Abstract
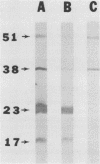
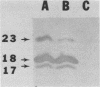
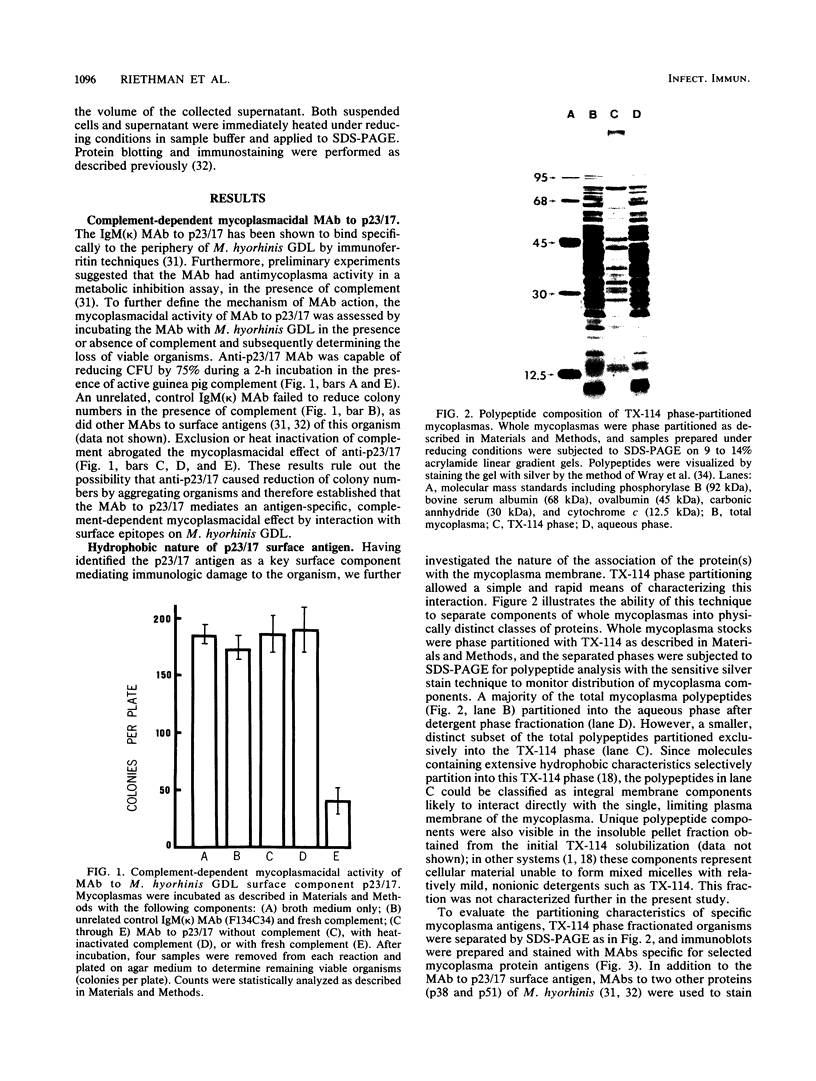
A previously defined immunoglobulin M(kappa) monoclonal antibody reacting with a surface epitope of Mycoplasma hyorhinis is shown in this report to mediate specific, complement-dependent mycoplasmacidal activity. Immunoblot analysis of mycoplasma components and their tryptic cleavage products showed that the epitope recognized was present on a protein with an apparent molecular weight of 23,000 (p23) and on a limit tryptic fragment of this protein with an apparent molecular weight of 18,000 (p18). Both p23 and p18 are shown by Triton X-114 phase fractionation to partition efficiently into the hydrophobic detergent phase. Other antigens bearing epitopes not expressed at the cell surface were present among the numerous hydrophilic proteins found in the aqueous phase. The external orientation and membrane association of the p23 antigen were further established by demonstrating that trypsin treatment of intact mycoplasmas generated the antigenic p18 fragment, which remained tightly associated with the organism. These results localize an epitope responsible for antibody-mediated mycoplasma killing onto a specific, surface-exposed region of an integral membrane protein of this organism. Since the monoclonal antibody used in this study does not bind to the surface of all strains of M. hyorhinis, the epitope identified also defines a structural marker of antigenic surface variation within this species, a feature previously observed during serological classification of the organism. Analysis of the antigenic and structural features of the p23 surface antigen may therefore be useful in establishing mechanisms of surface antigen variation among integral membrane proteins of mycoplasmas that could dictate important antigenic characteristics recognized during chronic disease caused by these agents.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER M., LEACH R. H. A MYCOPLASMA WHICH INDUCES ACIDITY AND CYTOPATHIC EFFECT IN TISSUE CULTURE. J Gen Microbiol. 1964 Feb;34:285–294. doi: 10.1099/00221287-34-2-285. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Proteolipid formation in Mycoplasma capricolum. Influence of cholesterol on unsaturated fatty acid acylation of membrane proteins. J Biol Chem. 1983 Oct 10;258(19):11814–11818. [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S. Phospholipids as acyl donors to membrane proteins of Mycoplasma capricolum. J Biol Chem. 1984 Sep 10;259(17):10771–10776. [PubMed] [Google Scholar]
- Dahl C. E., Sacktor N. C., Dahl J. S. Acylated proteins in Acholeplasma laidlawii. J Bacteriol. 1985 Apr;162(1):445–447. doi: 10.1128/jb.162.1.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Cassell G. H., Minion F. C., Wise K. S. Mycoplasma host-cell interactions resulting in chronic inflammation: acquisition of host antigens and other mechanisms. Isr J Med Sci. 1981 Jul;17(7):633–636. [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Gois M., Kuksa F., Franz J., Taylor-Robinson D. The antigenic differentiation of seven strains of Mycoplasma hyorhinis by growth-inhibition, metabolism-inhibition, latex-agglutination, and polyacrylamide-gel-electrophoresis tests. J Med Microbiol. 1974 Feb;7(1):105–115. doi: 10.1099/00222615-7-1-105. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. Monoclonal antibodies reacting with immunogenic mycoplasma proteins present in human hematopoietic cell lines. J Immunol. 1982 Dec;129(6):2734–2738. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nyström S., Johansson K. E., Wieslander A. Selective acylation of membrane proteins in Acholeplasma laidlawii. Eur J Biochem. 1986 Apr 1;156(1):85–94. doi: 10.1111/j.1432-1033.1986.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Proteolipids. Annu Rev Biochem. 1981;50:193–206. doi: 10.1146/annurev.bi.50.070181.001205. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F. Fatty acid binding: a new kind of posttranslational modification of membrane proteins. Curr Top Microbiol Immunol. 1983;102:101–129. doi: 10.1007/978-3-642-68906-2_3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Ramsay J. R., Roberts L. K. Characterization of the metabolism inhibition antigen of Mycoplasma arthritidis. Infect Immun. 1985 Aug;49(2):357–364. doi: 10.1128/iai.49.2.357-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Cassell G. H., Action R. T. Selective association of murine T lymphoblastoid cell surface alloantigens with Mycoplasma hyorhinis. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4479–4483. doi: 10.1073/pnas.75.9.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Minion F. C., Cheung H. C. Translocation of Thy-1 antigen and a fluorescent lipid probe during lymphoblastoid cell interaction with Mycoplasma hyorhinis. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S210–S218. doi: 10.1093/clinids/4.supplement_1.s210. [DOI] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Antigenic mimicry of mammalian intermediate filaments by mycoplasmas. Infect Immun. 1985 May;48(2):587–591. doi: 10.1128/iai.48.2.587-591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Monoclonal antibodies to Mycoplasma hyorhinis surface antigens: tools for analyzing mycoplasma-lymphoid cell interactions. Yale J Biol Med. 1983 Sep-Dec;56(5-6):623–629. [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983 Sep;41(3):1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]