Abstract
The Dahl salt-sensitive rat is a widely used model of human salt-sensitive forms of hypertension. The kidney plays an important role in the pathogenesis of Dahl salt-sensitive hypertension, but the molecular mechanisms involved remain a subject of intensive investigation. Gene expression profiling studies suggested that 11β-hydroxysteroid dehydrogenase type 1 might be dysregulated in the renal medulla of Dahl salt-sensitive rats. Additional analysis confirmed that renal medullary expression of 11β-hydroxysteroid dehydrogenase type 1 was downregulated by a high-salt diet in SS-13BN rats, a consomic rat strain with reduced blood pressure salt sensitivity, but not in Dahl salt-sensitive rats. 11β-Hydroxysteroid dehydrogenase type 1 is known to convert inactive 11-dehydrocorticosterone to active corticosterone. The urinary corticosterone/11-dehydrocorticosterone ratio as well as urinary excretion of corticosterone was higher in Dahl salt-sensitive rats than in SS-13BN rats. Knockdown of renal medullary 11β-hydroxysteroid dehydrogenase type 1 with small-interfering RNA attenuated the early phase of salt-induced hypertension in Dahl salt-sensitive rats and reduced urinary excretion of corticosterone. Knockdown of 11β-hydroxysteroid dehydrogenase type 1 did not affect blood pressure in SS-13BN rats. Long-term attenuation of salt-induced hypertension was achieved with small hairpin RNA targeting renal medullary 11β-hydroxysteroid dehydrogenase type 1. In summary, we have demonstrated that suppression of 11β-hydroxysteroid dehydrogenase type 1 expression in the renal medulla attenuates salt-induced hypertension in Dahl salt-sensitive rats.
Keywords: blood pressure, glucocorticoids, gene expression, gene therapy, RNA interference
an abnormally increased blood pressure-salt sensitivity is a common feature of essential hypertension, especially in African American patients (38). It is of substantial clinical and scientific interest to understand the mechanisms underlying the increase in blood pressure salt sensitivity. Much of what we know about salt-sensitive hypertension has been derived from studies of the Dahl salt-sensitive (SS) rat (3, 5, 32). Classic experiments have demonstrated that abnormalities in the kidneys may play a critical role in the development of Dahl SS hypertension (9, 11, 34, 45). Within the kidney, the medullary region has been shown to be important for long-term blood pressure regulation (4, 7). The molecular mechanisms involved in the development of salt-induced hypertension in the SS rat, however, appear to be complex (5, 33, 37) and remain poorly understood.
Programmatic efforts have been undertaken to generate consomic and congenic variants of the SS rat that may facilitate the study of the molecular pathophysiology of salt-sensitive hypertension (5, 6, 10). These efforts are important since the rat strains commonly used as controls for SS in physiological studies may contain many molecular differences from the SS rat that are unrelated to the hypertension phenotype. In one of the consomic strains generated, SS-13BN, chromosome 13 is the only chromosome that is of the Brown Norway origin, making SS-13BN genetically 98% identical to SS. Yet the blood pressure salt sensitivity is substantially reduced in SS-13BN (10). SS-13BN has become a valuable control strain for the study of the SS model (5, 6, 10, 13, 42).
We previously used cDNA microarrays to examine renal medullary gene expression profiles in SS and SS-13BN rats on diets containing 0.4 or 4% NaCl (22, 24, 25). It was found that following 3 days of dietary high-salt exposure, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) mRNA expression was decreased in the renal medulla of SS-13BN rats to ∼35% of the level seen on the 0.4% NaCl diet but did not change significantly in SS rats (22, 24). 11β-HSD1 converts inactive glucocorticoids (11-dehydrocorticosterone in rodents and cortisone in human) to active glucocorticoids (corticosterone in rodents and cortisol in human) (46). Experimental overexpression of 11β-HSD1 in the adipose tissue or the liver in mice led to the development of hypertension (28, 31).
A critical question that has not been addressed is whether abnormalities of endogenous 11β-HSD1 contribute functionally to any common forms of hypertension. The hypothesis of the present study was that the inability of the SS rat to suppress the renal medullary level of 11β-HSD1 expression contributed to salt-induced hypertension. We used a number of new approaches, including gene knockdown locally in the renal medulla, to test this hypothesis.
MATERIALS AND METHODS
(An expanded methods section is available in the online supplement.)1
Animals.
Male SS and consomic SS-13BN rats were generated as described previously (10, 25, 42). Rats were maintained on a purified AIN-76A rodent diet (Dyets, Bethlehem, PA) containing 0.4% NaCl and had free access to water. Male Sprague-Dawley rats were from Harlan (Indianapolis, IN). The animal protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Liquid chromatography-tandem mass spectrometry.
Liquid chromatography-tandem mass spectrometry analysis of corticosterone and 11-dehydrocorticosterone was performed using a reversed-phase analytical column and an API 4000 Q-trap tandem mass spectrometer as described previously (47). In some experiments, corticosterone was measured with enzyme immunoassay.
RNA extraction and real-time PCR.
Renal regional tissues were collected and RNA was extracted as described previously (24, 25, 43). Real-time PCR analysis using the Taqman chemistry was performed as described previously (21, 23, 27). 18S rRNA was used as an internal normalizer.
Small interfering RNA.
Small interfering RNA (siRNA) was designed and synthesized similar to that described previously (23, 26, 27). The rat 11β-HSD1 target sequence was 5′-aagcaaucagagguugggucauu-3′. Control siRNA targeting luciferase was from Dharmacon (Lafayette, CO).
Acute renal medullary interstitial injection.
The left kidney was exposed through a flank incision. Approximately 200 μl of siRNA preparation (20 μg) were injected over 1 min through a PE-10 tubing inserted into the renal medulla. The tubing was removed after injection. In some experiments, siRNA was combined with Optison microbubbles (1:1), and ultrasound (2.2 W/cm2, 1 MHz, and 20% duty cycle) (41) was applied on the kidney surface for 5 min after the injection. In other experiments, siRNA was combined with 12 μl of Oligofectamine (Invitrogen, Carlsbad, CA).
Acute intraureter injection.
The left ureter was exposed through a midline incision. Urine flow was temporarily stopped. siRNA was injected into the ureter to allow retrograde diffusion into the kidney. Oligofectamine was used in some experiments.
Small hairpin RNA plasmid.
Small hairpin RNA (shRNA) targeting rat 11β-HSD1 and a control shRNA containing two nucleotide substitutions were designed, cloned, and prepared as described previously (27). The 11β-HSD1 target sequence was 5′-aauaccacaugggcucccauu-3′. The two underlined nucleotides were swapped for the control shRNA.
Renal medullary interstitial injection of shRNA with polyethylenimine.
The rats were uninephrectomized, and a catheter was implanted in the renal medulla as described previously (29, 42, 47) ∼2 wk prior to the injection. shRNA plasmids (100 μg) were combined with in vivo-jetPEI (PolyPlus, New York, NY) at a ratio of 8 following the manufacturer's instructions and injected into the catheter over 1 min. In vivo-jetPEI is a polyethylenimine designed for in vivo transfection (1, 12).
Chronic measurement of arterial blood pressure.
Chronic monitoring of arterial blood pressure in freely moving rats using indwelling femoral arterial catheters was performed as described previously (29, 42, 47).
Statistical analysis.
Data were analyzed by Student's t-test, paired t-test, or analysis of variance followed by Holm-Sidak test. P < 0.05 was considered significant. Data shown are means ± SE.
RESULTS
Renal medullary expression of 11β-HSD1 in SS and SS-13BN rats exhibited distinct responses to a high-salt diet.
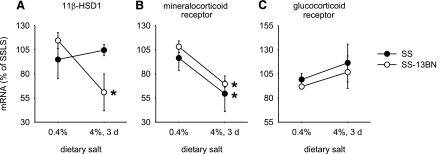
As shown in Fig. 1, Taqman real-time PCR analysis indicated a significant reduction, ∼45%, in the level of renal medullary 11β-HSD1 mRNA in SS-13BN rats in response to an increase in dietary salt intake, which was not observed in SS rats. The data confirmed the finding of previous cDNA microarray and Northern blot analyses (24). Renal cortical expression of 11β-HSD1 in SS-13BN rats did not change significantly.
Fig. 1.
Expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in the renal medulla was downregulated in SS-13BN rats by an increase in dietary salt intake but not in SS rats. Rats were maintained on the 0.4% NaCl diet or switched to the 4% NaCl diet for 3 days. A: renal medullary levels of 11β-HSD1 mRNA. *P < 0.05 vs. SS-13BN, 0.4%; n = 4. B: renal medullary levels of mineralocorticoid receptor mRNA were downregulated by the 4% NaCl diet in both SS and SS-13BN rats. *P < 0.05 vs. 0.4%; n = 4. C: renal medullary levels of glucocorticoid receptor mRNA; n = 4; SS-13BN, consomic rat strain with reduced blood pressure salt sensitivity; SS, Dahl salt-sensitive rat; d, day.
Corticosterone may act through stimulation of glucocorticoid or mineralocorticoid receptors. Renal medullary levels of mineralocorticoid receptor mRNA decreased significantly upon exposure to the high-salt intake (Fig. 1). The decrease of mineralocorticoid receptor mRNA levels occurred in both SS and SS-13BN. Glucocorticoid receptor mRNA levels remained relatively stable in both strains of rats.
The urinary level of active glucocorticoids was higher in SS than in SS-13BN.
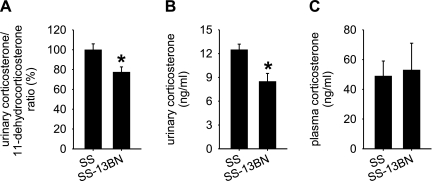
The higher expression level of 11β-HSD1 suggested that the kidneys of SS rats might contain increased levels of corticosterone. To test the possibility, we collected 24 h urine from SS and SS-13BN rats that had been exposed to the high-salt diet for 3 days. As shown in Fig. 2A, the corticosterone/11-dehydrocorticosterone ratio in the urine was significantly higher in SS rats compared with SS-13BN rats. The average ratio in SS rats was 1.5. Moreover, urinary concentrations of corticosterone (Fig. 2B) or 24 h urinary excretion of corticosterone were significantly higher in SS rats. The 24 h urine volume was not significantly different between the two rat strains [48 ± 5 ml/24 h in SS, 46 ± 4 ml/24 in SS-13BN, n = 5, not significant (NS)]. The difference in urinary excretion of corticosterone appeared to have originated locally from the kidneys because plasma concentrations of corticosterone were similar in SS and SS-13BN rats (Fig. 2C). Urinary volume or concentration or excretion of corticosterone was not significantly different between SS and SS-13BN on the 0.4% NaCl diet (114 ± 13 ng/24 h in SS, 95 ± 8 ng/24 h in SS-13BN, n = 5, NS).
Fig. 2.
The urinary corticosterone/11-dehydrocorticosterone ratio and corticosterone concentration were higher in SS rats than in SS-13BN rats. Rats had been on the 4% NaCl diet for 3 days. A: the urinary corticosterone/11-dehydrocorticosterone ratio was significantly higher in SS rats than in SS-13BN rats. *P < 0.05 vs. SS; n = 7. B: corticosterone concentration in 24 h urine was significantly higher in SS rats than in SS-13BN rats. *P < 0.05 vs. SS; n = 7. C: plasma levels of corticosterone were not significantly different between SS and SS-13BN rats; n = 5.
siRNA treatment significantly decreased the level of renal medullary 11β-HSD1 expression.
Pharmacological inhibitors specific for rat 11β-HSD1 are not available. We explored the possibility of using siRNA to reduce the expression level of 11β-HSD1 in the rat renal medulla in vivo.
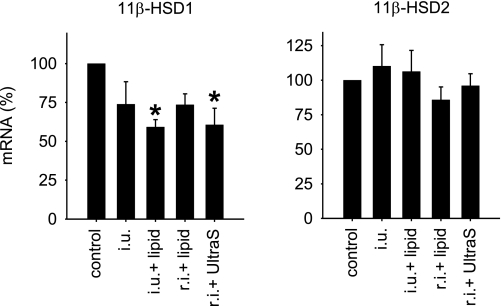
We examined several ways of delivering 11β-HSD1 siRNA into the renal medulla in Sprague-Dawley rats. At 3 days after injection, rat kidneys receiving 20 μg siRNA targeting 11β-HSD1 delivered through a medullary interstitial injection combined with the ultrasound/microbubble treatment or through an intraureter injection combined with Oligofectamine exhibited a statistically significant reduction of ∼45% in 11β-HSD1 mRNA expression in the renal medulla (Fig. 3). The effect was confined to the renal medulla, as 11β-HSD1 mRNA levels in the renal cortex were not significantly altered. 11β-HSD1 mRNA levels in the contralateral, uninfused kidneys were not affected either. Expression of 11β-HSD2, the isoform that inactivates glucocorticoids, was not affected by siRNA targeting 11β-HSD1 (Fig. 3).
Fig. 3.
Methods for delivering 11β-HSD1 siRNA to the renal medulla. 11β-HSD1 siRNA was delivered into the kidney by the indicated methods. Renal medullary levels of 11β-HSD1 (left) and 11β-HSD2 mRNA (right) were measured 3 days after siRNA treatment. The mRNA levels shown are relative to corresponding controls. *P < 0.05 vs. control; n = 3–4. i.u., Intraureter injection; lipid, Oligofectamine; r.i., renal medullary interstitial injection; UltraS, ultrasound/microbubble treatment.
Rats receiving the siRNA delivered through an interstitial injection combined with Oligofectamine or through an intraureter injection of the siRNA alone exhibited ∼25% reduction in 11β-HSD1 mRNA expression in the renal medulla (Fig. 3), but the reduction did not reach statistical significance. Renal interstitial injection of the siRNA alone did not result in appreciable reduction.
siRNA targeting renal medullary 11β-HSD1 attenuated the early phase of salt-induced hypertension in SS rats.
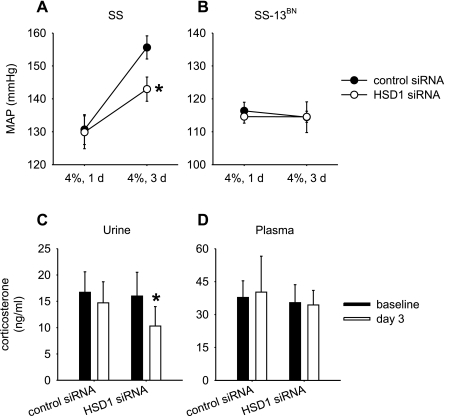
The siRNA targeting 11β-HSD1 or a control siRNA was delivered into the remaining kidney of uninephrectomized SS or SS-13BN rats through renal interstitial injection combined with the ultrasound/microbubble treatment. Uninephrectomy and arterial catheterization were performed on day 0, siRNA injection on day 10, the dietary content of NaCl increased from 0.4 to 4% on day 13, and mean arterial blood pressure (MAP) recorded on days 1, 3, and 5 after the dietary change.
As shown in Fig. 4A, SS rats treated with 11β-HSD1 siRNA and the control siRNA had similar levels of MAP of ∼130 mmHg on day 1 following the switch to the 4% NaCl diet. On day 3 of the 4% NaCl diet, the MAP of SS rats treated with the control siRNA was increased by 25 mmHg to 156 ± 4 mmHg. In contrast, the increase of MAP was significantly attenuated in SS rats treated with 11β-HSD1 siRNA, reaching only 143 ± 4 mmHg (P < 0.05 vs. control siRNA). On day 5 of the 4% NaCl diet, however, MAP was increased to similar levels in SS rats treated with either control (159 ± 5 mmHg) or 11β-HSD1 siRNA (153 ± 7 mmHg, NS vs. control siRNA). The same siRNA treatment did not have any significant effects on the blood pressure of SS-13BN rats (Fig. 4B).
Fig. 4.
Administration of 11β-HSD1 siRNA in the renal medulla attenuated salt-induced hypertension in SS rats, but not in SS-13BN rats. 11β-HSD1 siRNA or a control siRNA targeting luciferase was combined with microbubbles and injected into uninephrectomized rats through an acutely implanted renal medullary interstitial catheter. Ultrasound treatment was applied on the kidney surface. A: salt-induced increases of mean arterial blood pressure (MAP) were significantly attenuated by 11β-HSD1 siRNA in SS rats. Rats were switched to the 4% NaCl diet 3 days after the siRNA injection and MAP recorded. *P < 0.05 vs. control shRNA; n = 7–9. B: 11β-HSD1 siRNA did not significantly affect MAP in SS-13BN rats; n = 5–6. C: 11β-HSD1 siRNA significantly reduced urinary concentration of corticosterone in SS rats. Corticosterone was measured prior to (baseline) and 3 days after the injection of siRNA (day 3). *P < 0.05; n = 5. D: 11β-HSD1 siRNA did not affect plasma levels of corticosterone; n = 5.
The effect of 11β-HSD1 siRNA on blood pressure was accompanied by a reduction of the renal level of corticosterone. As shown in Fig. 4C, SS rats treated with 11β-HSD1 siRNA exhibited a statistically significant decrease in the urinary concentration of corticosterone. The decrease resembled the difference observed between SS and SS-13BN rats (Fig. 2). No significant changes in corticosterone levels were seen in the urine of rats treated with control siRNA or in the plasma of either treatment group (Fig. 4, C and D). Urine flow rates on the 4% NaCl diet were not significantly affected by the siRNA treatment (47 ± 3 ml/24 h for the control siRNA group, 46 ± 4 ml/24 h for the 11β-HSD1 siRNA group, n = 5, NS).
shRNA targeting renal medullary 11β-HSD1 led to a long-term attenuation of salt-induced hypertension in SS rats.
The effect of siRNA on salt-induced hypertension in SS rats was restricted to the early phase. We suspected that the effect was transient because chemically synthesized siRNA had a short lifetime in the tissue. We examined the effect of targeting 11β-HSD1 with a shRNA plasmid that might have a longer lifetime than siRNA. In vitro studies showed that the 11β-HSD1 shRNA plasmid, but not the control shRNA with two nucleotide substitutions, was highly effective in silencing 11β-HSD1 expression in cultured cells (27), supporting the efficiency of the 11β-HSD1 shRNA.
shRNA (100 μg) was combined with in vivo-jetPEI and injected into the renal medulla through a chronically implanted catheter. Uninephrectomy was performed on day 0, arterial and renal medullary interstitial catheters implanted on day 10, daily recording of MAP started on day 17, shRNA injected and the dietary NaCl content increased from 0.4% to 4% on day 21, and MAP recorded daily for another 11 days.
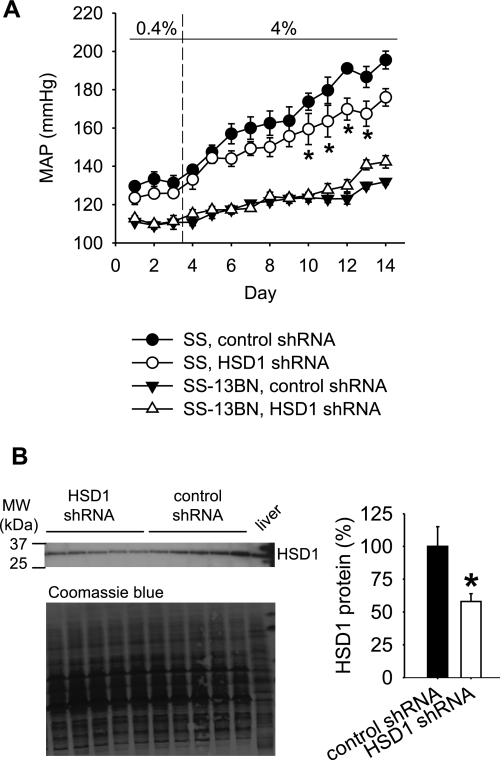
As shown in Fig. 5A, shRNA targeting 11β-HSD1 resulted in a statistically significant, long-term amelioration of salt-induced hypertension in SS rats. MAP began to diverge between the two groups of rats 3 days after the injection of shRNA and the start of the 4% NaCl diet. MAP was significantly lower in the group receiving 11β-HSD1 shRNA 7 days after the start of the 4% NaCl diet (144 ± 4 mmHg vs. 157 ± 5 mmHg in the group receiving the control shRNA). The difference in MAP remained significant for several more days and reached ∼20 mmHg. 11β-HSD1 shRNA did not lower MAP in SS-13BN rats (Fig. 5A). Curiously, there was a slight increase of MAP in SS-13BN treated with 11β-HSD1 shRNA toward the end of the recording period.
Fig. 5.
Long-term attenuation of salt-induced hypertension in SS rats by 11β-HSD1 shRNA administered in the renal medulla. 11β-HSD1 shRNA or a control shRNA with two nucleotide substitutions was combined with in vivo-jetPEI and injected into uninephrectomized rats through a chronically implanted renal medullary interstitial catheter just prior to changing the dietary salt content from 0.4 to 4%. A: salt-induced increases of MAP were significantly attenuated by 11β-HSD1 shRNA in SS rats. *P < 0.05 vs. control shRNA; n = 5 for control shRNA and 7 for 11β-HSD1 shRNA in SS rats, except for day 14 where n = 5 for 11β-HSD1 shRNA; n = 5 for SS-13BN rats. B: renal outer medullary levels of 11β-HSD1 protein, measured at 6 days after shRNA injection, were significantly reduced by 11β-HSD1 shRNA. A liver sample was loaded in the last lane as a positive control. *P < 0.05 vs. control shRNA; n = 5–6.
The 11β-HSD1 mRNA expression level in the outer medulla of the SS kidney receiving 11β-HSD1 shRNA was significantly reduced at the end of the experiment, which was 11 days after the shRNA injection (57 ± 8% in the 11β-HSD1 shRNA group vs. 100 ± 11% in the control shRNA group, n = 5–6, P < 0.05). It supported the longer-term efficacy of the shRNA treatment. 11β-HSD1 mRNA levels in the renal cortex did not appear to be altered (90 ± 10% in the 11β-HSD1 shRNA group vs. 100 ± 23% in the control shRNA group, NS). 11β-HSD1 levels in the outer medulla of SS-13BN rats also were not significantly altered by the shRNA (96 ± 4% in the 11β-HSD1 shRNA group vs. 100 ± 7% in the control shRNA group, NS), probably because 11β-HSD1 levels in SS-13BN were already suppressed by the high-salt diet.
An additional group of SS rats was prepared to examine the effect of 11β-HSD1 shRNA on the levels of 11β-HSD1 protein and urinary corticosterone at 6 days after the shRNA treatment. The 6-day time point was immediately prior to the detection of significant differences in MAP. As shown in Fig. 5B, the protein level of 11β-HSD1 in the outer medulla was significantly reduced by 11β-HSD1 shRNA by >40%. Corticosterone concentrations in 24 h urine samples tended to be decreased by 11β-HSD1 shRNA (6.6 ± 2.7 ng/ml in the 11β-HSD1 shRNA group vs. 11.1 ± 1.9 ng/ml in the control shRNA group, n = 5–6), although the difference did not reach statistical significance. Urine flow rates were not significantly altered by 11β-HSD1 shRNA (44 ± 6 ml/24 h in the 11β-HSD1 shRNA group, 47 ± 5 ml/24 h in the control shRNA group, NS).
DISCUSSION
The present study demonstrated that suppression of 11β-HSD1 expression in the renal medulla attenuated salt-induced hypertension in SS rats. It provided evidence for a functional role of endogenous 11β-HSD1 in the development of salt-sensitive forms of hypertension.
The importance of 11β-HSDs in the regulation of arterial blood pressure was first recognized when 11β-HSD2, which inactivates glucocorticoids, was found to be critical in protecting mineralocorticoid receptors in the distal nephron from stimulation by glucocorticoids (18, 36). Pharmacological inhibition or genetic deficiency of 11β-HSD2 leads to the development of hypertension (48). Studies in the last few years indicated that 11β-HSD1, which reactivates glucocorticoids, might also play an important role in blood pressure regulation. Overexpression of 11β-HSD1 specifically in the adipose tissue or the liver caused hypertension in mice, which might be due to activation of the angiotensin pathway (28, 31). Variants of 11β-HSD1 have been reported to be associated with the risk of hypertension in Pima Indians (17).
There was an ongoing interest in the role of 11β-HSDs in Dahl salt-sensitive hypertension. However, no conclusive functional evidence had been reported prior to the present study. Dehydrogenase, or glucocorticoid inactivating, activities of 11β-HSDs were reported to be generally lower in SS rats than in Dahl salt-resistant rats (16, 40). The urinary corticosterone/11-dehydrocorticosterone ratio, measured by a radioimmunoassay, was higher in SS rats than in Dahl salt-resistant rats (39). The mRNA levels of 11β-HSD1 and 11β-HSD2, however, were found to be similar in SS rats and Dahl salt-resistant rats (15). 11β-HSD1 is expressed in the kidney (2, 35), but the functional significance of renal 11β-HSD1 is virtually unknown.
We used consomic SS-13BN rats, instead of Dahl salt-resistant rats, as the control for SS. The comparison with SS-13BN rats allowed us to identify an inability of SS rats to suppress 11β-HSD1 in response to salt. It was consistent with the higher corticosterone/11-dehydrocorticosterone ratio in the urine. Knockdown of 11β-HSD1 specifically in the renal medulla using siRNA or shRNA provided strong evidence for the elusive functional role of 11β-HSD1 in Dahl salt-sensitive hypertension. Urinary corticosterone levels appeared to be decreased following 11β-HSD1 silencing and the high-salt treatment. It suggested, but did not prove, that 11β-HSD1 in the renal medulla of SS rats was a reductase. It would be interesting to further analyze the biochemistry of 11β-HSD1 and the related metabolism in SS and SS-13BN rats, particularly following 11β-HSD1 silencing and a high-salt challenge.
The shRNA experiment, compared with the siRNA experiment, included three technical advantages in addition to the longer-term efficacy of shRNA. First, the shRNA was designed to target a different region of 11β-HSD1 mRNA than the siRNA. Second, a shRNA containing two nucleotide substitutions was used as control. These two advantages helped to reduce any concerns about off-target effects of RNA interference (14). Third, a polymer reagent designed for in vivo transfection was used, which allowed us to inject shRNA into a chronically implanted catheter and avoid the anesthesia required for ultrasound treatment.
11β-HSD1 shRNA reduced the blood pressure of SS rats on the high-salt diet by ∼15–20 mmHg. The modesty of the effect is probably a reflection of the nature of the SS model. Dahl SS hypertension is widely considered a polygenic model (5, 33), as opposed to models of single gene diseases. The polygenic nature may be the strength of the SS model since human salt-sensitive forms of hypertension are also likely to be polygenic (33). The implication, however, is that any single molecular pathway will contribute at best to a fraction of the hypertensive phenotype.
Our general hypothesis is that the molecules and pathways involved in the pathophysiology of Dahl SS hypertension, and perhaps other polygenic, complex diseases, form a tree-like structure. Causal genes containing genetic variations resemble numerous leaves on the tree. The genetic variations interact with environmental factors and impact on various molecular pathways and intermediate mechanisms, represented by small branches on the tree. The intermediate mechanisms converge gradually to form larger branches, or shared pathways. Correcting abnormalities in an intermediate pathway, such as that involving 11β-HSD1, would, therefore, be expected to have modest effects on the overall phenotype.
Two important questions arise from the results of the present study and deserve further investigation in future studies. First, how does the apparent excess of glucocorticoids in the kidneys contribute to salt-induced hypertension in the SS rat? One possibility is that excess glucocorticoids stimulate mineralocorticoid receptors, similar to that occurs with 11β-HSD2 deficiencies. However, 11β-HSD1 protein seems to be localized in proximal tubules and interstitial cells (2, 35), rather than the distal nephron where mineralocorticoid receptors are expressed. A recent study indicated that hexose-6-phosphate dehydrogenase, which provides the NADPH needed for the reductase activity of 11β-HSD1, was not readily detectable in renal inner medullary interstitial cells (19). 11β-HSD1 located in other cells might be involved in the effect seen in the present study since knockdown of 11β-HSD1 significantly decreased (3 days after the siRNA treatment) or tended to decrease (6 days after the shRNA treatment) urinary concentrations of corticosterone.
Another possibility is that excess glucocorticoids may suppress endothelial nitric oxide synthase as we recently demonstrated (26). It is also possible that excess glucocorticoids may exacerbate oxidative stress (20). Nitric oxide deficiencies as well as oxidative stress in the renal medulla have been shown in physiological studies to contribute to Dahl SS hypertension (8, 30, 42). Yet another possibility is that excess glucocorticoids might upregulate local expression of angiotensinogen in the renal medulla, reminiscent of the observations in mice overexpressing 11β-HSD1 (28, 31). We recently found that angiotensinogen expression in the renal medulla was upregulated by a high-salt diet in SS rats but downregulated in SS-13BN rats (44).
Second, why is the SS rat unable to suppress renal medullary expression of 11β-HSD1 in response to an increase in dietary salt intake? 11β-HSD1 is located on chromosome 13, the chromosome that is substituted in SS-13BN, but we do not have genetic evidence to support the presence of different 11β-HSD1 alleles in SS and SS-13BN. It is possible that the inability of the SS rat to suppress 11β-HSD1 is the result of deficiencies in upstream genes or pathways that modulate the responsiveness of 11β-HSD1 expression. The key data point in the present study is that experimental suppression of 11β-HSD1 was sufficient to attenuate salt-induced hypertension in SS rats, mimicking the changes seen in SS-13BN rats. It does not mean that suppression of 11β-HSD1 is required for achieving salt resistance. For instance, a high-salt diet did not significantly alter renal interstitial levels of corticosterone or corticosterone/11-dehydrocorticosterone ratio in salt-insensitive Sprague-Dawley rats (47).
Genomic context may play an important role in determining the pathophysiological role of 11β-HSD1 or any other molecular pathways. A gene or pathway that is important for a phenotype in a given genomic-environmental context might not be important in another genomic-environmental context. Different genes or pathways may very well underlie the salt-resistance phenotype in different strains of animals, just as various animal models of hypertension may develop hypertension due to different mechanisms. This is consistent with the complex, polygenic nature of the blood pressure phenotype in humans. One would not expect all patients of essential hypertension to share the same genetic and molecular defects or all normotensive subjects to share the same protective mechanism.
GRANTS
The present study was supported by National Heart, Lung, and Blood Institute Grant R01 HL-077263 (M. Liang).
Supplementary Material
Address for reprint requests and other correspondence: M. Liang, Dept. of Physiology, Medical College of Wisconsin, 8701 Watertown Plank Rd., Milwaukee, WI 53226 (e-mail: mliang@mcw.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92: 7297–7301, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castello R, Schwarting R, Muller C, Hierholzer K. Immunohistochemical localization of 11-hydroxysteroid dehydrogenase in rat kidney with monoclonal antibody. Renal Physiol Biochem 12: 320–327, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Cowley AW Jr. Genetic and nongenetic determinants of salt sensitivity and blood pressure. Am J Clin Nutr 65: 587S–593S, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Cowley AW Jr. Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1–R15, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Cowley AW Jr. The genetic dissection of essential hypertension. Nat Rev Genet 7: 829–840, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Cowley AW Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand 181: 585–592, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cowley AW Jr, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension 25: 663–673, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW Jr, Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol 284: R1355–R1369, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Cowley AW Jr, Roman RJ. The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996. [PubMed] [Google Scholar]
- 10.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dahl LK, Heine M. Primary role of renal homografts in setting chronic blood pressure levels in rats. Circ Res 36: 692–696, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Demeneix B, Hassani Z, Behr JP. Towards multifunctional synthetic vectors. Curr Gene Ther 4: 445–455, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Drenjancevic-Peric I, Frisbee JC, Lombard JH. Skeletal muscle arteriolar reactivity in SS.BN13 consomic rats and Dahl salt-sensitive rats. Hypertension 41: 1012–1015, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Editorial. Whither RNAi? Nat Cell Biol 5: 489–490, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Franco-Saenz R, Shen P, Lee SJ, Cicila GT, Henrich WL. Regulation of the genes for 11beta-hydroxysteroid dehydrogenase type 1 and type 2 in the kidney of the Dahl rat. J Hypertens 17: 1089–1093, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Franco-Saenz R, Tokita Y, Latif S, Morris DJ. 11Beta-hydroxysteroid dehydrogenase in the Dahl rat. Am J Hypertens 10: 1004–1009, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Franks PW, Knowler WC, Nair S, Koska J, Lee YH, Lindsay RS, Walker BR, Looker HC, Permana PA, Tataranni PA, Hanson RL. Interaction between an 11betaHSD1 gene variant and birth era modifies the risk of hypertension in Pima Indians. Hypertension 44: 681–688, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242: 583–585, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Sanchez EP, Romero DG, de Rodriguez AF, Warden MP, Krozowski Z, Gomez-Sanchez CE. Hexose-6-phosphate dehydrogenase and 11beta-hydroxysteroid dehydrogenase-1 tissue distribution in the rat. Endocrinology 149: 525–533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, Matsumoto T. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res 92: 81–87, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Knoll KE, Pietrusz JL, Liang M. Tissue-specific transcriptome responses in rats with early streptozotocin-induced diabetes. Physiol Genomics 21: 222–229, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Liang M, Cowley AW, Greene AS. High throughput gene expression profiling: a molecular approach to integrative physiology. J Physiol 554: 22–30, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang M, Pietrusz JL. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol 27: 77–83, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Liang M, Yuan B, Rute E, Greene AS, Olivier M, Cowley AW Jr. Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics 12: 229–237, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Liang M, Yuan B, Rute E, Greene AS, Zou AP, Soares P, MCQuestion GD, Slocum GR, Jacob HJ, Cowley AW Jr. Renal medullary genes in salt-sensitive hypertension: a chromosomal substitution and cDNA microarray study. Physiol Genomics 8: 139–149, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Mladinov D, Pietrusz JL, Usa K, Liang M. Glucocorticoid response elements and 11β-hydroxysteroid dehydrogenases in the regulation of endothelial nitric oxide synthase expression. Cardiovasc Res 2008. Aug 20 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 27.Liu Y, Park F, Pietrusz JL, Jia G, Singh RJ, Netzel BC, Liang M. Suppression of 11β-hydroxysteroid dehydrogenase type 1 with RNA interference substantially attenuates 3T3–L1 adipogenesis. Physiol Genomics 32: 343–351, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Masuzaki H, Yamamoto H, Kenyon CJ, Elmquist JK, Morton NM, Paterson JM, Shinyama H, Sharp MG, Fleming S, Mullins JJ, Seckl JR, Flier JS. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest 112: 83–90, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW Jr. Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol Heart Circ Physiol 266: H1918–H1926, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Miyata N, Cowley AW Jr. Renal intramedullary infusion of l-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension 33: 446–450, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, Staels B, Seckl JR, Mullins JJ. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci USA 101: 7088–7093, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapp JP Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4: 753–763, 1982. [DOI] [PubMed] [Google Scholar]
- 33.Rapp JP Genetic analysis of inherited hypertension in the rat. Physiol Rev 80: 135–172, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Roman RJ Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol Renal Fluid Electrolyte Physiol 251: F57–F65, 1986. [DOI] [PubMed] [Google Scholar]
- 35.Rundle SE, Funder JW, Lakshmi V, Monder C. The intrarenal localization of mineralocorticoid receptors and 11 beta-dehydrogenase: immunocytochemical studies. Endocrinology 125: 1700–1704, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 2: 821–824, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Stoll M, Cowley AW Jr, Tonellato PJ, Greene AS, Kaldunski ML, Roman RJ, Dumas P, Schork NJ, Wang Z, Jacob HJ. A genomic-systems biology map for cardiovascular function. Science 294: 1723–1726, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan JM, Prewitt RL, Ratts TE. Sodium sensitivity in normotensive and borderline hypertensive humans. Am J Med Sci 295: 370–377, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Takeda Y, Inaba S, Furukawa K, Miyamori I. Renal 11beta-hydroxysteroid dehydrogenase in genetically salt-sensitive hypertensive rats. Hypertension 32: 1077–1082, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Takeda Y, Miyamori I, Yoneda T, Iki K, Hatakeyama H, Takeda R. Gene expression of 11 beta-hydroxysteroid dehydrogenase in the mesenteric arteries of genetically hypertensive rats. Hypertension 23: 577–580, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation 105: 1233–1239, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res 18: 404–411, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Z, Greene AS, Usa K, Matus IR, Bauwens J, Pietrusz JL, Cowley AW Jr, Liang M. Renal regional proteomes in young Dahl salt-sensitive rats. Hypertension 51: 899–904, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Tobian L Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension 17: I52–I58, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 25: 831–866, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Usa K, Singh RJ, Netzel BC, Liu Y, Raff H, Liang M. Renal interstitial corticosterone and 11-dehydrocorticosterone in conscious rats. Am J Physiol Renal Physiol 293: F186–F192, 2007. [DOI] [PubMed] [Google Scholar]
- 48.White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 18: 135–156, 1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.