Abstract
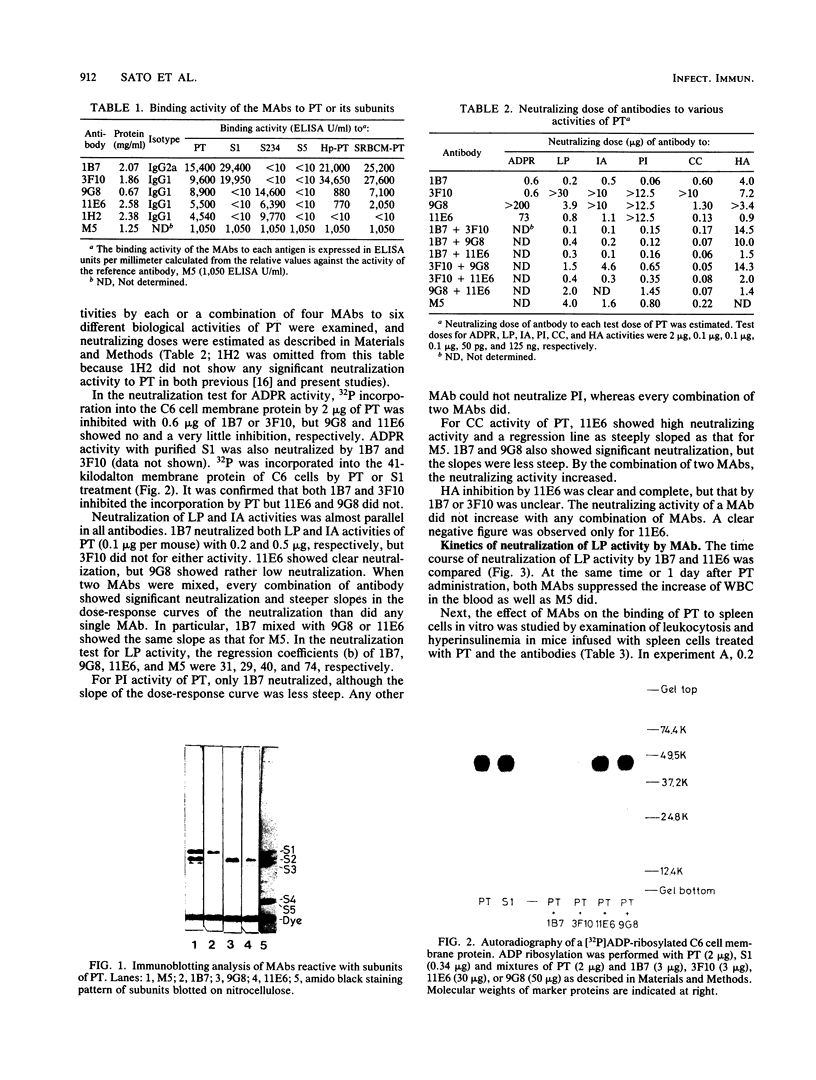
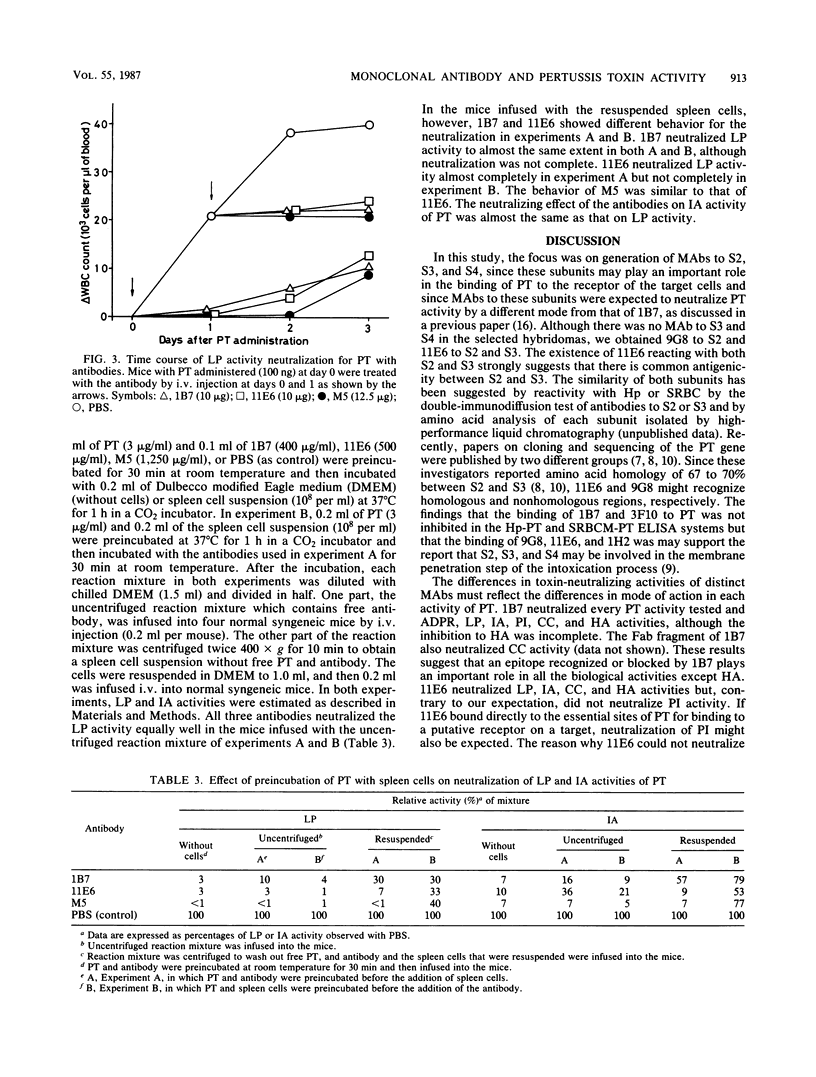
Two distinct monoclonal antibodies, one to pertussis toxin subunit S2, called 9G8, and another to subunits S2 and S3, called 11E6, were generated from the hybridomas of myeloma SP2/0 and spleen cells of BALB/c mice immunized mainly with the subunit S234 complex. Binding ability of 9G8 and 11E6 to the subunits was confirmed by the enzyme-linked immunosorbent assay and immunoblotting analysis. Generation of 11E6 bound to both S2 and S3 might mean that there is common antigenicity between S2 and S3. Neutralizing activities of 9G8 and 11E6 on various biological activities of pertussis toxin, including ADP-ribosyltransferase and leukocytosis-promoting, islet-activating, permeability-increasing. Chinese hamster ovary (CHO) cell-clustering, and hemagglutinating activities, were compared with those of anti-S1 monoclonal antibodies 1B7 and 3F10, which were isolated and characterized in a previous study (H. Sato, A. Ito, J. Chiba, and Y. Sato, Infect. Immun. 46:422-428, 1984). 1B7 and 3F10 neutralized ADP-ribosyltransferase activity of pertussis toxin or S1, but 9G8 and 11E6 did not. 1B7 showed very potent neutralization against leukocytosis-promoting, islet-activating, permeability-increasing, and CHO cell-clustering activities of pertussis toxin, but 3F10 did not, although anti-ADP-ribosyltransferase activities of both antibodies were identical. 11E6 neutralized leukocytosis-promoting, islet-activating, CHO cell-clustering, and hemagglutinating activities but not permeability-increasing activity. 9G8 showed slight neutralization of leukocytosis-promoting and CHO cell-clustering activities. Specific activities of 1B7 and 11E6 in each neutralization test were higher than or almost comparable to those of polyclonal antibodies to pertussis toxin. The neutralizing mechanism of 1B7 and 11E6 in leukocytosis-promoting activity was compared. 11E6 seemed to interfere with the binding of pertussis toxin to receptors on mouse spleen cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A., Morse S. I. Interaction of lymphoid and nonlymphoid cells with the lymphocytosis-promoting factor of Bordetella pertussis. Infect Immun. 1973 Mar;7(3):461–467. doi: 10.1128/iai.7.3.461-467.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S., Asakawa S., Kurokawa M. A preliminary report on the neutralization of the lymphocytosis-promoting factor (LPF) with anti-LPF serum. Jpn J Med Sci Biol. 1970 Feb;23(1):67–70. doi: 10.7883/yoken1952.23.67. [DOI] [PubMed] [Google Scholar]
- Katada T., Amano T., Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982 Apr 10;257(7):3739–3746. [PubMed] [Google Scholar]
- Katada T., Tamura M., Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983 Jul 1;224(1):290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locht C., Barstad P. A., Coligan J. E., Mayer L., Munoz J. J., Smith S. G., Keith J. M. Molecular cloning of pertussis toxin genes. Nucleic Acids Res. 1986 Apr 25;14(8):3251–3261. doi: 10.1093/nar/14.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Tomasi M., Schiavo G., Rappuoli R. Hydrophobic photolabelling of pertussis toxin subunits interacting with lipids. FEBS Lett. 1986 Jan 6;194(2):301–304. doi: 10.1016/0014-5793(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogimori K., Ito K., Tamura M., Satoh S., Ishii S., Ui M. Chemical modification of islet-activating protein, pertussis toxin. Essential role of free amino groups in its lymphocytosis-promoting activity. Biochim Biophys Acta. 1984 Sep 28;801(2):220–231. doi: 10.1016/0304-4165(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Hashimura N., Ishii S., Ui M. Structure-function relationship of islet-activating protein, pertussis toxin: biological activities of hybrid toxins reconstituted from native and methylated subunits. Biochemistry. 1986 Mar 25;25(6):1355–1363. doi: 10.1021/bi00354a025. [DOI] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Sato H., Ito A., Chiba J., Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984 Nov;46(2):422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect Immun. 1984 Nov;46(2):415–421. doi: 10.1128/iai.46.2.415-421.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y. Protective antigens of Bordetella pertussis mouse-protection test against intracerebral and aerosol challenge of B. pertussis. Dev Biol Stand. 1985;61:461–467. [PubMed] [Google Scholar]
- Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect Immun. 1983 Jul;41(1):313–320. doi: 10.1128/iai.41.1.313-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Braaten B. A., Daynes R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J Immunol. 1984 Jan;132(1):354–362. [PubMed] [Google Scholar]
- Stanker L. H., Vanderlaan M., Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985 Jan 21;76(1):157–169. doi: 10.1016/0022-1759(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]





