Abstract
Aim
To identify all cases of Neurofibromatosis type 1 in Northern Ireland under 16 years of age, document age, modes of presentation and any complications that occurred.
Methods
All cases of Neurofibromatosis type 1 in children less than 16 years of age were identified from the records in the Department of Medical Genetics. From the records and by direct contact with the patient's parents the relevant clinical information was obtained.
Results
Seventy-five children aged sixteen years or less were identified (prevalence of 17.6 per 100,000 (1 in 5681) of the population under 16 years of age). 45 (57%) had an affected first degree relative and 32 (43%) had no family history. 54 (72%) had at least one complication, 18 (24%) had 2; 9 (12%) had 3 and 3 (4%) had 4 complications. The most common complication was learning difficulties, which was seen in 37 (49.3%) cases. 11 (14.7%) patients had a malignancy; of whom 5 (6.7%) had an optic glioma (2 identified after diagnosis) and 3 (4%) had a CNS malignancy.
Conclusion
Children with NF1 should be seen yearly by a health professional or team until after puberty and have a thorough clinical examination. The minimum prevalence is 1 in 5681 (17.6 per 100,000). We suggest a checklist is used to review nine important features; height, weight, head circumference, examination of the skin, blood pressure, ophthalmology examination (includes visual fields), examination of the spine, and for early / late puberty and consider referral to educational psychology. Educational authorities should identify all individuals with NF1 as they are at high risk of developing learning difficulties.
Keywords: Neurofibromatosis, malignant, tumours, prevalence
INTRODUCTION
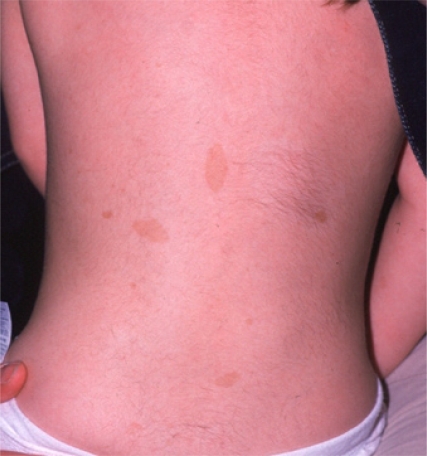
Neurofibromatosis type 1 (NF1) is an autosomal dominant condition, which can cause growths, either on peripheral nerves or in the central nervous system, as well as multiple café au lait spots on the skin (figure 1). The condition is one of the most common genetic conditions; it occurs in around 1 in 4000 of the population and is noted for its variation of clinical expression1,2. It is associated with a significant reduction in life expectancy3. The gene for NF1 was localised to the long arm of chromosome 17 in 19874. Neurofibromatosis type 1 occurs in every racial and ethnic group and affects both sexes equally.
Fig 1.
Café-au-lait spots
The diagnosis of NF1 can be made using the National Institutes of Health (NIH) diagnostic criteria5 (Table I). NF1 is associated with many other clinical features that can occur at all ages. Morbidity, due to complications of NF1, occurs mainly in adults but occasionally in children. NF1 can have an adverse effect on the child's life. In this study, the population of individuals less than 16 years of age with NF1 in Northern Ireland was identified and the main causes of morbidity recorded.
Table I.
NIH Diagnostic criteria for type 1 neurofibromatosis.
| Two or more of the following criteria are required for diagnosis: |
|---|
| 1. Six or more café-au-lait spots over 5 mm in prepubertal individuals and over 15 mm in postpubertal individuals |
| 2. Two or more neurofibromas of any type or one plexiform neurofibroma. |
| 3. Freckling in the axilla or groin. |
| 4. Optic glioma. |
| 5. Two or more Lisch nodules. |
| 6. Presence of a distinct osseous lesion, sphenoid wing, dysplasia or thinning of a long bone with or without pseudoarthrosis. |
| 7. A first degree relative who meets the above criteria for NF1. |
METHODS
Northern Ireland has well developed paediatric, childhood dermatology and neurology services that liaise closely with Medical Genetics. Virtually all patients with NF1 in Northern Ireland (population 1.68 million, of whom 425,250 are aged under 16 years4) are referred to the Department of Medical Genetics in Belfast City Hospital for genetic counselling. A register of all cases of NF1 has been maintained since 1990. From the register, all patients under 16 years of age who were diagnosed as having NF1 were identified and the diagnosis verified using the diagnostic criteria (Table I). From the medical records, the presenting symptom of NF1 and all the relevant clinical signs and symptoms were documented. The parents of each patient were contacted and information was obtained on the child's behaviour and school performance. Any complications that required medical or educational intervention and were present on the prevalence day were recorded. Learning difficulties were said to be present if the child had a statement of educational needs, or was placed in special schooling.
RESULTS
Seventy-five children aged sixteen years or less were identified. This gives a prevalence of 17.6 per 100,000 (1 in 5681) of the population under 16 years of age. Figure 2 shows the incidence of NF1 in Northern Ireland from 1997-2002 and shows the number of cases that were the result of new mutations. Of the 75 cases, 38 were male and 37 female. The mean age was 9.3 years (range 0.9 - 16.8 years).
Fig 2.
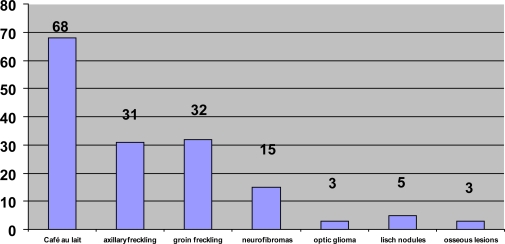
Frequency of major features found at diagnosis Features as %. (Café au lait 91%; axillary freckling 41%; groin freckling 43%; neurofibromas 20%; optic glioma 4%; Lisch nodules 6%; osseous lesions 4%)
The age of diagnosis ranged from 3 months to 15.5 years (mean 4.6 years). Some children were diagnosed early as there was a family history and these children were kept under review. 45 (57%) had an affected first degree relative and 32 (43%) had no family history. Features seen are documented in Table II, and their frequency at diagnosis in figure 1. Café au lait spots were found in most of the 75 cases, a significant number had freckling in the axilla or the groin, 15 had neurofibromas and a small number had optic gliomas, Lisch nodules or bone lesions. In our cohort, 99 complications were documented in the 75 children. Fifty-four children (72%) had at least one complication. The types of complications and their frequency are documented in Table III and the majority occurred before 10 years of age.
Table II.
Type and frequency of complications detected
| Complication | No. (%) of children |
|---|---|
| Learning difficulties | 37 (49.3) |
| Optic glioma | 5 (6.7) |
| Epilepsy | 3 (4) |
| Plexiform neurofibromas | 7(9.3) |
| Pseudoarthrosis | 4 (5.3) |
| Scoliosis | 6 (8) |
| CNS malignancy | 3 (4) |
| Non CNS malignancy | 3 (4) |
| Short stature | 5(6.7) |
| Large head | 19(25.3) |
| Hypertension | 1 (1.3) |
| Other non tumour complications | 3 (4) |
Table III.
Frequency of children with one or more complication
| Number of complications | Number (%) of children |
|---|---|
| 1 | 24 (32) |
| 2 | 18 (24) |
| 3 | 9 (12) |
| 4 | 3 (4) |
| Total no. of complications | 99 |
DISCUSSION
In Northern Ireland, most cases of NF1 are referred to the specialist clinic for neurofibromatosis in the Department of Medical Genetics. Northern Ireland has a stable population with negligible patient drift to Great Britain or the Republic of Ireland. Together with the well developed and multiple referral and ascertainment routes, we believe, therefore, that this study shows a rigorous attempt at as complete ascertainment that is possible and allows calculation of a fairly accurate minimum prevalence figure. In the specialist clinic, the patient is offered genetic counselling and any complications of the condition are identified. At present the guidelines of the National Institute of Health are adhered to. A comprehensive clinical examination and ocular assessment with fundoscopy is performed. By identifying the incidence and types of complications seen in cases that attend the clinic, these guidelines can be evaluated and recommendations can be made about any screening that should be undertaken.
We believe we have identified most of the significantly affected cases of NF1 in children less than 16 years of age in Northern Ireland. Although the prevalence rate of 17.6/100,000 is lower than a recent study in Germany which showed a prevalence of 30/100,000 live births7, we believe our result is accurate and represents a minimum prevalence figure for childhood NF. The majority of other calculated prevalence figures include adults and it is likely that some adults with a mild skin phenotype or with complications such as phaeochromocytoma, which are recognised later in life, clearly would not be identified in our cohort. Figure 3 shows the incidence of NF1 during the period 1997-2002 and shows that the number of new cases increased over that time. This is most likely due to better awareness about the condition, referrals, and clinic facilities, rather than a true increase in the number of cases. Undoubtedly the accuracy of the prevalence will improve with continued searching for cases and a repeat ascertainment study in a few years time.
Fig 3.
Number of new cases identified from 1997-2002
A study in a large cohort of NF1 patients in the north west of England8 found that neurofibromas and Lisch nodules were more common than our results suggest. It is likely that the Lisch nodules were not looked for on diagnosis and because of the average young age of diagnosis (4.6 years) the neurofibromas had not developed.
This study shows that children with NF1 can develop a wide range of complications. These findings are useful in counselling families with NF1 - the most common complication is mild to moderate learning difficulties. All children with NF1 should identified by the education authorities in view of their high risk of developing learning difficulties. Other complications, like short stature, neurofibromas, large head and hypertension should be detected by a thorough clinical examination as recommended in the NIH guidelines (Table I). This also recommends regular ophthalmic examination for optic gliomas preferably by an ophthalmologist. There are no screening procedures for epilepsy. Riccardi1 recommended that all patients with NF1 should have an MRI or CT scan once, however this would not be an effective screening procedure and in this study the low rate of CNS malignancy would suggest that regular radiological investigations would have a low pick up rate and subject the child to unnecessary investigations.
Most of the children had either one or two complications but a small number suffered from multiple complications. This study did not include a more detailed look at the patterns of severity, unlike Huson et al9 who divided their patients into 4 grades of severity. In their cohort of patients they found that the younger new mutations were more seriously affected than older new mutations. There was no difference in degrees of severity when they looked at parental status. The difference was attributed to ascertainment. No other study has offered any explanation to explain the different ways the NF1 gene is expressed in different patients. We suggest a checklist of nine features, which can be used by specialists or GP's and will cover the majority of potential problems with NF1 (Table IV). A recent paper on guidelines for diagnosis and management of NF lists a useful onset age for many of the manifestations (table V), and this will alert paediatricians and other health professionals involved with children with a diagnosis of NF, on when to expect complications to arise11.
Table IV.
Suggested check list for yearly examination on patients with Neurofibromatosis type 1
|
Table V.
Frequency and age of onset of major clinical manifestations of neurofibromatosis 1*
| Clinical manifestation | Frequency (%) | Age of onset |
|---|---|---|
| Café au lait patches | >99 | Birth to 12 y |
| Skin-fold freckling | 85 | 3 y to adolescence |
| Lisch nodules | 90–95 | >3 y |
| Cutaneous neurofibromas | >99 | >7 y (usually late adolescence) |
| Plexiform neurofibromas | 30 (visible) − 50 (on imaging) | Birth to 18 y |
| Disfiguring facial plexiform neurofibromas | 3–5 | Birth to 5 y |
| Malignant peripheral nerve sheath tumour | 2–5 (8–13% lifetime risk) | 5–75 y |
| Scoliosis | 10 | Birth to 18 y |
| Scoliosis requiring surgery | 5 | Birth to 18 y |
| Pseudarthrosis of tibia | 2 | Birth to 3 y |
| Renal artery stenosis | 2 | Lifelong |
| Phaeochromocytoma | 2 | >10 y |
| Severe cognitive impairment (IQ <70) | 4–8 | Birth |
| Learning problems | 30–60 | Birth |
| Epilepsy | 6–7 | Lifelong |
| Optic pathway glioma | 15 (only 5% symptomatic) | Birth to 7 y (up to 30 y) |
| Cerebral gliomas | 2–3 | Lifelong |
| Sphenoid wing dysplasia | <1 | Congenital |
| Aqueduct stenosis | 1.5 | Lifelong |
reproduced with permission of the BMJ publishing group Ltd from table 4 of reference 11
CONCLUSIONS
In this study of individuals under 16 years of age with NF1 we believe we achieved nearly total ascertainment in Northern Ireland and calculated a minimum prevalence rate of 1 in 5681 children under the age of 16 years. This group of patients suffered a wide variety of complications. After examining the frequency of complications we believe that the NIH guidelines are appropriate for detecting these complications.
A study into the management of children with NF1 at a London teaching hospital10 found that there was a need amongst health-care professionals for evidence-based guidelines. Our study provides an almost complete ascertainment of NF1 cases in a geographical area and provides a sound evidence base for management of NF1 in children.
The authors have no conflict of interest.
REFERENCES
- 1.Riccardi VM. 2nd Ed. Baltimore: The Johns Hopkins University Press; 1992. Neurofibromatosis; phenotype, natural history and pathogenesis. [Google Scholar]
- 2.Biggart JH. Generalised neurofibromatosis. Ulster Med J. 1941;10(2):77–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68(5):1110–8. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Faryniarz AG, Chao MV, et al. Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell. 1987;49(5):589–94. doi: 10.1016/0092-8674(87)90534-4. [DOI] [PubMed] [Google Scholar]
- 5.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development. Arch Neurol. 1988;45(5):575–8. [PubMed] [Google Scholar]
- 6.Northern Ireland Statistics & Research Agency. Census 2001. Population report and mid-year estimates. Available online from: http://www.nisranew.nisra.gov.uk/census/Census2001Output/PopulationReport/populationreport1.html. Last accessed June 2008.
- 7.Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrolment. Arch Dermat. 2005;141(1):71–4. doi: 10.1001/archderm.141.1.71. [DOI] [PubMed] [Google Scholar]
- 8.McGaughran JM, Harris DI, Donnai D, Teare D, MacLeod R, Westerbeek R, et al. A clinical study of type 1 neurofibromatosis in North West England. J Med Genet. 1999;36:197–203. [PMC free article] [PubMed] [Google Scholar]
- 9.Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. J Med Genet. 1989;26(11):712–21. doi: 10.1136/jmg.26.11.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgstein B. Confusion in the management of Neurofibromatosis type 1- the need for evidence. BAACH News. 2005 Winter;:19–23. [Google Scholar]
- 11.Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, Upadhyaya M, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44(2):81–8. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]