Abstract
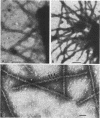
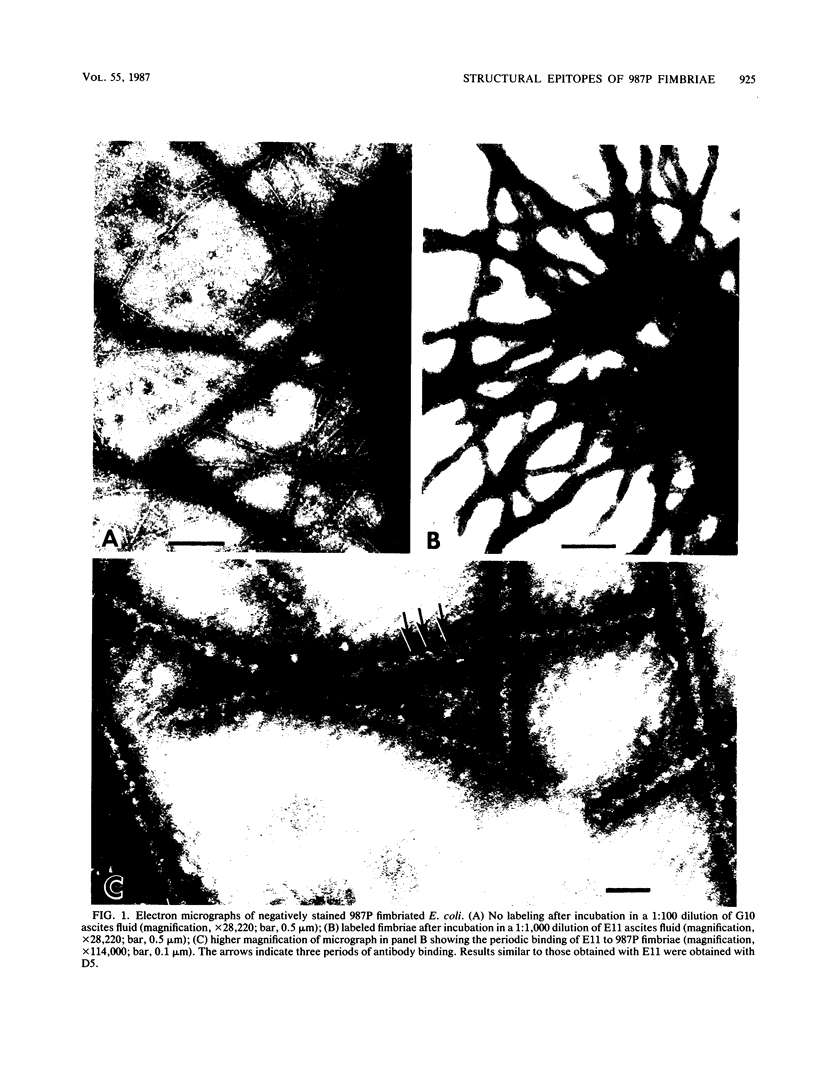
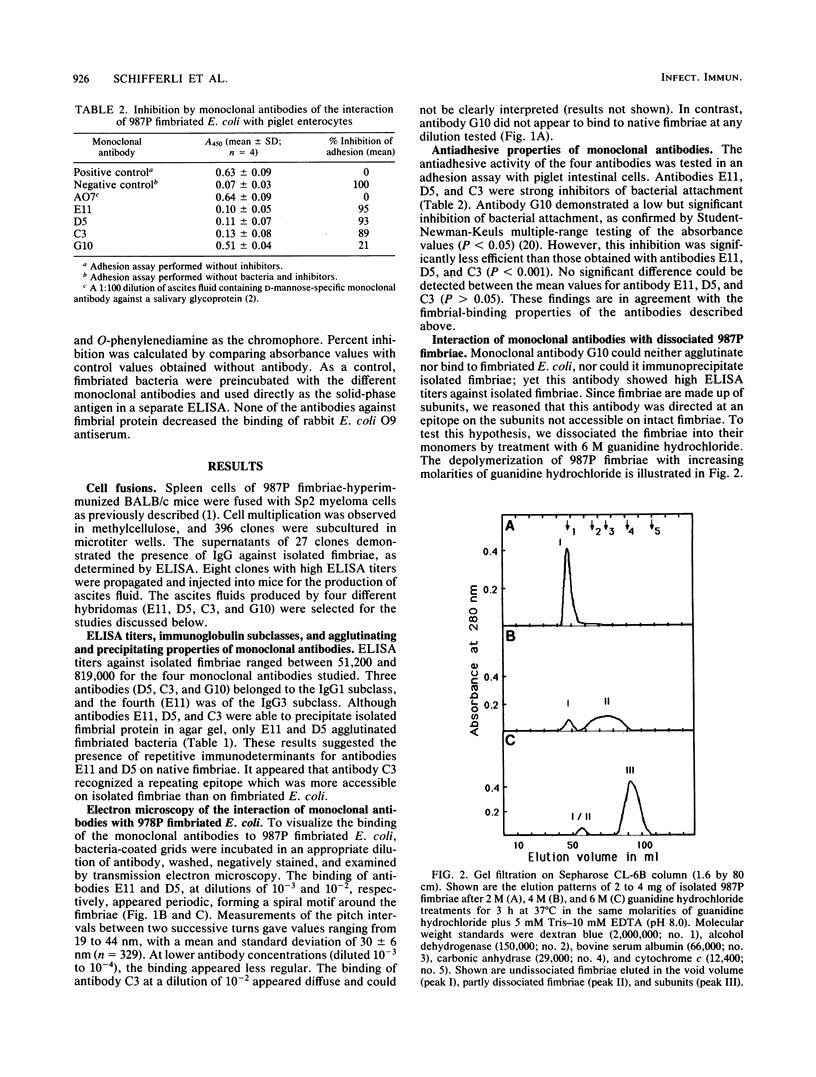
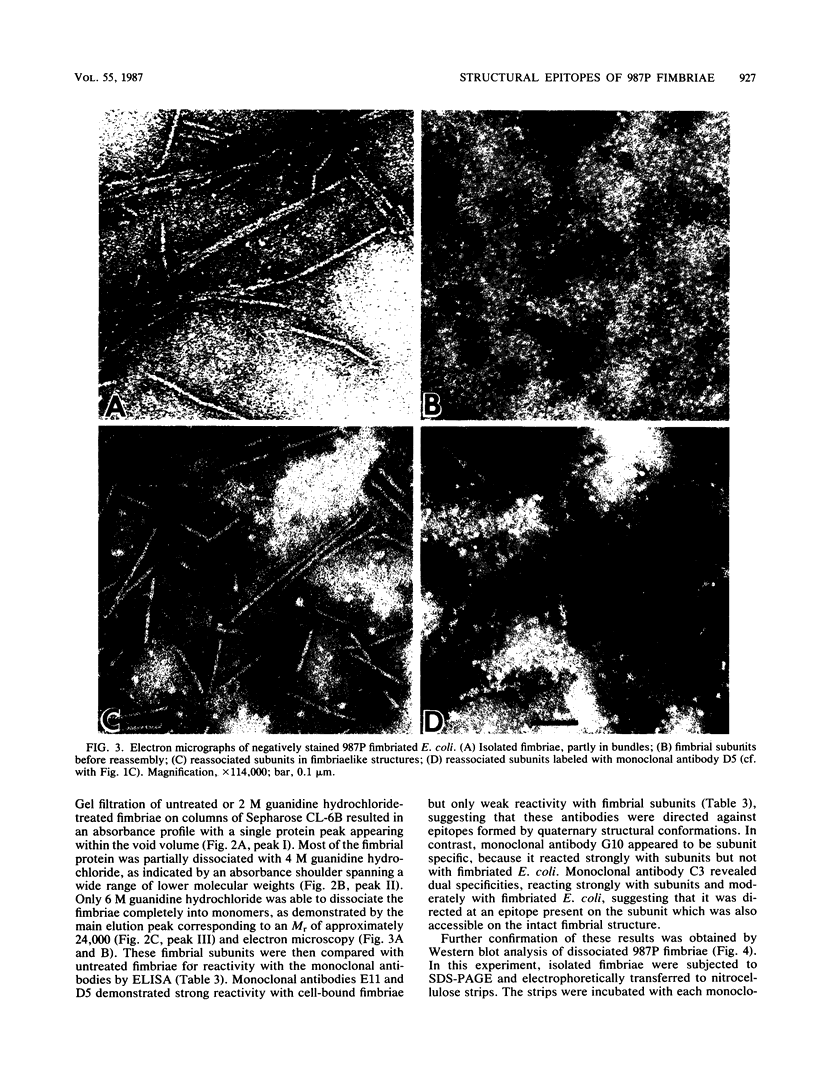
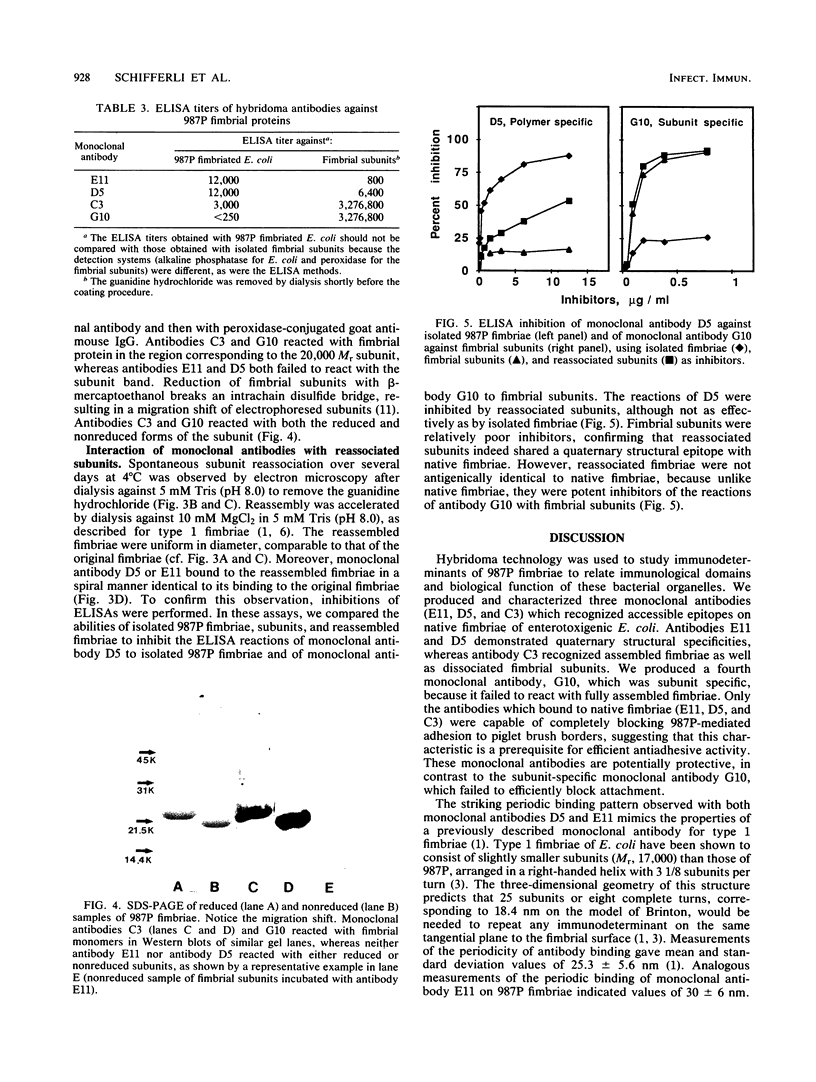
The relationship between the structure and biological function of 987P fimbriae of a strain of enterotoxigenic Escherichia coli (O9:K103:H-) from piglets was investigated. A set of four monoclonal antibodies was prepared from the spleen cells of mice immunized with isolated 987P fimbriae. Antibodies E11, D5, and C3, but not G10, reacted in enzyme-linked immunosorbent assays with 987P fimbriae-bearing E. coli. Electron microscopy showed that E11 and D5 reacted in a discrete periodic pattern forming a spiral motif along the length of the fimbriae. The results of enzyme-linked immunosorbent assays were in agreement with these results; antibodies E11 and D5 reacted at a high dilution (1:12,000) with native fimbriae on the surface of E. coli, whereas antibody C3 reacted at an intermediate dilution (1:3,000) and G10 failed to react at all (less than 1:250). In contrast, C3 and G10 reacted at a dilution of 1:3,276,000 with the fimbrial subunits derived by treating the isolated fimbriae with 6 M guanidine hydrochloride, whereas E11 and D5 reacted with the subunits at much lower dilutions of 1:800 and 1:6,400, respectively. Moreover, fimbriae reassembled from the subunits regained reactivity with antibodies D5 and E11, indicating that these antibodies are directed against quaternary conformational epitopes. Only the three antibodies (D5, E11, and C3) that recognized epitopes accessible on intact fimbriae were able to efficiently block the adhesion of 987P fimbriated E. coli to piglet enterocytes. These results indicate that certain epitopes of 987P fimbriae are dependent on quaternary structural conformation, whereas others are present on monomeric subunits; some of the latter appear to remain accessible on fully assembled fimbriae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Hasty D. L., Simpson W. A., Beachey E. H. Antiadhesive properties of a quaternary structure-specific hybridoma antibody against type 1 fimbriae of Escherichia coli. J Exp Med. 1983 Oct 1;158(4):1114–1128. doi: 10.1084/jem.158.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Hasty D. L., Simpson W. A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986 Feb;51(2):405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. In vitro adhesion of piliated Escherichia coli to small intestinal villous epithelial cells from rabbits and the identification of a soluble 987P pilus receptor-containing fraction. Infect Immun. 1982 Jun;36(3):1192–1198. doi: 10.1128/iai.36.3.1192-1198.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D. C., Eisenstein B. I. Antigenic quantitation of type 1 fimbriae on the surface of Escherichia coli cells by an enzyme-linked immunosorbent inhibition assay. Infect Immun. 1982 Nov;38(2):764–773. doi: 10.1128/iai.38.2.764-773.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foged N. T., Klemm P., Elling F., Jorsal S. E., Zeuthen J. Monoclonal antibodies to K88ab, K88ac and K88ad fimbriae from enterotoxigenic Escherichia coli. Microb Pathog. 1986 Feb;1(1):57–69. doi: 10.1016/0882-4010(86)90032-x. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E. Factors affecting expression of the Escherichia coli pilus K99. Infect Immun. 1980 Apr;28(1):190–194. doi: 10.1128/iai.28.1.190-194.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Nagy B., Moon H. W. Colonization of porcine small intestine by Escherichia coli: colonization and adhesion factors of pig enteropathogens that lack K88. J Infect Dis. 1977 Apr;135(4):531–539. doi: 10.1093/infdis/135.4.531. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Richter P. Escherichia coli 987P pilus: purification and partial characterization. J Bacteriol. 1981 May;146(2):784–789. doi: 10.1128/jb.146.2.784-789.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo, adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect Immun. 1977 Apr;16(1):344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E., To C. C., Brinton C. C. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating dams with purified pili. Infect Immun. 1978 Jul;21(1):269–274. doi: 10.1128/iai.21.1.269-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Courtney H. S., Schifferli D. M., Beachey E. H. Enzyme-linked immunosorbent assay for adherence of bacteria to animal cells. J Clin Microbiol. 1986 Oct;24(4):512–516. doi: 10.1128/jcm.24.4.512-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Abraham S. N., Beachey E. H. Influence of trimethoprim and sulfamethoxazole on the synthesis, expression, and function of type 1 fimbriae of Escherichia coli. J Infect Dis. 1986 Sep;154(3):490–496. doi: 10.1093/infdis/154.3.490. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Scraba D. G., Paranchych W. Formation of 9-nm filaments from pilin monomers obtained by octyl-glucoside dissociation of Pseudomonas aeruginosa pili. J Bacteriol. 1982 Sep;151(3):1508–1513. doi: 10.1128/jb.151.3.1508-1513.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]