Abstract
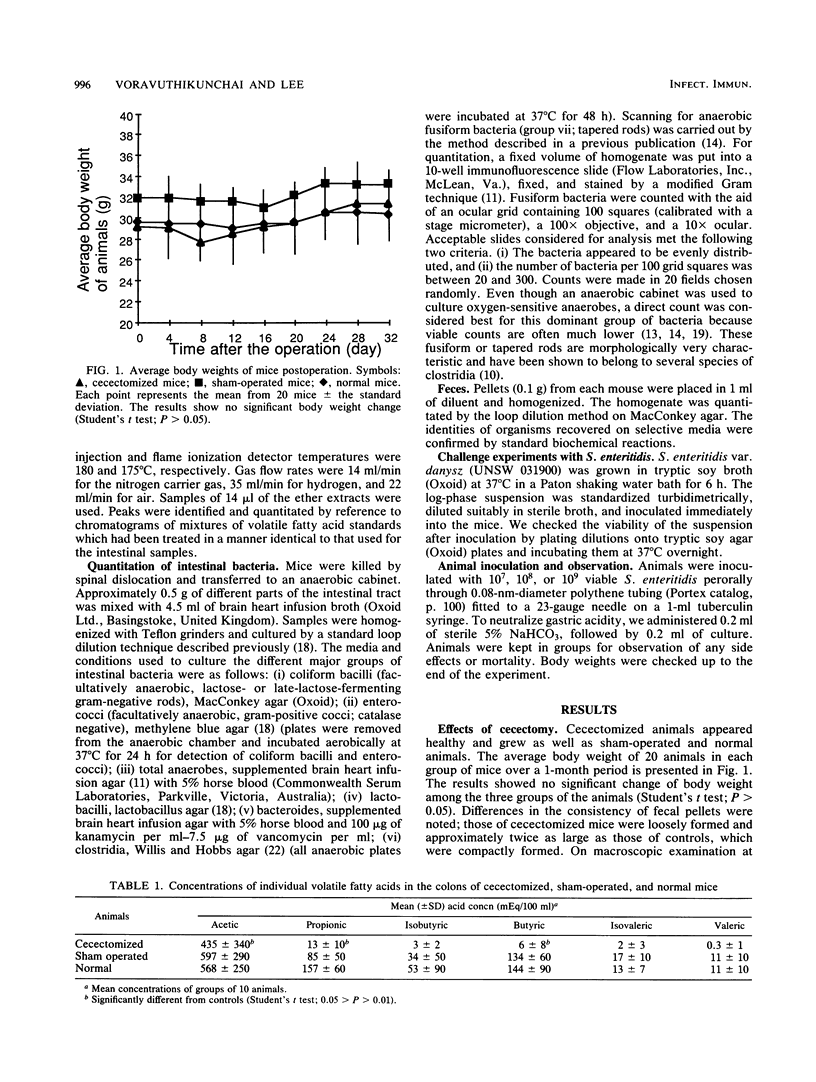
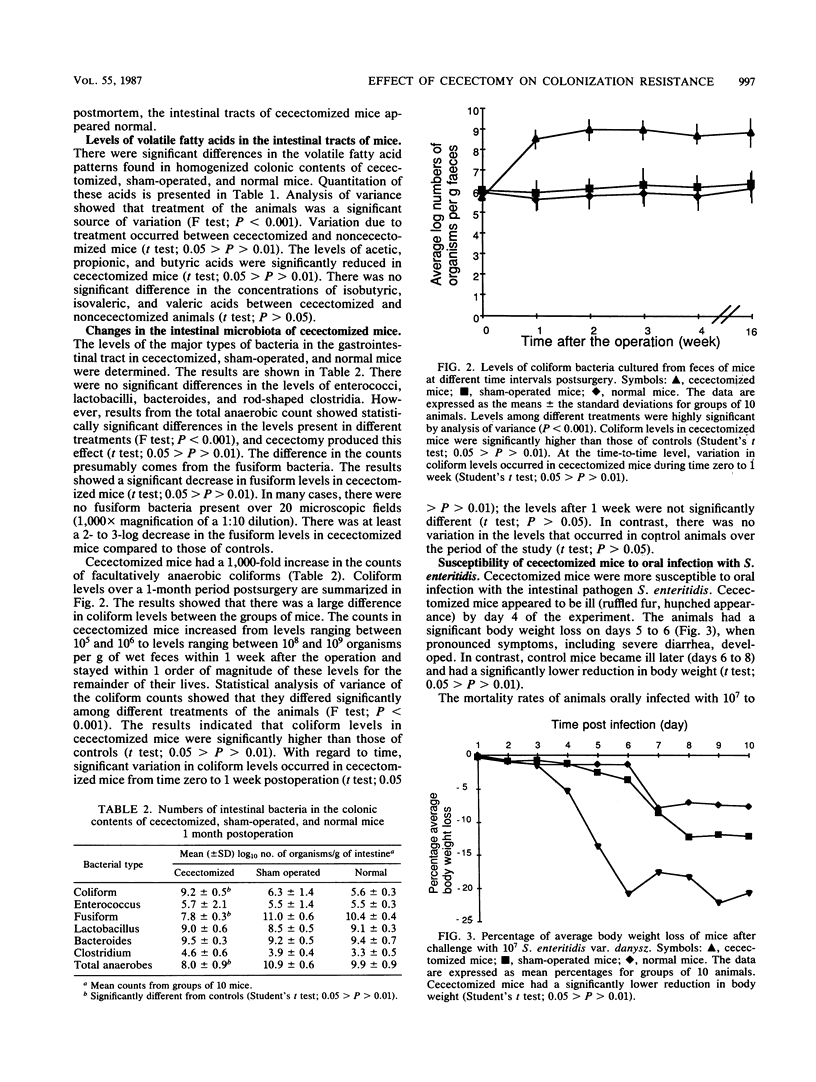
Removal of the cecum from normal mice caused a major perturbation of the microbial ecology of the gastrointestinal tract. There was a permanent reduction in colonization resistance resulting in a 1,000-fold increase in the concentration of facultatively anaerobic coliform bacteria. The animals were significantly more susceptible to peroral challenge by the intestinal pathogen Salmonella enteritidis. Coincident with this increase in coliform counts and susceptibility to salmonellae was a decrease in the numbers of strictly anaerobic fusiform bacteria that dominate the rodent intestinal tract, resulting in reduced levels of acetic, propionic, and butyric acids. Cecectomized mice are likely to be a useful model for study of the interaction between intestinal pathogens and the normal microbiota and for studies of translocation of bacteria into host tissues after loss of colonization resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHNHOFF M., MILLER C. P. Enhanced susceptibility to Salmonella infection in streptomycin-treated mice. J Infect Dis. 1962 Sep-Oct;111:117–127. doi: 10.1093/infdis/111.2.117. [DOI] [PubMed] [Google Scholar]
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. I. FACTORS WHICH INTERFERE WITH THE INITIATION OF INFECTION BY ORAL INOCULATION. J Exp Med. 1964 Nov 1;120:805–816. doi: 10.1084/jem.120.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyssen H., Parmentier G. Biohydrogenation of sterols and fatty acids by the intestinal microflora. Am J Clin Nutr. 1974 Nov;27(11):1329–1340. doi: 10.1093/ajcn/27.11.1329. [DOI] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Fekete J., Vickerman M. M., Carey K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983 Feb;39(2):686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg M. P., Bakker M., Verschoor-Burggraaf A. Effects of the human intestinal flora on germ-free mice. J Appl Bacteriol. 1981 Feb;50(1):95–106. doi: 10.1111/j.1365-2672.1981.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Hazenberg M. P., Custers-van Lieshout L. M., Engels W., Kock-van Dalen A. C. The clostridial flora of conventional mice. Z Versuchstierkd. 1977;19(3):167–174. [PubMed] [Google Scholar]
- Koopman J. P., Kennis H. M., van Druten J. A. The role of the cecum in the gastro-intestinal colonization resistance in mice. Z Versuchstierkd. 1979;21(4):192–196. [PubMed] [Google Scholar]
- Lee A., Gemmell E. Changes in the mouse intestinal microflora during weaning: role of volatile fatty acids. Infect Immun. 1972 Jan;5(1):1–7. doi: 10.1128/iai.5.1.1-7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Gordon J., Lee C. J., Dubos R. The mouse intestinal microflora with emphasis on the strict anaerobes. J Exp Med. 1971 Feb 1;133(2):339–352. doi: 10.1084/jem.133.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- MILLER C. P., BOHNHOFF M. CHANGES IN THE MOUSE'S ENTERIC MICROFLORA ASSOCIATED WITH ENHANCED SUSCEPTIBILITY TO SALMONELLA INFECTION FOLLOWING STREPTOMYCIN TREATMENT. J Infect Dis. 1963 Jul-Aug;113:59–66. doi: 10.1093/infdis/113.1.59. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J Exp Med. 1968 Jul 1;128(1):97–110. doi: 10.1084/jem.128.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Archibald R. D. The derivation and use of mice which do not harbour lactobacilli in the gastrointestinal tract. Can J Microbiol. 1984 Jun;30(6):849–853. doi: 10.1139/m84-131. [DOI] [PubMed] [Google Scholar]
- WILLIS A. T., HOBBS G. Some new media for the isolation and identification of Clostridia. J Pathol Bacteriol. 1959 Apr;77(2):511–521. doi: 10.1002/path.1700770223. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971 Sep;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]



