Abstract
Endothelial nitric oxide (NO) synthase (eNOS) expression and activity are decreased in fetal lambs with persistent pulmonary hypertension (PPHN). We sought to determine the impact of mechanical ventilation with O2 with or without inhaled NO (iNO) or recombinant human SOD (rhSOD) on eNOS in the ductal ligation model of PPHN. PPHN lambs and age-matched controls were ventilated with 100% O2 for 24 h alone or combined with 20 ppm iNO continuously or a single dose of rhSOD (5 mg/kg) given intratracheally at delivery. In 1-day spontaneously breathing lambs, eNOS expression in resistance pulmonary arteries increased relative to fetal levels. eNOS expression increased in control lambs ventilated with 100% O2, but not in PPHN lambs. Addition of iNO or rhSOD increased eNOS expression and decreased generation of reactive oxygen species (ROS) in PPHN lambs relative to those ventilated with 100% O2 alone. However, only rhSOD restored eNOS function, increased tetrahydrobiopterin (BH4), a critical cofactor for eNOS function, and restored GTP cyclohydrolase I expression in isolated vessels and lungs from PPHN lambs. These data suggest that ventilation of PPHN lambs with 100% O2 increases ROS production, blunts postnatal increases in eNOS expression, and decreases available BH4 in PPHN lambs. Although the addition of iNO or rhSOD diminished ROS production and increased eNOS expression, only rhSOD improved eNOS function and levels of available BH4. Thus therapies designed to decrease oxidative stress and restore eNOS coupling, such as rhSOD, may prove useful in the treatment of PPHN in newborn infants.
Keywords: reactive oxygen species, biopterin
as part of the normal physiological transition at birth, the pulmonary vascular resistance decreases through complex pathways allowing pulmonary blood flow to increase by 10-fold. Physical and biochemical processes that contribute to the normal newborn pulmonary transition include mechanical distension of the lungs and increased Po2, which stimulate endothelial nitric oxide (NO) synthase (eNOS) (28, 34, 40). eNOS converts l-arginine to l-citrulline and NO, which in turn activates soluble guanylate cyclase in vascular smooth muscle cells to generate cGMP, ultimately leading to vasodilation (1). Emerging evidence continues to increase the understanding of the complex regulation of eNOS expression and activity, including the potential effects of O2 (12, 33).
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome occurring in 2–6 per 1,000 live births, with a significant risk of death, as well as short- and long-term morbidity (19, 44). It is caused by multiple disease processes, which lead to an abnormal transition at birth, resulting in continued elevated pulmonary vascular resistance, right- to left-sided extrapulmonary shunting of deoxygenated blood, and hypoxemia. Pathological findings include pulmonary vascular remodeling and smooth muscle hyperplasia in the absence of significant lung parenchyma pathology (15, 29), changes that may be the result of prolonged fetal stress and hypoxia. Clinical management strategies include mechanical ventilation with high levels of inspired O2 and inhaled NO (iNO). Although iNO decreases the need for extracorporeal membrane oxygenation support, it has not been proven to improve survival, and ∼50% of infants have a limited or transient response (7, 14, 32).
O2 stimulates NO production by fetal pulmonary artery (PA) endothelial cells (12), dilates the pulmonary vasculature, and increases pulmonary eNOS expression in the fetal lamb (2). Although O2 is widely used as a pulmonary vasodilator in the clinical setting of PPHN, the effects of prolonged exposure to high O2 concentrations in combination with mechanical ventilation are not well characterized. Emerging evidence in adult and neonatal disease states raises concern about the potential for oxidative stress inducing significant lung parenchymal and vascular injury (10, 21, 22, 26, 38, 41, 43). Even during normal aerobic metabolism, eukaryotic cells produce reactive oxygen species (ROS), such as superoxide and H2O2, which must be tightly regulated to prevent undesired cellular injury. Multiple cell types present in the lung, particularly during inflammation, can produce ROS, which may affect vascular tone and stimulate vascular smooth muscle cell growth (20). ROS may also directly and indirectly interact with eNOS and NO, such as superoxide combining with NO to form the potent oxidant peroxynitrite (9, 12).
SOD catalyzes the dismutation of superoxide into H2O2 and O2, serving as an antioxidant and playing an important role in vascular tone, lung function, and metabolism of NO (9, 11, 17). Our hypothesis is that ROS play a critical role in the pathophysiology of PPHN and that ROS scavengers such as SOD may represent new therapeutic agents. We recently reported that a dose of recombinant human SOD (rhSOD) at or shortly after birth improved oxygenation in lambs with PPHN created by antenatal ligation of the ductus arteriosus (23). The goal of the present studies is to further investigate the pathways involved in PPHN, particularly those relating to the effects of SOD, on eNOS expression and function. A better understanding of these pathways will advance the effort to determine the potential harm of ROS and the therapeutic value of ROS scavengers, such as SOD, in the management of PPHN.
MATERIALS AND METHODS
Fetal surgery and ventilation protocols for neonatal sheep.
The Laboratory Animal Care Committees at the State University of New York at Buffalo and Northwestern University approved this study. Pregnant ewes and newborn lambs were obtained from the Swartz family farm (Attica, NY). One-day spontaneously breathing (1DSB) lambs were healthy newborn lambs that delivered spontaneously at comparable gestation to the experimental lambs, fed normally, breathed room air, and then at ∼24 h of life were anesthetized with thiopental sodium (Pentothal) and killed by rapid exsanguination through a direct cardiac puncture. Pulmonary hypertension was established by antenatal duct ligation in lambs, as previously described (23). Ewes with twin gestations were selected for fetal ductal ligation surgery, so that the additional fetus could serve as a control. Briefly, fetal surgery was performed on anesthetized pregnant ewes at 126 days gestation: the fetal head and left upper extremity were exposed, a left thoracotomy was performed, and the ductus arteriosus was ligated. The fetal chest was closed, the fetus was returned to the uterus, and the ewe's uterine and abdominal incisions were closed.
At 135 days gestation (full term = 145 days), the pregnant ewes were anesthetized with thiopental sodium and halothane, and the fetal lambs were delivered by cesarean section to avoid unattended spontaneous deliveries. Fetal control and ligated (PPHN) lambs were anesthetized and killed as described above before their first breath. Additional lambs were delivered by cesarean section, placed under servo-controlled radiant warmers, intubated, given Infasurf (3 ml/kg; ONY, Amherst, NY), and ventilated with 100% O2 alone, 100% O2 with 20 ppm iNO, or 100% O2 with 5 mg/kg intratracheal rhSOD (1 mg = 3,850 U of activity; Savient Pharmaceuticals, Iselin, NJ). Ventilator settings (peak inspiratory pressure and rate) were adjusted to maintain arterial Pco2 between 35 and 50 mmHg. The subsequent care protocols are described in detail elsewhere (23). After 24 h of ventilation, lambs were anesthetized and killed as described above. The heart and lungs were removed en bloc, and fifth-generation PA (500 μm ID) were dissected and isolated. Tissue samples were frozen in liquid nitrogen and stored at −80°C until analysis.
Quantitative RT real-time PCR.
Frozen isolated PA tissue was ground on liquid nitrogen, and RNA was isolated utilizing the Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA). RNA was quantified using the Quant-it RiboGreen assay (Molecular Probes/Invitrogen, Carlsbad, CA). cDNA was prepared from total RNA utilizing the iScript cDNA Synthesis Kit (Bio-Rad). Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad) with the iCycler iQ real-time PCR detection system (Bio-Rad), with 40 cycles of real-time data collection at 95°C for 20 s and 59.6°C for 1 min, followed by melt-curve analysis to verify the presence of a single product. Primers were kindly provided by Dr. Girija G. Konduri and have been previously described (6). The sequences of the primers are as follows: 5′ CCTCACCGCTACAACATCC 3′ (sense) and 5′ GCACAGCCAGGTTGATCTC 3′ (antisense) for eNOS and 5′ CGGACACGGACAGGATTGACAG 3′ (sense) and 5′ ATGCCAGAGTCTCGTTCGTTATCG 3′ (antisense) for 18S RNA. For eNOS and 18S primers, 75- to 150-bp-long amplicons were produced, and there was a single product on melt-curve analysis with good correlation for efficiency and standard curves (r2 ≥ 0.98). PCR product size was verified by agarose gel electrophoresis, and all samples were analyzed in duplicate. For each reaction, negative controls containing reaction mix and primers without cDNA were performed to verify that primers and reaction mixtures were free of template contamination. Relative eNOS amounts were normalized to 18S expression using the cycle threshold (ΔΔCT) method (25). Data are fold values relative to fetal control lambs.
Western blot analysis.
Isolated frozen lung and PA tissue was homogenized, and total protein was collected using the PARIS kit (Ambion, Austin, TX), as previously described (10). Protein concentration was measured using the Bradford method (3). Total protein (40 μg) was separated on a 4–20% SDS-polyacrylamide gel (Bio-Rad) and then transferred from the gel to a nitrocellulose membrane (Amersham, Arlington Heights, IL). Western blot was then performed as previously described (10). Briefly, membranes were blocked for 1 h at room temperature with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (1× TBST) and then incubated overnight at 4°C with the primary antibody in 5% milk + 1× TBST at an appropriate dilution [1:1,000 for mouse anti-eNOS (BD Transduction, San Jose, CA), 1:200 for goat anti-GTP cyclohydrolase I (GTP-CH1; Santa Cruz Biotechnology, Santa Cruz, CA), and 1:2,000 for mouse β-actin (Sigma, St. Louis, MO)]. The membranes were then washed and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Pierce, Rockford, IL) diluted 1:1,000 in 5% milk + 1× TBST. Membranes were then washed and exposed via chemiluminescence (Pierce). Bands were analyzed using a Digital Science Image Station (Kodak, Rochester, NY). eNOS expression within each Western blot was normalized to β-actin. Data are fold values relative to fetal control lambs.
Immunohistochemistry.
Lung sections were prepared and stained as described previously (10). Briefly, the right middle lobe of the lung was removed, and OCT compound (VWR Scientific, West Chester, PA) was pushed gently into the deflated lobe and allowed to solidify on ice for 15–20 min. Blocks were prepared and cut into 8- to 10-μm sections that were mounted onto charged slides for staining and stored at −80°C. Sections were subsequently fixed with acetone (Sigma) for 10 min at 4°C, allowed to air dry, and then washed with 1× PBS (Mediatech, Herndon, VA). Sections were blocked with 5% BSA (Sigma) + 1× TBST at room temperature for 1 h and then stained overnight at 4°C with anti-eNOS antibody (BD Transduction) at a 1:100 dilution in 5% BSA + 1× TBST. After sections were further incubated in Alexa Fluor 488 anti-mouse antibody (Molecular Probes/Invitrogen) at a 1:200 dilution in 5% BSA, the tissue localization and expression of eNOS were visualized with a Nikon Eclipse TE-300 fluorescent microscope with excitation at 495 nm and emission at 519 nm. Fluorescent images were captured using a CoolSnap digital camera with Metamorph imaging software (Molecular Devices, Sunnyvale, CA).
In situ analysis of superoxide generation.
Frozen lung sections were exposed to 5 μM dihydroethidium (DHE; Molecular Probes/Invitrogen) in PBS. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min. Ethidium-stained slices were observed by fluorescence microscopy with excitation at 518 nm and emission at 605 nm. Fluorescent images were captured as described above. Tissue sections were processed and imaged in parallel.
Isolated vessel studies.
Fifth-generation intralobar PA (500 μm ID) were isolated, dissected with care to preserve the integrity of the endothelium, cut into ∼1- to 2-mm-long, 1- to 2-mg rings, and studied using standard tissue bath techniques, as described previously (39). Rings were suspended in water-jacketed chambers filled with aerated (94% O2-6% CO2) Krebs-Ringer solution. To obtain a continuous recording of isometric force generation, we tied each vessel ring to a force displacement transducer (model UC2, Statham Instruments, Hato Rey, PR) that was connected to a recorder (Gould Instrument Systems, Valley View, OH). After the arterial rings were mounted, they were allowed to equilibrate for 20 min in the bathing solution. A micrometer was used to stretch the tissues repeatedly in small increments over the following 45 min until resting tone remained stable at a passive tension of 0.8 g for PA isolated from control lambs and 1.0 g for PA isolated from PPHN lambs. Preliminary experiments determined that this procedure provided an optimal length for generation of active tone to exogenous norepinephrine (NE). Wet tissue weights were obtained at the end of each experiment, and contraction responses were normalized to tissue weight.
The following pharmacological agents were used: indomethacin, dl-propranolol, norepinephrine hydrochloride (NE), and N-nitro-l-arginine (l-NA). Indomethacin was dissolved in ethyl alcohol. Sonication was used to dissolve l-NA in warmed Krebs solution. All other drugs were dissolved in distilled water. All drugs were purchased from Sigma Aldrich (St. Louis, MO). Isolated PA were pretreated with indomethacin (10−5 M) to block endogenous prostaglandins and with propranolol (10−6 M) to block β-adrenergic receptors. Two arterial rings from each lamb were pretreated with l-NA (10−3 M) and then constricted with NE (3 × 10−7 M). Four arterial rings from each lamb were constricted with NE (3 × 10−7 M) without l-NA. This concentration of NE provided 40–50% of contraction force (expressed as grams of force per grams of tissue weight) generated by 118 mM KCl. The starting tensions generated by NE in the protocols with and without l-NA were recorded and corrected for wet tissue weight. The mean starting tension (expressed in grams of force per gram of wet tissue weight) from two to four PA rings per protocol was studied from each animal, and mean tension was used for analysis.
Determination of total biopterin levels by HPLC.
Lung biopterin content was measured by HPLC analysis and a differential oxidation method, as previously described (24, 26). Briefly, frozen lung tissue was ground to a powder on liquid nitrogen. The powder was then dissolved into biopterin extraction buffer (50 mM Tris, 1 mM DTT, and 1 mM EDTA). Tetrahydrobiopterin (BH4) levels were determined by the difference between the acid extraction [total biopterins: biopterin + BH4 + dihydrobiopterin (BH2)] and the alkaline extraction (BH2 + biopterin). A 5-μm C-18 reverse chromatography column (Waters, Milford, MA) was used for HPLC with a solvent system of 5% methanol (Fisher Scientific, Pittsburgh, PA)-95% water at a flow rate of 1 ml/min. Biopterins were detected by fluorescence at 350 nm for excitation and 450 nm for emission. Peak areas were compared with a biopterin standard curve (Sigma) and then normalized for micrograms of total protein in each sample as measured by the Bradford assay (3).
Statistical analysis.
Real-time PCR and Western blot results were normalized to fetal controls. DHE staining was normalized to 1DSB lambs. Values are means ± SE, with each n representing a single lamb. Results were analyzed by ANOVA with Bonferroni's post hoc analysis using Prism software (GraphPad Software, San Diego, CA). Statistical significance was set at P < 0.05.
RESULTS
eNOS expression is increased in resistance PA after birth.
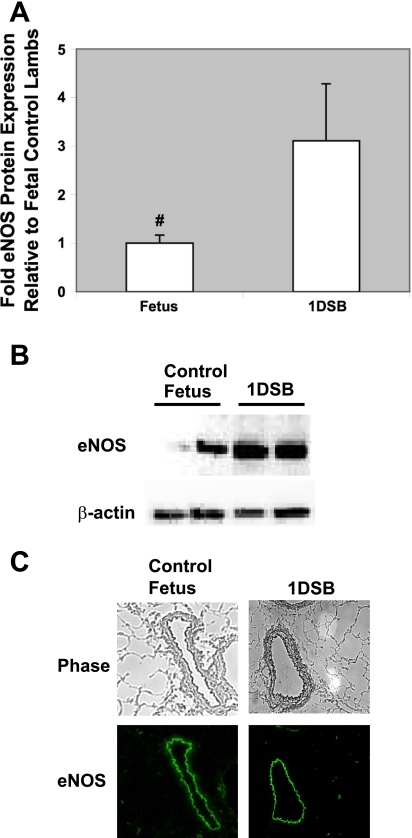
Significant literature implies that eNOS plays a critical role in normal transition after birth, and multiple studies have demonstrated that eNOS activity increases after birth, both as a consequence of increased O2 and mechanical forces (2, 8, 39). Consistent with these studies, eNOS RNA was increased in resistance PA from 1DSB lambs compared with fetal controls (Table 1). Similarly, eNOS protein expression in resistance PA from 1DSB lambs was increased compared with fetal controls (3.1 ± 1.1-fold, P < 0.05; Fig. 1, A and B). In contrast, there was no change in neuronal (nNOS) or inducible NO synthase (iNOS) protein expression in 1DSB lambs compared with fetal controls (see supplemental Fig. 1 in the online version of this article). Furthermore, as shown in Fig. 1C, eNOS expression was confined to the endothelial layer of the vessels in control and 1DSB lambs (Fig. 1C). In contrast, expression of nNOS and iNOS is largely confined to the smooth muscle layer of the vessels in control and 1DSB lambs (see supplemental Figs. 2 and 3). Ventilation with 100% O2 also significantly increased eNOS mRNA in control lambs compared with fetal lambs (Table 1). As shown in Fig. 2, ventilation with 100% O2 also significantly increased eNOS protein in control lambs compared with fetal lambs (2.5 ± 0.4-fold, P < 0.05), and this increase was similar in magnitude to that in the 1DSB lambs. The increase in eNOS protein expression in the control lambs ventilated with 100% O2 is an effect specific to eNOS, inasmuch as nNOS protein expression was decreased and there was no change in iNOS protein expression in these lambs (see supplemental Fig. 1). Inasmuch as eNOS appears to be the primary NOS isoform that is upregulated in the pulmonary endothelium after birth and the primary NOS isoform that is impacted by mechanical ventilation with O2, the remainder of the studies presented here will focus on regulation of eNOS in the neonatal pulmonary vasculature.
Table 1.
eNOS mRNA expression in ovine resistance pulmonary arteries
| Sample | n | eNOS mRNA Expression |
P |
|||
|---|---|---|---|---|---|---|
| vs. Control Fetus | vs. PPHN Fetus | vs. 1DSB | vs. Control 100% O2 | |||
| Control lambs | ||||||
| Fetus | 5 | 1±0.28 | NS | <0.01 | ||
| 1DSB | 7 | 17.1±7.0 | <0.01 | |||
| 100% O2 | 6 | 8.6±1.6 | <0.05 | NS | ||
| 100% O2 + iNO | 4 | 6.9±2.7 | <0.05 | NS | ||
| 100% O2 + rhSOD | 4 | 21.6±11.8 | <0.01 | NS | ||
| PPHN lambs | ||||||
| Fetus | 9 | 0.7±0.2 | NS | <0.001 | ||
| 100% O2 | 4 | 1.9±0.7 | NS | <0.05 | <0.05 | |
| 100% O2 + iNO | 4 | 15.2±5.4 | <0.01 | NS | ||
| 100% O2 + rhSOD | 4 | 18.7±10.4 | <0.01 | NS | ||
Endothelial nitric oxide (NO) synthase (eNOS) values are means ± SE, expressed as fold relative to fetal control. 1DSB, 1-day spontaneously breathing lambs; PPHN, persistent pulmonary hypertension of the newborn; iNO, inhaled NO; rhSOD, recombinant human SOD; NS, not significant.
Fig. 1.
eNOS protein is increased in resistance pulmonary arteries (PA) after birth. Ovine resistance PA were harvested from fetal control lambs (n = 7) and 1-day spontaneously breathing (1DSB) lambs (n = 5). A: Western blot analysis of PA endothelial nitric oxide (NO) synthase (eNOS) protein expression, with β-actin normalization. Values are means ± SE relative to fetal control lambs. #P < 0.05 vs. 1DSB. B: representative Western blots for eNOS and β-actin in resistance PA. C: localization of eNOS expression within PA endothelium. Top: phase-contrast images of frozen lamb lung sections. Magnification ×20. Bottom: corresponding sections stained for eNOS, with immunofluorescence shown as green.
Fig. 2.
eNOS protein expression is impaired in newborn lambs with persistent pulmonary hypertension (PPHN) ventilated with 100% O2, but eNOS is rescued by inhaled NO (iNO) or recombinant human SOD (rhSOD). Ovine resistance PA were harvested from 1DSB lambs (n = 5), fetal nonventilated control and PPHN lambs (n = 9), control and PPHN lambs ventilated with 100% O2 for 24 h (n = 4), control and PPHN lambs ventilated with 100% O2 + iNO for 24 h (n = 4), and control and PPHN lambs ventilated with 100% O2 + rhSOD for 24 h (n = 4). A: Western blot analysis of PA eNOS protein expression, with β-actin normalization. Values are means ± SE relative to control. *P < 0.05 vs. PPHN 100% O2. +P < 0.05 vs. PPHN 100% O2 + iNO. #P < 0.05 vs. 1DSB. B: representative Western blots for eNOS and β-actin in resistance PA. F, fetal nonventilated lambs; 100, lambs ventilated with 100% O2; NO, lambs ventilated with 100% O2 + iNO; S, lambs ventilated with 100% O2 + rhSOD.
Fig. 3.
eNOS protein expression by immunohistochemistry is rescued by iNO and rhSOD in PPHN resistance PA endothelium. eNOS expression is localized within PA endothelium. Left: phase-contrast images of representative frozen PPHN lamb lung sections. Remodeled, thickened vessel wall is consistent with PPHN phenotype. Magnification ×20. Right: corresponding sections stained for eNOS, with immunofluorescence shown as green.
eNOS expression does not increase in PPHN lambs ventilated with 100% O2.
In contrast to our observations in control lambs, ventilation of PPHN lambs with 100% O2 did not increase eNOS mRNA or protein relative to fetal nonventilated PPHN lambs. eNOS mRNA and protein expression was also significantly less in ventilated PPHN lambs than healthy 1DSB lambs and control lambs ventilated with 100% O2 (Table 1, Fig. 2). Furthermore, Fig. 3 shows significant vessel remodeling with marked smooth muscle hypertrophy in fetal and ventilated PPHN. Similar to control lambs, eNOS expression was confined to the endothelial layer of the vessels.
Treatment with iNO or rhSOD rescues eNOS expression in PPHN lambs.
Treatment of control lambs with iNO did not cause any further increase in eNOS mRNA relative to ventilation of control lambs with 100% O2 alone (Table 1). In contrast, addition of iNO significantly increased eNOS mRNA in PPHN lambs relative to PPHN lambs ventilated with 100% O2 alone (Table 1; 15.2 ± 5.4 vs. 1.9 ± 0.7 fold, P < 0.05). Ventilation with 100% O2 + iNO in PPHN lambs produced a less dramatic increase in eNOS protein. Ventilation with 100% O2 + iNO significantly increased eNOS protein expression in PPHN lambs relative to fetal PPHN lambs and PPHN lambs ventilated with 100% O2 alone (P < 0.05; Fig. 2A). However, protein expression remained significantly less than that in comparably ventilated control lambs (2.0 ± 0.3 vs. 4.3 ± 0.8 fold, P < 0.05). eNOS expression in PPHN lambs ventilated with 100% O2 + iNO remained confined to the endothelial layer of the vessels (Fig. 3).
Similar to the results we observed for lambs treated with iNO, treatment of control lambs with a single dose of rhSOD did not increase eNOS mRNA relative to control lambs ventilated with 100% O2 alone (Table 1). rhSOD significantly increased eNOS mRNA in PPHN lambs relative to PPHN lambs ventilated with 100% O2 alone (Table 1; 18.6 ± 10.4 vs. 1.9 ± 0.7 fold, P < 0.05). In contrast to iNO treatment, ventilation with 100% O2 + rhSOD increased eNOS protein equally in control and PPHN lambs (Fig. 2A; 4.1 ± 1.0 and 4.1 ± 1.1 fold in control and PPHN lambs, respectively). The increases were significantly greater in the PPHN lambs than in fetal PPHN lambs and PPHN lambs ventilated with 100% O2 alone (Fig. 2, A and B; P < 0.05). Furthermore, this eNOS expression in the PPHN lambs treated with 100% O2 + rhSOD was appropriately localized in the endothelial layer of the vessels (Fig. 3).
Ventilation with 100% O2 increases ROS in PPHN lambs, but treatment with iNO or rhSOD decreases ROS.
Ventilation with high levels of O2 was associated with accumulation of ROS, as measured by DHE fluorescence, in PPHN lambs that was greater than ROS accumulation in 1DSB control and fetal nonventilated PPHN lambs (Fig. 4; P < 0.05). Various strategies were employed, including treatment with iNO or rhSOD, in an attempt to reduce oxidative stress in these lambs. These strategies were equally effective at decreasing oxidative stress compared with ventilation with 100% O2 alone in PPHN lambs, as measured by DHE fluorescence (1.8 ± 0.8 and 2.2 ± 0.9 fold in 100% O2 + iNO and 100% O2 + rhSOD lambs, respectively; P < 0.05 vs. 100% O2 PPHN lambs; Fig. 4).
Fig. 4.
Oxidative stress in ventilated PPHN lambs is increased by ventilation with 100% O2 and reduced by iNO or rhSOD. Unfixed frozen lung sections from 1DSB lambs (n = 4), fetal nonventilated PPHN lambs (n = 3), PPHN lambs ventilated with 100% O2 for 24 h (n = 3), PPHN lambs ventilated with 100% O2 + iNO (n = 3), and PPHN lambs ventilated with 100% O2 + rhSOD (n = 3) were incubated with dihydroethidium (DHE) and imaged using fluorescence microscopy. Conversion of DHE by superoxide to ethidium results in red nuclear fluorescence. A: fluorescent intensity of each image was quantified using Metamorph imaging software. Values are means ± SE (5–7 vessels quantified per animal imaged) relative to 1DSB lambs. *P < 0.05 vs. PPHN 100% O2. #P < 0.05 vs. 1DSB. B: representative fluorescent images, with DHE fluorescence in red. Magnification ×20.
Treatment with rhSOD, but not iNO, restores endogenous eNOS function in PPHN lambs ventilated with 100% O2.
Recent studies demonstrated that ventilation with 100% O2 increases NE-induced contractility of resistance PA in control and PPHN lambs relative to fetal and 1DSB lambs (21, 23). As shown in Fig. 5, ventilation of PPHN lambs with 100% O2 with iNO or rhSOD significantly reduced NE-induced contractility, restoring it to levels similar to those observed in fetal and 1DSB control lambs. The difference in the contraction response of the resistance PA to NE alone vs. NE + l-NA, an eNOS inhibitor, was subsequently used as a measure of endogenous eNOS function. In Fig. 5, we demonstrated that addition of l-NA, an eNOS inhibitor, had no effect on vessel contractility in fetal PPHN lambs or those ventilated with 100% O2 or 100% O2 + iNO. However, treatment with a single dose of rhSOD restored the contraction response to l-NA, indicating restored endogenous eNOS function.
Fig. 5.
Endogenous eNOS function is restored in PPHN lambs ventilated with 100% O2 and treated with a single dose of rhSOD. Contraction response of 5th-generation PA was measured to 300 nM norepinephrine (NE) with and without pretreatment with the NOS inhibitor N-nitro-l-arginine (l-NA, 1 mM) and expressed as grams of tension per gram of wet tissue weight. *P < 0.05 vs. PPHN 100% O2. †P < 0.05 vs. corresponding vessel without l-NA.
Treatment with a single dose of rhSOD, but not iNO, increases BH4 in PPHN lambs ventilated with 100% O2.
Decreased levels of BH4, a key cofactor for eNOS function, can lead to uncoupling of eNOS in PA endothelial cells (26). In addition, recent reports indicate that biopterin pools may be altered in other models of pulmonary hypertension (13, 16, 31). We hypothesized that rhSOD may improve eNOS function through an increase in available BH4. Using HPLC, we determined that the total biopterin pool (BH4 + BH2 + biopterin), but not the BH2 + biopterin pool, was increased in 1DSB lamb lungs relative to fetal controls. This suggests that BH4 specifically increases as part of normal postnatal transition (Fig. 6; P < 0.05 vs. control fetuses). However, BH4 levels in the lungs of ventilated PPHN lambs were significantly decreased compared with 1DSB lambs (Fig. 6; P < 0.05). Addition of iNO did not significantly increase BH4 levels. In contrast, treatment of PPHN lambs with a single dose of rhSOD increased BH4 to levels comparable to those observed in 1DSB lambs (Fig. 6).
Fig. 6.
Tetrahydrobiopterin (BH4) is decreased in PPHN lambs ventilated with 100% O2, but BH4 is rescued by treatment with rhSOD. Ovine lungs were harvested from fetal control lambs (n = 9), 1DSB lambs (n = 5), fetal PPHN lambs (n = 9), PPHN lambs ventilated with 100% O2 for 24 h (n = 4), PPHN lambs ventilated with 100% O2 + iNO for 24 h (n = 3), and PPHN lambs ventilated with 100% O2 + rhSOD for 24 h (n = 4). Total biopterins [BH4 + dihydrobiopterin (BH2) + biopterin] were measured by HPLC with an acid extraction. Oxidized biopterins (BH2 + biopterin) were measured by HPLC with an alkaline extraction. BH4 was determined by the difference between total and oxidized biopterins. Values are means ± SE. *P < 0.05 vs. PPHN 100% O2. #P < 0.05 vs. 1DSB.
Treatment with a single dose of rhSOD, but not iNO, increases GTP-CH1 in PPHN lambs ventilated with 100% O2.
Since treatment with rhSOD normalized BH4 levels in the PPHN lambs ventilated with 100% O2, we hypothesized that rhSOD might impact GTP-CH1 expression or activity in these animals. GTP-CH1, the rate-limiting enzyme in the synthesis of BH4, has previously been demonstrated to be developmentally regulated in the perinatal vasculature (30). In Fig. 7, we demonstrate that GTP-CH1 increased dramatically in the lung tissue within 24 h of birth, as shown by the large increase in the 1DSB lambs compared with fetal control lambs. GTP-CH1 expression was decreased in the PPHN lambs ventilated with 100% O2 relative to the healthy 1DSB lambs (Fig. 7). Similar to the BH4 results, treatment with rhSOD, but not iNO, was sufficient to restore GTP-CH1 expression to levels comparable to those in the healthy 1DSB lambs (Fig. 7).
Fig. 7.
GTP cyclohydrolase expression is decreased in PPHN lambs ventilated with 100% O2 but rescued by treatment with rhSOD. Ovine lung tissue was harvested from 1DSB lambs (n = 5), fetal nonventilated control and PPHN lambs (n = 6), PPHN lambs ventilated with 100% O2 for 24 h (n = 4), PPHN lambs ventilated with 100% O2 + iNO for 24 h (n = 4), and PPHN lambs ventilated with 100% O2 + rhSOD for 24 h (n = 4). Lung GTP cyclohydrolase protein expression was analyzed by Western blot, with β-actin normalization. Values are means ± SE relative to fetal control lambs. *P < 0.05 vs. PPHN 100% O2. #P < 0.05 vs. 1DSB.
DISCUSSION
PPHN is a clinical syndrome with multiple etiologies that involve complex interrelated pathways that we are just beginning to elucidate. The ductal ligation lamb model of PPHN increases fetal PA pressures, leads to pulmonary vascular remodeling, and produces a clinical and histological disease process consistent with that seen in idiopathic PPHN (27, 46). Previous studies utilizing the ductal ligation model suggest that ROS may play a significant role in the pathogenesis of PPHN (4, 18, 23, 45). These findings raise concerns about the impact of traditional therapies on disease progression. Mechanical ventilation with high concentrations of O2 is typically utilized in clinically significant PPHN to minimize hypoxemia. The purpose of the present study was to examine the effects of mechanical ventilation, O2, iNO, and rhSOD on endogenous eNOS expression and function in the lamb model of PPHN.
Since total lung tissue includes multiple different components, such as vessels, airways, and parenchymal tissue, the studies presented here focused on the effects on endogenous eNOS in the resistance PA. In control lambs, eNOS protein expression increased after birth (Fig. 1, A and B), consistent with results reported previously (2, 8, 35). When control lambs were delivered and ventilated with 100% O2 for 24 h, the increase in eNOS protein was similar to that in spontaneously breathing lambs. Our findings suggest that the ROS generated by ventilating control lambs with high O2 for 24 h are not sufficient to impair endogenous eNOS expression. However, we recently reported in control lambs that hyperoxic ventilation increased expression and activity of the cGMP-specific phosphodiesterase PDE5, indicating that other components of the NO-cGMP pathway may be affected under these conditions (10).
In contrast, we found the impact of mechanical ventilation with O2 to be significantly different in the PPHN lambs. In fetal PPHN lambs, we found trends toward decreased eNOS mRNA and protein expression compared with fetal controls. In contrast to previous reports, these differences did not reach statistical significance, likely because the lambs in the present study were delivered 9 days after in utero ligation, compared with 10 days in previous studies (35, 42). Despite this difference, the PPHN lambs in the present study demonstrate all the histopathological and clinical features of PPHN (Figs. 3 and 5) (21, 23). The earlier time point of delivery was chosen because of an unacceptably high rate of fetal loss due to preterm labor and stillbirth.
After delivery and ventilation of PPHN lambs, eNOS protein was suppressed relative to healthy 1DSB lambs and control lambs ventilated with 100% O2 (Fig. 2). This finding correlated with significantly greater ROS production (Fig. 4) and increased contractile responses to NE (Fig. 5). It should be noted that the resting tension used for the arteries isolated from the PPHN lambs was higher than that used for the controls. This is not surprising, given the higher intravascular pressure in vivo and known remodeling of these vessels. We also noted that contractile responses in PPHN arteries were unaffected by addition of the eNOS inhibitor l-NA, suggesting that the endogenous eNOS was not producing NO (Fig. 8). We previously demonstrated increased ROS production, associated with decreased endogenous SOD activity and increased protein expression of the p67phox subunit of NADPH oxidase, in fetal nonventilated PPHN lambs (4). The present study suggests that, when delivered and ventilated with high levels of O2, PPHN lambs are particularly susceptible to further increases in ROS production and ROS-mediated damage, which lead to continued suppression of eNOS expression and function.
Fig. 8.
Model for effects of hyperoxia and rhSOD on eNOS in resistance PA. Exposure to hyperoxia leads to increased reactive species (ROS) production, which impairs eNOS expression and function, likely by decreasing available BH4. rhSOD scavenges ROS, thereby restoring normal BH4 levels and normal eNOS expression and function.
The only pulmonary vasodilator approved by the US Food and Drug Administration for infants with PPHN is iNO. As such, we sought to determine whether addition of iNO would restore endogenous eNOS expression and function in PPHN lambs. When PPHN lambs were ventilated with iNO, there was a significant increase in eNOS mRNA and protein relative to PPHN lambs ventilated with 100% O2 alone, which was comparable to the increase in 1DSB lambs (Table 1, Fig. 2). However, the lack of l-NA enhancement of vascular contractility suggests that this endogenous eNOS remains relatively inactive in these lambs. It is particularly interesting that iNO successfully upregulates eNOS expression, but not function, in the PPHN lambs. Previous studies demonstrated that exogenous NO upregulates eNOS mRNA levels in fetal intrapulmonary artery endothelial cells (47) but inhibits eNOS activity by protein nitration (5). Our results are consistent with these findings in isolated PA endothelial cells. Furthermore, we present new data that ventilation of PPHN lambs with 100% O2 leads to decreased BH4 levels and treatment with exogenous NO is unable to rescue these BH4 levels (Fig. 6) and GTP-CH1 expression (Fig. 7). Since BH4 represents a critical cofactor for eNOS function, the decreased BH4 levels in the PPHN lambs treated with iNO likely also contribute to the decreased eNOS function in the isolated vessel studies (Fig. 5). The relative inability of iNO to induce endogenous eNOS function in PPHN lambs may explain in part why ∼50% of infants with PPHN either fail to respond or do not sustain their response to iNO (7, 14, 32).
We previously demonstrated decreased SOD activity and increased NADPH oxidase expression in PPHN lambs (4). If this decreased SOD activity affects endogenous eNOS expression and/or function, then restoration of SOD activity should restore endogenous eNOS expression and function. We found that administration of a single dose of rhSOD at birth increased eNOS mRNA and protein expression levels relative to PPHN lambs ventilated with 100% O2 alone. This increase was comparable to 1DSB lambs and control lambs ventilated with 100% O2 + rhSOD (Table 1, Fig. 2). We also found that contractile responses to l-NA were restored, suggesting that rhSOD restored endogenous eNOS function (Fig. 5). Furthermore, treatment with rhSOD increased BH4 levels in the PPHN lambs relative to the lambs ventilated with 100% O2 alone and the lambs treated with iNO (Fig. 6) and increased GTP-CH1 expression relative to the lambs ventilated with 100% O2 alone and the lambs treated with iNO. Thus it is our hypothesis that rhSOD counteracts the effects of hyperoxia on eNOS and BH4/GTP-CH1 in the PA endothelial cell (Fig. 8).
The difference in BH4 levels in the rhSOD lambs likely plays a key role in the restoration of eNOS function in these lambs (Fig. 5). The oxidized biopterins (BH2 + biopterin) do not change across any of the ventilation groups (Fig. 6). This is in contrast to previously published data in another ovine model of chronic pulmonary hypertension (13). However, other groups have demonstrated that GTP-CH1, the rate-limiting enzyme for BH4 production, is developmentally regulated and increases at 12–24 h after birth in the pulmonary vasculature. However, after 24 h, GTP-CH1 levels fall, reaching a nadir at 3 days of life (30). This decrease places the neonate at risk for having limited BH4 pools. It has been shown that significant deficits in BH4 biosynthesis, such as in GTP-CH1 knockout mice, lead to pulmonary hypertension and pulmonary vascular remodeling, even in normoxia (16, 31). Finally, postnatal cotreatment with BH4 and a superoxide mimetic, MnTMPyP, has been reported to improve endothelial function and increase vessel relaxation in a porcine model of pulmonary hypertension (30). Thus the ability of rhSOD in the present study to restore GTP-CH1 expression and subsequently restore BH4 levels and eNOS function in our ovine model of pulmonary hypertension is consistent with the larger body of data regarding the impact of BH4 on pulmonary hypertension. It is of particular interest that only rhSOD is sufficient to restore GTP-CH1 expression and BH4 levels in the PPHN lambs (Figs. 6 and 7) when both rhSOD and iNO reduce oxidative stress in the PPHN lambs, as evidenced by DHE staining (Fig. 5). However, we would hypothesize that iNO, when delivered with 100% O2 in the context of PPHN, may increase reactive nitrogen species and protein nitration, which impacts eNOS function directly as well as BH4 biosynthesis. Furthermore, recent studies in the literature have demonstrated that GTP-CH1 expression and BH4 production are upregulated by H2O2 (36, 37). Since rhSOD acts to convert superoxide to H2O2, it is reasonable to hypothesize that the H2O2 produced by rhSOD is in part responsible for the increase in GTP-CH1 expression we observed (Fig. 7). This hypothesis would also further explain why iNO is less effective at normalizing GTP-CH1 expression and BH4 production, inasmuch as it likely clears ROS by reacting with them directly to produce reactive nitrogen species as opposed to the H2O2 produced by rhSOD. Thus the ability of rhSOD to restore eNOS expression and function as well as BH4 levels may explain in part the significant increase in oxygenation we recently reported when PPHN lambs are treated with rhSOD (23).
Thus our studies indicate that rhSOD is at least as effective as iNO on eNOS expression and function in resistance PA from PPHN lambs, suggesting that superoxide conversion and clearance have a significant role in eNOS expression and function and a significant effect on BH4 levels. Although iNO has been a successful therapy in PPHN, a significant portion of infants do not respond or sustain their response to iNO, nor does iNO significantly impact mortality or morbidity due to PPHN (14, 32). The data presented here suggest that the complex pathophysiology of PPHN is likely due, in part, to inhibition of eNOS expression and function by ROS, such as superoxide. This pathophysiology is further negatively impacted by the most common PPHN treatment, i.e., mechanical ventilation with high levels of O2. The data presented here suggest that treatment with rhSOD is able to enhance eNOS expression and function through enhancement of ROS clearance and restoration of BH4 levels (Fig. 8). Therefore, rhSOD may represent a future adjunctive or alternative therapy to iNO in the clinical management of severe PPHN.
GRANTS
These studies have been funded by National Heart, Lung, and Blood Institute Grants HL-086715 (K. N. Farrow) and HL-54705 (R. H. Steinhorn) and the Department of Pediatrics, University of Buffalo (S. Lakshminrusimha).
Supplementary Material
Acknowledgments
The authors thank Eugenia Mata-Greenwood for providing assistance with the biopterin assay protocol, Edmund A. Egan (ONY Laboratories) for providing calfactant (Infasurf), and INO Therapeutics for providing iNO and INOvent.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black SM, Johengen MJ, Ma ZD, Bristow J, Soifer SJ. Ventilation and oxygenation induce endothelial nitric oxide synthase gene expression in the lungs of fetal lambs. J Clin Invest 100: 1448–1458, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 4.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brennan LA, Wedgwood S, Bekker JM, Black SM. Nitric oxide activates p21ras and leads to the inhibition of endothelial NO synthase by protein nitration. DNA Cell Biol 22: 317–328, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar I, Eis A, Konduri GG. Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension. Pediatr Res 63: 67–72, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 342: 469–474, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Dukarm RC, Steinhorn RH, Morin FC 3rd. The normal pulmonary vascular transition at birth. Clin Perinatol 23: 711–726, 1996. [PubMed] [Google Scholar]
- 9.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24: 1367–1373, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102: 226–233, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 35: 236–256, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury. I. Basic mechanisms and in vivo monitoring of ROS. Circulation 108: 1912–1916, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 290: L1069–L1077, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Group NINOS. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336: 597–604, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Haworth SG Pulmonary vascular remodeling in neonatal pulmonary hypertension. Chest 93: 133S–138S, 1988. [PubMed] [Google Scholar]
- 16.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation 111: 2126–2133, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600–1619, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Konduri GG, Bakhutashvili I, Eis A, Pritchard K Jr. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, Wright LL, Van Meurs K, Stork E, Kirpalani H, Peliowski A. A randomized trial of early vs. standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics 113: 559–564, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 85: 753–766, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC 3rd, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 59: 137–141, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, Morin FC 3rd. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 62: 313–318, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 174: 1370–1377, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, Black SM, Steinhorn RH. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol 290: L232–L241, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin FC Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245–250, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Morin FC, Egan EA. Pulmonary hemodynamics in fetal lambs during development at normal and increased oxygen tension. J Appl Physiol 73: 213–218, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Murphy JD, Rabinovitch M, Goldstein JD, Reid LM. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr 98: 962–967, 1981. [DOI] [PubMed] [Google Scholar]
- 30.Nandi M, Leiper J, Arrigoni F, Hislop A, Vallance P, Haworth S. Developmental regulation of GTP-CH1 in the porcine lung and its relationship to pulmonary vascular relaxation. Pediatr Res 59: 767–772, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Nandi M, Miller A, Stidwill R, Jacques TS, Lam AA, Haworth S, Heales S, Vallance P. Pulmonary hypertension in a GTP-cyclohydrolase 1-deficient mouse. Circulation 111: 2086–2090, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JD, Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM, Thusu KG, Zellers TM, Wylam ME, Zaslavsky A. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med 336: 605–610, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Shaul PW Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64: 749–774, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Shaul PW, Wells LB. Oxygen modulates nitric oxide production selectively in fetal pulmonary endothelial cells. Am J Respir Crit Care Med 11: 432–438, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC 3rd. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 272: L1005–L1012, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu S, Hiroi T, Ishii M, Hagiwara T, Wajima T, Miyazaki A, Kiuchi Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through activation of the Jak2 tyrosine kinase pathway in vascular endothelial cells. Int J Biochem Cell Biol 40: 755–765, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu S, Shiota K, Yamamoto S, Miyasaka Y, Ishii M, Watabe T, Nishida M, Mori Y, Yamamoto T, Kiuchi Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through the induction of GTP-cyclohydrolase I and increases nitric oxide synthase activity in vascular endothelial cells. Free Radic Biol Med 34: 1343–1352, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med 32: 2496–2501, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Steinhorn RH, Morin FC 3rd, Gugino SF, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol Heart Circ Physiol 264: H2162–H2167, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Teitel D, Iwamoto H, Rudolph A. Changes in the pulmonary circulation during birth-related events. Pediatr Res 27: 372–378, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Tzao C, Nickerson PA, Russell JA, Gugino SF, Steinhorn RH. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr Pulmonol 31: 97–105, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 107: 642–647, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile L, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 105: 14–20, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wild LM, Nickerson PA, Morin FC 3rd. Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res 25: 251–257, 1989. [DOI] [PubMed] [Google Scholar]
- 47.Yuhanna IS, MacRitchie AN, Lantin-Hermoso RL, Wells LB, Shaul PW. Nitric oxide (NO) upregulates NO synthase expression in fetal intrapulmonary artery endothelial cells. Am J Respir Cell Mol Biol 21: 629–636, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.