Abstract
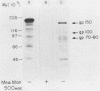
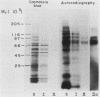
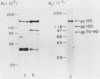
Type 1 fimbriated (mannose-specific) Escherichia coli cells bind to mannose residues on human polymorphonuclear leukocytes (PMN); this leads to phagocytosis of the bacteria. To identify the mannose-containing receptors on the PMN, the cells were surface labeled with 125I and lysed in 0.5% Nonidet P-40, and the lysate was fractionated by affinity chromatography on a column of Sepharose-bound fimbriae. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography of the material eluted from the column with 500 mM methyl-alpha-mannoside revealed two radioactive bands of Mr 70,000 to 80,000 (gp70-80) and 100,000 (gp100). Another weak band of Mr 150,000 (gp150) was observed after prolonged exposure of the gel. Upon blotting of the glycoproteins separated by polyacrylamide gel electrophoresis and overlaying of the blots with concanavalin A, gp150 appeared as the major band. Membrane preparations of the PMN were enriched in gp70-80, gp100, and gp150, in comparison with the cell homogenates, further suggesting that these glycoproteins are surface components. Fractionation of the membrane preparations on the immobilized fimbriae followed by concanavalin A overlay of blots of the methyl-alpha-mannoside-eluted material revealed that gp150 was the major component in this fraction. The eluted fraction, obtained from a cell lysate (4.4 micrograms/ml), inhibited by 70% the agglutination of yeasts by the intact bacteria. Our results suggest that the three surface glycoproteins isolated by us serve as receptors for mannose-specific E. coli on PMN and may be involved in the lectin-mediated phagocytosis of the bacteria.
Full text
PDF


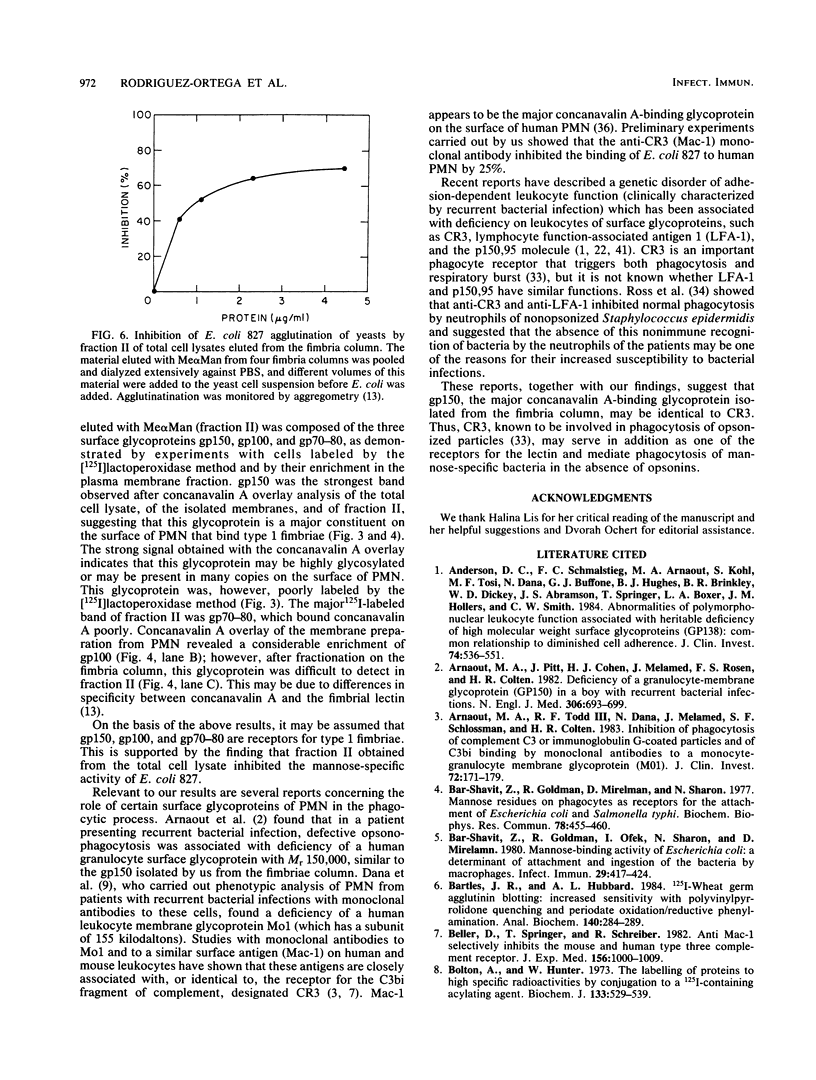

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalstieg F. C., Arnaout M. A., Kohl S., Tosi M. F., Dana N., Buffone G. J., Hughes B. J., Brinkley B. R., Dickey W. D. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest. 1984 Aug;74(2):536–551. doi: 10.1172/JCI111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Pitt J., Cohen H. J., Melamed J., Rosen F. S., Colten H. R. Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982 Mar 25;306(12):693–699. doi: 10.1056/NEJM198203253061201. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Goldman R., Ofek I., Sharon N., Mirelman D. Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun. 1980 Aug;29(2):417–424. doi: 10.1128/iai.29.2.417-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Bartles J. R., Hubbard A. L. 125I-wheat germ agglutinin blotting: increased sensitivity with polyvinylpyrrolidone quenching and periodate oxidation/reductive phenylamination. Anal Biochem. 1984 Jul;140(1):284–292. doi: 10.1016/0003-2697(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana N., Todd R. F., 3rd, Pitt J., Springer T. A., Arnaout M. A. Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984 Jan;73(1):153–159. doi: 10.1172/JCI111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A., Lis H., Gershoni J. M., Sharon N. Identification of glycoproteins that are receptors for peanut agglutinin on immature (cortical) mouse thymocytes. FEBS Lett. 1986 Jan 1;194(1):28–32. doi: 10.1016/0014-5793(86)80045-x. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Davis F. E., Palade G. E. Protein blotting in uniform or gradient electric fields. Anal Biochem. 1985 Jan;144(1):32–40. doi: 10.1016/0003-2697(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Hjorth R., Jonsson A. K., Vretblad P. A rapid method for purification of human granulocytes using percoll. A comparison with dextran sedimentation. J Immunol Methods. 1981;43(1):95–101. doi: 10.1016/0022-1759(81)90040-5. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Springer T. A., Schmalstieg F. C., Loo L. S., Anderson D. C. Defective natural killer cytotoxicity and polymorphonuclear leukocyte antibody-dependent cellular cytotoxicity in patients with LFA-1/OKM-1 deficiency. J Immunol. 1984 Dec;133(6):2972–2978. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Koellreutter B., Wuersch P. Oligomannoside-type glycopeptides inhibiting adhesion of Escherichia coli strains mediated by type 1 pili: preparation of potent inhibitors from plant glycoproteins. Infect Immun. 1986 May;52(2):428–436. doi: 10.1128/iai.52.2.428-436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Perry A., Ofek I., Silverblatt F. J. Enhancement of mannose-mediated stimulation of human granulocytes by type 1 fimbriae aggregated with antibodies on Escherichia coli surfaces. Infect Immun. 1983 Mar;39(3):1334–1345. doi: 10.1128/iai.39.3.1334-1345.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Ross G. D., Thompson R. A., Walport M. J., Springer T. A., Watson J. V., Ward R. H., Lida J., Newman S. L., Harrison R. A., Lachmann P. J. Characterization of patients with an increased susceptibility to bacterial infections and a genetic deficiency of leukocyte membrane complement receptor type 3 and the related membrane antigen LFA-1. Blood. 1985 Oct;66(4):882–890. [PubMed] [Google Scholar]
- Rottini G., Cian F., Soranzo M. R., Albrigo R., Patriarca P. Evidence for the involvement of human polymorphonuclear leucocyte mannose-like receptors in the phagocytosis of Escherichia coli. FEBS Lett. 1979 Sep 15;105(2):307–312. doi: 10.1016/0014-5793(79)80636-5. [DOI] [PubMed] [Google Scholar]
- Schmalstieg F. C., Rudloff H. E., Anderson D. C. Binding of the adhesive protein complex (LFA-1/Mac-1/p150,95) to concanavalin A. J Leukoc Biol. 1986 Feb;39(2):193–203. doi: 10.1002/jlb.39.2.193. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Chen K. Y., Canellakis E. S. Isolation and characterization of the plasma membrane of L-1210 cells. Iodination as a marker for the plasma membrane. Biochim Biophys Acta. 1975 Aug 20;401(2):196–212. doi: 10.1016/0005-2736(75)90304-1. [DOI] [PubMed] [Google Scholar]