wells C. L., Pruett T. L., Sorenson J. J., Simmons R. L. Succinic acid production by Bacteroides fragilis. A potential bacterial virulence factor. Arch Surg. 1987 Jan;122(1):93–98. doi: 10.1001/archsurg.1987.01400130099015. [DOI] [PubMed] [Google Scholar]
- Simchowitz L. Intracellular pH modulates the generation of superoxide radicals by human neutrophils. J Clin Invest. 1985 Sep;76(3):1079–1089. doi: 10.1172/JCI112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Peterson P. K., Schmeling D., Bracke J., Kim Y., Quie P. G. Opsonization of four Bacteroides species: role of the classical complement pathway and immunoglobulin. Infect Immun. 1980 Mar;27(3):784–792. doi: 10.1128/iai.27.3.784-792.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



