Abstract
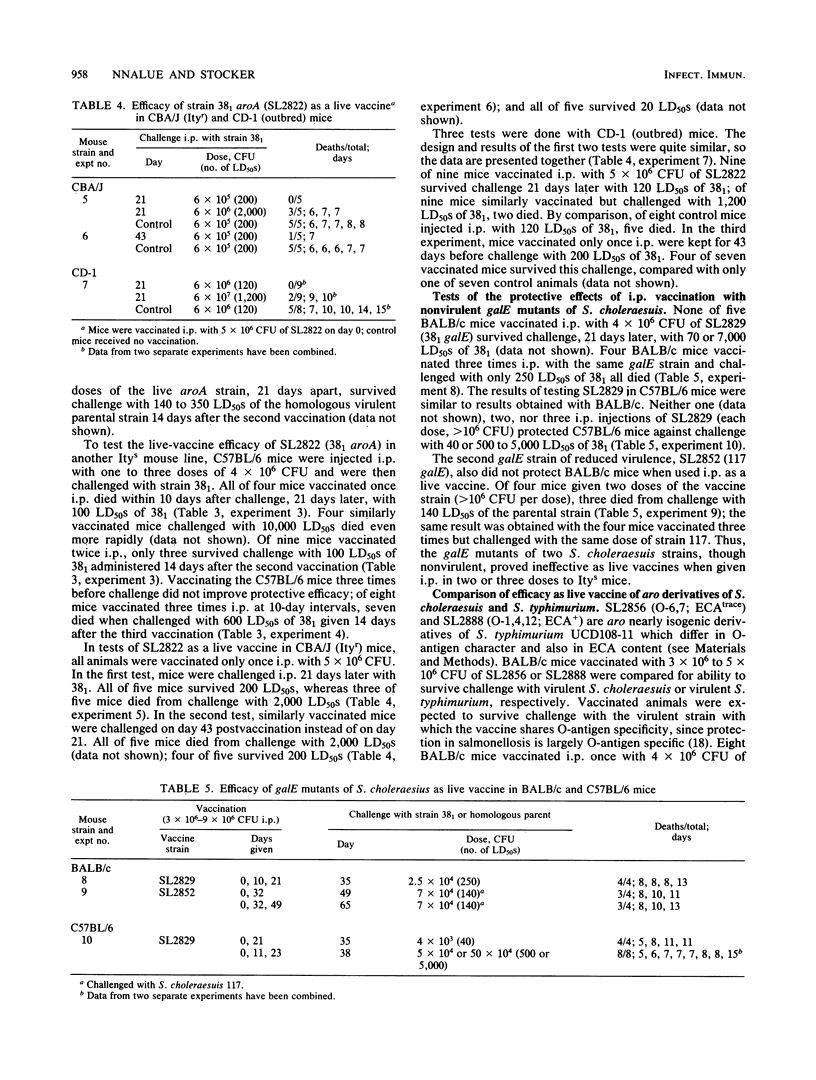
Aromatic compound-dependent (aro) derivatives of three mouse-virulent strains of Salmonella choleraesuis (Salmonella cholerae-suis) were constructed and shown to be nonvirulent for mice (intraperitoneal [i.p.] 50% lethal dose [LD50], greater than 5 X 10(6) CFU). A pur derivative, and a thy derivative, each of a different virulent parent, remained moderately virulent (i.p. LD50S for BALB/c mice, ca. 10(5) and 5 X 10(4) CFU, respectively). Tested as live vaccines i.p., the aro strains were ineffective in salmonella-susceptible BALB/c and C57BL/6 mice but were somewhat effective in salmonella-resistant CBA/J mice and in outbred CD-1 mice. The pur and thy strains were effective as live vaccines in BALB/c mice when given in sublethal doses. Two previously isolated nonvirulent galE derivatives of S. choleraesuis (i.p. LD50 in BALB/c mice, greater than 10(6) CFU) were also ineffective as live vaccines in BALB/c and C57BL/6 mice. The main antigenic difference between S. choleraesuis (O-6,7) and S. typhimurium (O-4,12) is in O-antigen character, thought to largely determine the specificity of protection in salmonellosis. Paired, nearly isogenic O-6,7 and O-4,12 derivatives were constructed from an aro S. typhimurium strain of proven efficacy as a live vaccine. Used as live vaccines, the O-4,12 member protected BALB/c mice against challenge with virulent S. typhimurium, whereas the O-6,7 member did not protect against virulent S. choleraesuis. However, BALB/c mice vaccinated with the O-6,7 member and mice vaccinated with an aro S. choleraesuis strain were protected against challenge with a moderately virulent (LD50, 5 X 10(4) CFU) O-6,7 derivative of an S. typhimurium strain.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence of Bacterium typhosum: the virulence of mutants. Br J Exp Pathol. 1950 Dec;31(6):714–724. [PMC free article] [PubMed] [Google Scholar]
- Baskerville A., Dow C. Pathology of experimental pneumonia in pigs produced by Salmonella cholerae-suis. J Comp Pathol. 1973 Apr;83(2):207–215. doi: 10.1016/0021-9975(73)90044-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M., Stocker B. A. Transduction by phage P1kc in Salmonella typhimurium. Virology. 1974 Aug;60(2):503–514. doi: 10.1016/0042-6822(74)90344-4. [DOI] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Germanier R. Immunity in Experimental Salmonellosis I. Protection Induced by Rough Mutants of Salmonella typhimurium. Infect Immun. 1970 Sep;2(3):309–315. doi: 10.1128/iai.2.3.309-315.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Jochimsen B., Koduri K. R. Microbial models and regulatory elements in the control of purine metabolism. Ciba Found Symp. 1977;(48):23–41. doi: 10.1002/9780470720301.ch3. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO H., HONDA T., KAWAKAMI M., MITSUHASHI S. Studies on the experimental salmonellosis. VI. Longlasting immunity of mouse immunized with live vaccine of Salmonella enteritidis. Jpn J Exp Med. 1961 Jun;31:187–190. [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Ivánovics G., Marjai E., Dobozy A. The growth of purine mutants of Bacillus anthracis in the body of the mouse. J Gen Microbiol. 1968 Sep;53(2):147–162. doi: 10.1099/00221287-53-2-147. [DOI] [PubMed] [Google Scholar]
- KONDO E., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. IV. ACTIVE TRANSDUCING BACTERIOPHAGE P1 CM PRODUCED BY THE COMBINATION OF R FACTOR WITH BACTERIOPHAGE P1. J Bacteriol. 1964 Nov;88:1266–1276. doi: 10.1128/jb.88.5.1266-1276.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman M. B., Stocker B. A., Roantree R. J. Comparison of the virulence of O:9,12 and O:4,5,12 Salmonella typhimurium his+ transductants for mice. Infect Immun. 1977 Feb;15(2):491–499. doi: 10.1128/iai.15.2.491-499.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman M. B., Stocker B. A., Roantree R. J. Evaluation of the immune response directed against the Salmonella antigenic factors O4,5 and O9. Infect Immun. 1979 Dec;26(3):956–965. doi: 10.1128/iai.26.3.956-965.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACCREADY R. A., REARDON J. P., SAPHRA I. Salmonellosis in Massachusetts; a sixteen-year experience. N Engl J Med. 1957 Jun 13;256(24):1121–1128. doi: 10.1056/NEJM195706132562401. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H. Participation of lipopolysaccharide genes in the determination of the entobacterial common antigen: analysis in Salmonella groups B and C1. J Bacteriol. 1974 Sep;119(3):765–770. doi: 10.1128/jb.119.3.765-770.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Stocker B. A. Relation of lipopolysaccharide character to P1 sensitivity in Salmonella typhimurium. Virology. 1974 Aug;60(2):491–502. doi: 10.1016/0042-6822(74)90343-2. [DOI] [PubMed] [Google Scholar]
- Robertsson J. A., Lindberg A. A., Hoiseth S., Stocker B. A. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983 Aug;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- SAPHRA I., WINTER J. W. Clinical manifestations of salmonellosis in man; an evaluation of 7779 human infections identified at the New York Salmonella Center. N Engl J Med. 1957 Jun 13;256(24):1128–1134. doi: 10.1056/NEJM195706132562402. [DOI] [PubMed] [Google Scholar]
- SMITH H. W. THE IMMUNIZATION OF MICE, CALVES AND PIGS AGAINST SALMONELLA DUBLIN AND SALMONELLA CHOLERAE-SUIS INFECTIONS. J Hyg (Lond) 1965 Mar;63:117–135. doi: 10.1017/s0022172400045022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Smith H. W., Tucker J. F. The virulence of trimethoprim-resistant thymine-requiring strains of Salmonella. J Hyg (Lond) 1976 Feb;76(1):97–108. doi: 10.1017/s0022172400054991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USHIBA D., SAITO K., AKIYAMA T., NAKANO M., SUGIYAMA T., SHIRONO S. Studies on experimental typhoid: bacterial multiplication and host cell response after infection with Salmonella enteritidis in mice immunized with live and killed vaccines. Jpn J Microbiol. 1959 Apr;3:231–242. doi: 10.1111/j.1348-0421.1959.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Valtonen M. V., Larinkari U. M., Plosila M., Valtonen V. V., Mäkelä P. H. Effect of enterobacterial common antigen on mouse virulence of Salmonella typhimurium. Infect Immun. 1976 Jun;13(6):1601–1605. doi: 10.1128/iai.13.6.1601-1605.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- Weissbluth M., Shulman S. T., Holson B., Lerner C. Salmonella cholera-suis: a distinctive bacterial pathogen. J Pediatr. 1981 Mar;98(3):423–426. doi: 10.1016/s0022-3476(81)80710-x. [DOI] [PubMed] [Google Scholar]
- Wray C., Sojka W. J., Morris J. A., Brinley Morgan W. J. The immunization of mice and calves with gal E mutants of Salmonella typhimurium. J Hyg (Lond) 1977 Aug;79(1):17–24. doi: 10.1017/s0022172400052803. [DOI] [PMC free article] [PubMed] [Google Scholar]


