Abstract
Rabbit antiserum raised against the rough mutant of Escherichia coli O111:B4, designated J5, was examined for cross-reactivity to an E. coli clinical isolate (A2385). In whole-cell enzyme-linked immunosorbent assays, J5 antiserum reacted to a greater extent with A2385 grown for 5 h than with the same bacteria grown for 19 h, while the homologous antiserum reacted similarly with bacteria grown for different lengths of time. J5 antiserum reacted to the greatest extent with lipopolysaccharide (LPS) from A2385 grown for up to 10 h, and reactivity greatly diminished thereafter; homologous antiserum showed no difference in reaction over time. LPS from smooth bacteria grown for 19 h showed no reaction with J5 antiserum in immunoblots, while LPS from A2385 grown for 5 or 10 h showed a positive reaction. Little or no difference among the three LPS samples could be seen when homologous antiserum was used. Mice vaccinated with J5 LPS before lethal challenge with live A2385 were protected from this challenge, whereas most nonimmunized mice died. Toxicity tests in mice showed LPS from A2385 grown for 19 h to be twice as lethal as LPS from A2385 grown for 3 h. Mice vaccinated with J5 LPS were protected to a greater extent when challenged with a lethal dose of LPS from A2385 grown for 3 h than when challenged with LPS from A2385 grown for 19 h. The results reported here may explain the means by which J5 vaccination (active or passive) sometimes protects against heterologous challenge.
Full text
PDF



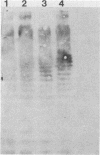
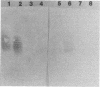
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Brade H. A 28,000-dalton protein of normal mouse serum binds specifically to the inner core region of bacterial lipopolysaccharide. Infect Immun. 1985 Dec;50(3):687–694. doi: 10.1128/iai.50.3.687-694.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Braude A. I. Endotoxic immunity. Adv Intern Med. 1980;26:427–445. [PubMed] [Google Scholar]
- Davis C. E., Ziegler E. J., Arnold K. F. Neutralization of meningococcal endotoxin by antibody to core glycolipid. J Exp Med. 1978 Apr 1;147(4):1007–1017. doi: 10.1084/jem.147.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. L., Ferguson R. M. Immunotherapy of gram-negative bacterial sepsis: enhanced survival in a guinea pig model by use of rabbit antiserum to Escherichia coli J5. Surgery. 1982 Aug;92(2):212–219. [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., DuBuy J. B., Woodward C. L. Experimental gram-negative bacterial sepsis: reevaluation of the ability of rough mutant antisera to protect mice. Proc Soc Exp Biol Med. 1978 Jul;158(3):482–490. doi: 10.3181/00379727-158-40231. [DOI] [PubMed] [Google Scholar]
- Johns M. A., Bruins S. C., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Serological response to lipid A and the lipopolysaccharide of Re mutants. Infect Immun. 1977 Jul;17(1):9–15. doi: 10.1128/iai.17.1.9-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M., Skehill A., McCabe W. R. Immunization with rough mutants of Salmonella minnesota. IV. Protection by antisera to O and rough antigens against endotoxin. J Infect Dis. 1983 Jan;147(1):57–67. doi: 10.1093/infdis/147.1.57. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Rietschel E. T. Endotoxins of Gram-negative bacteria. Pharmacol Ther. 1981;15(3):383–402. doi: 10.1016/0163-7258(81)90051-6. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Menkes E. Type-specific vs. cross-protective vaccination for gram-negative bacterial pneumonia. J Infect Dis. 1981 Dec;144(6):599–603. doi: 10.1093/infdis/144.6.599. [DOI] [PubMed] [Google Scholar]
- Sakulramrung R., Domingue G. J. Antigenic and immunogenic characteristics of subcellular fractions and whole cells of a rough E. coli 0111 (J5) mutant. Immunobiology. 1985 May;169(4):372–388. doi: 10.1016/S0171-2985(85)80018-8. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Kania S. A., Warren H. S. Cross-reactivity of rabbit antibodies to lipopolysaccharides of Escherichia coli J5 and other gram-negative bacteria. J Infect Dis. 1985 Nov;152(5):954–964. doi: 10.1093/infdis/152.5.954. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann M., Hahn H. Antiserum against Escherichia coli J5: a re-evaluation of its in vitro and in vivo activity against heterologous gram-negative bacteria. Infection. 1985 May-Jun;13(3):140–145. doi: 10.1007/BF01642875. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Novitsky T. J., Ketchum P. A., Roslansky P. F., Kania S., Siber G. R. Neutralization of bacterial lipopolysaccharides by human plasma. J Clin Microbiol. 1985 Oct;22(4):590–595. doi: 10.1128/jcm.22.4.590-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]