Abstract
Monoclonal antibodies against 12 of the 17 IATS serotype strains of Pseudomonas aeruginosa were produced. Eighty-seven hybridoma clones were isolated, and the antibodies secreted were found to be reactive with both Formalin-fixed whole cells and purified lipopolysaccharide of homologous strains in enzyme-linked immunosorbent assays. Among these monoclonal antibodies, the predominant antibody class was immunoglobulin M (IgM) (76%), although antibodies of the IgG2a and IgG3 isotypes were also produced. The monoclonal antibodies could further be divided into two groups based on their ability to agglutinate whole cells of homologous strains. The agglutinating monoclonal antibodies were found to immunoblot with the O side chains of homologous lipopolysaccharide, while the nonagglutinating monoclonal antibodies were found to be reactive with outer membrane protein-associated lipopolysaccharide. The applicability of monoclonal antibodies for serotyping was examined, and several antibodies were found to agglutinate whole cells and immunoblot with the O antigen of corresponding serotypes of clinical isolates from cystic fibrosis patients. In conclusion, a set of monoclonal antibodies against the IATS serotype strains of P. aeruginosa have been produced. These antibodies represent a bank of invaluable immunological reagents which may have application in serotyping, epitope mapping, lipopolysaccharide structural determination, and studies of protection against P. aeruginosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe C., Tanamoto K. I., Homma J. Y. Infection protective property of the common antigen (OEP) of Pseudomonas aeruginosa and its chemical composition. Jpn J Exp Med. 1977 Oct;47(5):393–402. [PubMed] [Google Scholar]
- Barclay G. R., Yap P. L., McClelland D. B., Jones R. J., Roe E. A., McCann M. C., Micklem L. R., James K. Characterisation of mouse monoclonal antibodies produced by immunisation with a single serotype component of a polyvalent Pseudomonas aeruginosa vaccine. J Med Microbiol. 1986 Feb;21(1):87–90. doi: 10.1099/00222615-21-1-87. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Gidney M. A., Perry M. B., Duncan J. R., Cherwonogrodzky J. W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect Immun. 1984 Nov;46(2):389–393. doi: 10.1128/iai.46.2.389-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin N. I., Lindberg A. A. Monoclonal antibodies specific for Shigella flexneri lipopolysaccharides: clones binding to type I and type III:6,7,8 antigens, group 6 antigen, and a core epitope. Infect Immun. 1986 Jul;53(1):103–109. doi: 10.1128/iai.53.1.103-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R., Lam J. S., Lam K., Costerton J. W. Influence of culture conditions on expression of the mucoid mode of growth of Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jan;19(1):8–16. doi: 10.1128/jcm.19.1.8-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect Immun. 1984 Mar;43(3):795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Holme T. Immunological characterization of Vibrio cholerae O:1 lipopolysaccharide, O-side chain, and core with monoclonal antibodies. Infect Immun. 1985 Aug;49(2):275–280. doi: 10.1128/iai.49.2.275-280.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
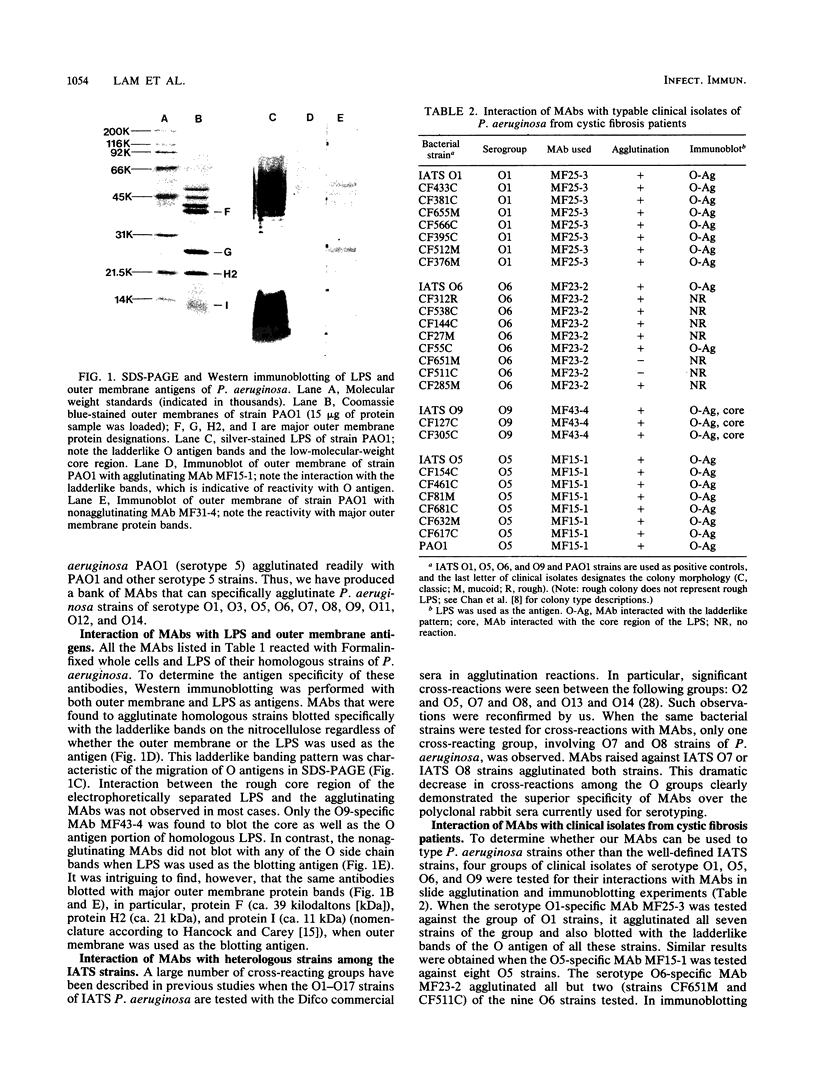
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wieczorek A. A., Mutharia L. M., Poole K. Monoclonal antibodies against Pseudomonas aeruginosa outer membrane antigens: isolation and characterization. Infect Immun. 1982 Jul;37(1):166–171. doi: 10.1128/iai.37.1.166-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly J. R., Chen Y. Y., Ramsay D. Serogrouping and subtyping of Legionella pneumophila with monoclonal antibodies. J Clin Microbiol. 1983 Nov;18(5):1040–1046. doi: 10.1128/jcm.18.5.1040-1046.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B. M., Cross A. S., Futrovsky S. L., Sidberry H. F., Sadoff J. C. Monoclonal antibodies reactive with K1-encapsulated Escherichia coli lipopolysaccharide are opsonic and protect mice against lethal challenge. Infect Immun. 1986 May;52(2):617–619. doi: 10.1128/iai.52.2.617-619.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Knirel Y. A., Vinogradov E. V., Shashkov A. S., Dmitriev B. A., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic antigens of Pseudomonas aeruginosa. The structure of the O-specific polysaccharide chains of Ps.aeruginosa O:2 (Lanyi) lipopolysaccharides. Eur J Biochem. 1982 Jun 15;125(1):221–227. doi: 10.1111/j.1432-1033.1982.tb06672.x. [DOI] [PubMed] [Google Scholar]
- Kusama H. Serological classification of Pseudomonas aeruginosa by a slide agglutination test. J Clin Microbiol. 1978 Aug;8(2):181–188. doi: 10.1128/jcm.8.2.181-188.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., Mutharia L. M., Hancock R. E., Høiby N., Lam K., Baek L., Costerton J. W. Immunogenicity of Pseudomonas aeruginosa outer membrane antigens examined by crossed immunoelectrophoresis. Infect Immun. 1983 Oct;42(1):88–98. doi: 10.1128/iai.42.1.88-98.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. D. A short-duration polyethylene glycol fusion technique for increasing production of monoclonal antibody-secreting hybridomas. J Immunol Methods. 1985 Aug 2;81(2):223–228. doi: 10.1016/0022-1759(85)90207-8. [DOI] [PubMed] [Google Scholar]
- MacIntyre S., Lucken R., Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Apr;52(1):76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre S., McVeigh T., Owen P. Immunochemical and biochemical analysis of the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Feb;51(2):675–686. doi: 10.1128/iai.51.2.675-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer H., Schmidt G. Chemistry and biology of the enterobacterial common antigen (ECA). Curr Top Microbiol Immunol. 1979;85:99–153. doi: 10.1007/978-3-642-67322-1_3. [DOI] [PubMed] [Google Scholar]
- Meadow P. M., Rowe P. S., Wells P. L. Characterization of polyagglutinating and surface antigens in Pseudomonas aeruginosa. J Gen Microbiol. 1984 Mar;130(3):631–644. doi: 10.1099/00221287-130-3-631. [DOI] [PubMed] [Google Scholar]
- Moll A., Kusecek B., Pluschke G., Morelli G., Kamke M., Jann B., Jann K., Achtman M. A reexamination of the O1 lipopolysaccharide antigen group of Escherichia coli. Infect Immun. 1986 Aug;53(2):257–263. doi: 10.1128/iai.53.2.257-263.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Surface localization of Pseudomonas aeruginosa outer membrane porin protein F by using monoclonal antibodies. Infect Immun. 1983 Dec;42(3):1027–1033. doi: 10.1128/iai.42.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Parr T. R., Jr, Poole K., Crockford G. W., Hancock R. E. Lipopolysaccharide-free Escherichia coli OmpF and Pseudomonas aeruginosa protein P porins are functionally active in lipid bilayer membranes. J Bacteriol. 1986 Feb;165(2):523–526. doi: 10.1128/jb.165.2.523-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh A., Pitt T., Roberts D., Hodson M. E., Batten J. C. The relationship of phenotype changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am Rev Respir Dis. 1983 May;127(5):605–608. doi: 10.1164/arrd.1983.127.5.605. [DOI] [PubMed] [Google Scholar]
- Peters H., Jürs M., Jann B., Jann K., Timmis K. N., Bitter-Suermann D. Monoclonal antibodies to enterobacterial common antigen and to Escherichia coli lipopolysaccharide outer core: demonstration of an antigenic determinant shared by enterobacterial common antigen and E. coli K5 capsular polysaccharide. Infect Immun. 1985 Nov;50(2):459–466. doi: 10.1128/iai.50.2.459-466.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt T. L. A comparison of flagellar typing and phage typing as means of subdividing the O groups of Pseudomonas aeruginosa. J Med Microbiol. 1981 Aug;14(3):261–270. doi: 10.1099/00222615-14-3-261. [DOI] [PubMed] [Google Scholar]
- Pitt T. L., MacDougall J., Penketh A. R., Cooke E. M. Polyagglutinating and non-typable strains of Pseudomonas aeruginosa in cystic fibrosis. J Med Microbiol. 1986 Mar;21(2):179–186. doi: 10.1099/00222615-21-2-179. [DOI] [PubMed] [Google Scholar]
- Pluschke G., Moll A., Kusecek B., Achtman M. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and monoclonal antibodies as tools for the subgrouping of Escherichia coli lipopolysaccharide O18 and O23 antigens. Infect Immun. 1986 Jan;51(1):286–293. doi: 10.1128/iai.51.1.286-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J. C., Wright D. C., Futrovsky S., Sidberry H., Collins H., Kaufmann B. Characterization of mouse monoclonal antibodies directed against Pseudomonas aeruginosa lipopolysaccharides. Antibiot Chemother (1971) 1985;36:134–146. doi: 10.1159/000410478. [DOI] [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y., Tomibe K. A new common polysaccharide antigen of strains of Pseudomonas aeruginosa detected with a monoclonal antibody. J Infect Dis. 1985 Dec;152(6):1290–1299. doi: 10.1093/infdis/152.6.1290. [DOI] [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y., Tomibe K. Characterization of a human monoclonal antibody to lipopolysaccharides of Pseudomonas aeruginosa serotype 5: a possible candidate as an immunotherapeutic agent for infections with P. aeruginosa. J Infect Dis. 1985 Nov;152(5):965–970. doi: 10.1093/infdis/152.5.965. [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki M., Kawamura T., Fujinaga S., Masuho Y., Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J Infect Dis. 1984 Oct;150(4):570–576. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Samra Z. An antigen common to a wide range of bacteria. I. The isolation of a 'common antigen' from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):143–149. doi: 10.1111/j.1699-0463.1980.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Stoll B. J., Pollack M., Young L. S., Koles N., Gascon R., Pier G. B. Functionally active monoclonal antibody that recognizes an epitope on the O side chain of Pseudomonas aeruginosa immunotype-1 lipopolysaccharide. Infect Immun. 1986 Sep;53(3):656–662. doi: 10.1128/iai.53.3.656-662.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawara R. J., Prato C., Sippel J. E. Monoclonal antibodies against Neisseria meningitidis lipopolysaccharide. Infect Immun. 1983 Dec;42(3):863–868. doi: 10.1128/iai.42.3.863-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]