Abstract
Thrombin is a procoagulant inflammatory agonist that can disrupt the endothelium-lumen barrier in the lung by causing contraction of endothelial cells and promote pulmonary cell proliferation. Both contraction and proliferation require increases in cytosolic Ca2+ concentration ([Ca2+]cyt). In this study, we compared the effect of thrombin on Ca2+ signaling in human pulmonary artery smooth muscle (PASMC) and endothelial (PAEC) cells. Thrombin increased the [Ca2+]cyt in both cell types; however, the transient response was significantly higher and recovered quicker in the PASMC, suggesting different mechanisms may contribute to thrombin-mediated increases in [Ca2+]cyt in these cell types. Depletion of intracellular stores with cyclopiazonic acid (CPA) in the absence of extracellular Ca2+ induced calcium transients representative of those observed in response to thrombin in both cell types. Interestingly, CPA pretreatment significantly attenuated thrombin-induced Ca2+ release in PASMC; this attenuation was not apparent in PAEC, indicating that a PAEC-specific mechanism was targeted by thrombin. Treatment with a combination of CPA, caffeine, and ryanodine also failed to abolish the thrombin-induced Ca2+ transient in PAEC. Notably, thrombin-induced receptor-mediated calcium influx was still observed in PASMC after CPA pretreatment in the presence of extracellular Ca2+. Ca2+ oscillations were triggered by thrombin in PASMC resulting from a balance of extracellular Ca2+ influx and Ca2+ reuptake by the sarcoplasmic reticulum. The data show that thrombin induces increases in intracellular calcium in PASMC and PAEC with a distinct CPA-, caffeine-, and ryanodine-insensitive release existing only in PAEC. Furthermore, a dynamic balance between Ca2+ influx, intracellular Ca2+ release, and reuptake underlie the Ca2+ transients evoked by thrombin in some PASMC. Understanding of such mechanisms will provide an important insight into thrombin-mediated vascular injury during hypertension.
Keywords: sarcoplasmic and endoplasmic reticulum, Ca2+ store
thrombin, a major effector protease of the coagulation cascade, is best known for its role in the conversion of fibrinogen into fibrin. Thrombin can operate as an inflammatory agent by stimulating endothelial cells (EC) to produce and/or secrete chemotactic factors by promoting monocyte recruitment and by increasing endothelial permeability (8, 9). At the same time, thrombin regulates the inflammation processes by blocking aggregation of platelets, adhesion of monocytes to endothelium, and by releasing nitric oxide (NO) from EC (35).
In the lung, thrombin has a number of effects not limited to coagulation, which may play significant roles in the development of lung injury and pulmonary hypertension. Of particular interest are its actions related to chronic thromboembolic pulmonary hypertension where it plays inherent roles in the formation of the persistent thrombus and where it may endure for prolonged periods of time in the pulmonary artery. Increased luminal native α-thrombin was shown to increase the clearance of albumin across the pulmonary endothelial monolayer causing morphological changes and resulting in pulmonary artery endothelial cell (PAEC) contraction and formation of intercellular gaps (14). In ensuing studies, PAEC contraction and disruption of the endothelial barrier by thrombin has been attributed to 1) increased Ca2+ influx and/or release (15, 18, 26, 27, 38), 2) cytoskeletal rearrangements (3, 6, 41), 3) altered adenylate cyclase function (11), 4) activation of canonical transient receptor potential channels (TRPC) (15, 22, 30, 33, 36, 37), and 5) mitochondrial membrane potential loss (5). Thrombin also acts as a mitogen, promoting the proliferation of fibroblasts (4, 17, 34) or airway smooth muscle cells (20). Ca2+ signaling is therefore central to the function of thrombin; increased Ca2+ influx and/or release from intracellular stores, such as endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR), is known to modulate the structure and contractility of pulmonary endothelial and smooth muscle cells (22, 32, 33). Intercellular gaps forming due to PAEC contraction could then expose pulmonary artery smooth muscle cells (PASMC) to thrombin. Activation of protease-activated receptors (PAR) on PASMC will then have vasoactive and mitogenic effects.
In the current study, we compared the effect of thrombin on Ca2+ signaling in human PAEC and PASMC. Our results show that thrombin-mediated intracellular Ca2+ mobilization involves different stores in PAEC and PASMC. Importantly, we identify a novel thrombin-releasable store in PAEC that is insensitive to cyclopiazonic acid (CPA), caffeine, and ryanodine, which may be crucial in understanding the pathogenesis of diseases such as chronic thromboembolic pulmonary hypertension (CTEPH), where pulmonary arteries are particularly exposed to thrombin.
MATERIALS AND METHODS
Cell preparation and culture.
Human PASMC and PAEC from normal subjects purchased from Lonza were used for experiments. Cryopreserved cells were plated onto cover slips or Petri dishes and incubated in a humidified atmosphere of 5% CO2 in air at 37°C in smooth muscle growth medium (SMGM; Lonza) for PASMC and in endothelium growth medium (EGM; Lonza) for PAEC. SMGM was composed of smooth muscle basal medium supplemented with 5% FBS, human epidermal growth factor, human fibroblast growth factor, and insulin. EGM was composed of endothelium basal medium supplemented with 2% FBS, human epidermal growth factor, human fibroblast growth factor, and insulin. PASMC and PAEC were used for experiments between passages 2 and 6. The morphology of the cells was examined using phase contrast microscopy.
Measurement of [Ca2+]cyt.
Cytosolic Ca2+ concentration ([Ca2+]cyt) was measured in single human PASMC and PAEC using ratiometric Ca2+ label fura 2-AM. Cells grown on cover slips were loaded with fura 2-AM (4 μM) in the dark at room temperature for 30 min before being transferred to a perfusion chamber on the Nikon TMS microscope stage. Loaded cells were superfused with modified Krebs solution (MKS) for 30 min at 32°C to remove residual extracellular dye and to allow intracellular esterases to cleave cytosolic fura 2-AM into active fura 2. The MKS contained (in mM) 138 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 10 HEPES, 1.8 CaCl2, and 10 glucose, pH 7.4. In Ca2+-free MKS, CaCl2 was replaced by equimolar MgCl2.
Fura 2 fluorescence (excited by a xenon lamp at 510 nm and emission recorded at 340 and 380 nm) was collected using a ×40 Nikon UV-Fluor objective and a charge-coupled device camera. The fluorescence signals emitted from the cells were captured every 2 s using InCytIM2 software (Intracellular Imaging). [Ca2+]cyt was measured as the ratio of fura 2 fluorescence emission at 340 nm and 380 nm (F340/F380) normalized to baseline (F0) and is reported as F/F0.
RT-PCR.
Total RNA was extracted from human PASMC and PAEC following cell lysis with TRIzol (Invitrogen). Addition of chloroform followed by centrifugation was used to separate the aqueous phase containing the RNA; RNA was then precipitated using isopropyl alcohol. RNA concentration and quality were determined from OD260–280 measurements. SuperScript First-Strand Synthesis System (Invitrogen) was used to synthesize cDNA. RNA (2 μg) was first treated with DNase I and the reaction stopped with 10% 25 mM EDTA (65°C 10 min). The reverse transcription (RT) reaction consisted of three phases: 1) 10 μl DNase-treated RNA, oligo(dT) dNTP (1 μl, 10 mM) at 65°C for 5 min; 2) first-strand buffer (4 μl, 5×), 0.1 M DTT (2 μl), RNaseOUT heated to 42°C for 2 min; and 3) SuperScript II reverse transcriptase added to the samples for 50 min at 42°C and 15 min at 70°C. For negative controls, DNase-RNase-free H2O was added to the RT reaction instead of SuperScript II.
Platinum PCR SuperMix (Invitrogen) was used to perform all PCR reactions. The mixture contained 22 U/ml complexed recombinant Taq DNA polymerase with Platinum Taq antibody, 22 mM Tris·HCl (pH 8.4), 55 mM KCl, 1.65 mM MgCl2, 220 μM dNTP, and stabilizers. Total volume of each reaction tube was 25 μl. PCR primer sequences are included in Table 1. The specificity of the sense and antisense oligonucleotides were examined using the NCBI-BLAST program. PCR was performed according to the following sequence: 5 min at 95°C followed by 35°C cycles of denaturation at 95°C for 30 s, primer annealing at 55 for 30 s, and primer extension at 72°C for 20 s and ending with 72°C for 7 min. PCR products were electrophoresed through 1.2% agarose gels containing 10 μM Gelstar Nucleic Acid stain (Lonza) and were visualized by ultraviolet illumination. A positive control GAPDH was used to semiquantify the RT-PCR products. The net intensity values of cDNA bands was measured by Image J software, and the PCR products were normalized to the GAPDH product from the same cDNA sample and PCR reaction and run on the same gel.
Table 1.
Primer sequences used for RT-PCR
| Gene Name (Accession No.) | Product Size, bp | Sequence (Sense/Antisense) | Location, nt |
|---|---|---|---|
| PAR-1 (NM_001992) | 708 | 5′-tgtgaactgatcatgtttatg-3′/ | 2542–2562 |
| 5′-ttcgtaagataagagatatgt-3′ | 3249–3229 | ||
| PAR-2 (NM_00542) | 428 | 5′-gtgagaggctgactttctc-3′/ | 55–73 |
| 5′-agggtgcttcttcttagttc-3′ | 482–462 | ||
| PAR-3 (NM_004101) | 599 | 5′-gaaagccctcatctttgcag-3′/ | 188–207 |
| 5′-aggtgaaaggatggacgatg-3′ | 786–766 | ||
| PAR-4 (NM_003950) | 244 | 5′-ggcaacctctatggtgccta-3′/ | 1121–1140 |
| 5′-ttcgacccagtacagccttc-3′ | 1364–1345 | ||
| GAPDH (NM_002046) | 243 | 5′-gacaacgaatttggctacagc-3′/ | 1045–1065 |
| 5′-gatggtacatgacaaggtgc-3′ | 1287–1268 |
Chemicals.
Human thrombin was purchased from Enzyme Research Laboratories (South Bend, IN). All other chemicals were purchased from Sigma (St. Louis, MO) and prepared as stock solutions in their respective solvents. CPA was dissolved in DMSO to make a stock solution of 10 mM. Aliquots of the stock solution were then diluted to the final concentration and used the day of each experiment. pH values were readjusted to 7.4 after the addition of drugs to the Ca2+-free solution.
Statistics and data analysis.
Data are expressed as means ± SE; n represents the number of cells. Statistical analysis was performed using paired Student's t-test. Differences were considered to be significant at P < 0.05.
RESULTS
Comparable PAR expression in PASMC and PAEC.
PASMC and PAEC from normal subjects (Fig. 1A) were first evaluated for expression of PAR subtypes. The mRNA expression for all PAR subtypes (PAR 1–4) was present in both PASMC (Fig. 1B) and PAEC (Fig. 1C). Semiquantitative analysis, when expression was normalized to GAPDH intensity (Fig. 1D), indicated no statistically significant differences in receptor subtype expression between the two cell lines. Indeed, PAR-2 and PAR-3 expression levels were higher in PASMC, and PAR-4 expression was higher in PAEC. Overall, these data indicate that the mRNA expression level of all PAR is comparable between PASMC and PAEC.
Fig. 1.
The mRNA expression levels of protease-activated receptors (PAR) are comparable in human pulmonary artery smooth muscle cells (PASMC) and pulmonary artery endothelial cells (PAEC). A: phase contrast image of cultured human PASMC (left) and PAEC (right). B and C: RT-PCR amplified products for PAR-1, PAR-2, PAR-3, PAR-4, and GAPDH in PASMC. M, 1,000-bp DNA ladder. RT-PCR-amplified products of GAPDH in the presence (+RT) or absence (−RT) of transcriptase are shown as controls. D: summarized data (n = 3–5) showing the mRNA levels of PAR 1–4, normalized to GAPDH, in PASMC (black bars) and PAEC (gray bars).
Thrombin causes kinetically different [Ca2+]cyt transients in PAEC and PASMC.
We directly compared the thrombin-induced increases in [Ca2+]cyt in PASMC and PAEC. Application of thrombin (10 nM) caused large transient increases in [Ca2+]cyt in both cell types (Fig. 2A), but the transients differed with respect to both their amplitude and duration (Fig. 2B). Peak amplitude of the Ca2+ transient was significantly greater in PASMC (2.72 ± 0.11 F/F0, n = 11) than in PAEC (2.28 ± 0.12 F/F0, n = 19; P < 0.05). Thrombin-induced Ca2+ transients also decayed faster in PASMC than in PAEC. Fitting of the recovery from the peak response to the baseline with an exponential decay function indicated that the duration of the Ca2+ transients was 162.4 ± 15.2 s for PASMC (n = 11) and 458.3 ± 31.2 s for PAEC (n = 18; P < 0.001). These results suggest a differential regulation of the release and reuptake of Ca2+ mechanisms in PAEC and PASMC.
Fig. 2.
Different kinetics of thrombin-induced transient cytosolic Ca2+ concentration ([Ca2+]cyt) increases in PASMC and PAEC. A: representative data depict the transient increase in [Ca2+]cyt induced by 10 nM thrombin in PASMC (top) and PAEC (bottom); [Ca2+]cyt elevations are described as the F/F0 ratio of fura 2 fluorescence. B: summarized data showing the amplitude (left) and duration (right) of thrombin-induced [Ca2+]cyt transients in PASMC (black bars, n = 11) and PAEC (gray bars, n = 18–19). *P < 0.05, ***P < 0.001 vs. PASMC.
We also calculated the area under the curve (AUC) for the [Ca2+]cyt transients in human PASMC and PAEC. AUC was equal to 214.9 ± 26.3 for PAEC and 77.1 ± 10.7 for PASMC (P < 0.0001). The values of AUC indicated that there was substantially more thrombin-induced Ca2+ release in PAEC than in PASMC, reflecting the slower kinetics of the recovery phase of the [Ca2+]cyt transients in PAEC that we have described.
Differential role of CPA-sensitive Ca2+ stores in thrombin-induced Ca2+ release.
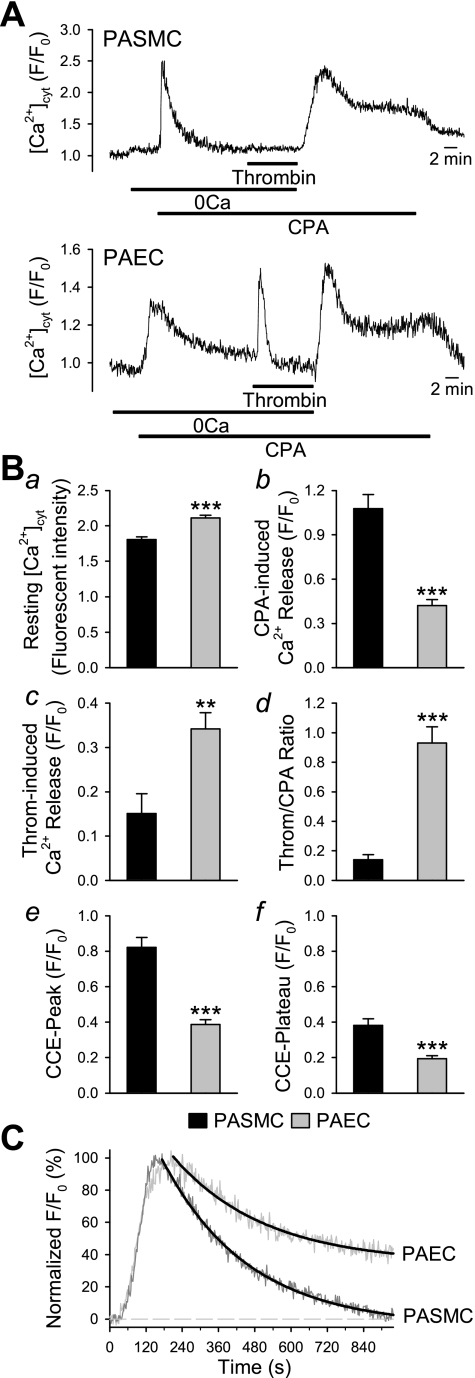
As shown in Fig. 2, the thrombin-mediated increase in [Ca2+]cyt in PAEC has a much slower kinetic of “recovery” (reuptake or extrusion from the cytoplasm) to basal [Ca2+]cyt than observed in PASMC. In PASMC, the more transient nature of the change in [Ca2+]cyt suggests that thrombin-mediated Ca2+ release and influx take place rapidly and are likely to overlap. To determine if the differential kinetics are due to the relative contribution of different intracellular Ca2+ stores, experiments were designed to determine the importance of ER/SR Ca2+ stores to the thrombin-induced increase in [Ca2+]cyt. Figure 3A shows data from PASMC and PAEC exposed to thrombin in the presence of 10 μM CPA, an inhibitor of the SR/ER Ca2+-ATPase pump that depletes Ca2+ stored in the IP3-sensitive SR/ER.
Fig. 3.
Cyclopiazonic acid (CPA) treatment significantly inhibited thrombin-induced Ca2+ release in PASMC, but not in PAEC. A: representative traces of [Ca2+]cyt changes (denoted by fluorescence ratio, F/F0) in PASMC (top) and PAEC (bottom) treated with CPA (10 μM) and thrombin (10 nM) in the presence and absence (0Ca) of extracellular Ca2+. B: summarized data showing the resting [Ca2+]cyt [basal fluorescence intensity (a), the amplitude of CPA-induced Ca2+ release (b), the amplitude of thrombin-induced Ca2+ release (c), the ratio of thrombin- and CPA-induced Ca2+ release (d), and the peak (e) and plateau (f) amplitude of CCE in PASMC (black bars; n = 15) and PAEC (gray bars; n = 16)]. C: time course of the decay phase of CPA-induced transient increase in [Ca2+]cyt in PASMC and PAEC superfused with Ca2+-free solution. **P < 0.01, ***P < 0.001.
Detailed analysis of these experiments showed that resting [Ca2+]cyt was significantly lower in PASMC than in PAEC (P < 0.001) (Fig. 3Ba). In both cell types, CPA caused a transient [Ca2+]cyt increase in the absence of extracellular Ca2+ (Fig. 3, A and Bb), but its amplitude was significantly greater in PASMC (1.08 ± 0.09 F/F0) than in PAEC (0.42 ± 0.04; P < 0.001). Furthermore, the CPA-induced Ca2+ transient showed different kinetics in PASMC and PAEC (Fig. 3C), reflecting those observed in response to thrombin in Fig. 2. Subsequent exposure to thrombin in Ca2+-free+CPA solution caused a substantial rapid Ca2+ release in all PAEC, whereas only a very small proportion of PASMC showed a brief, low amplitude response to thrombin (Fig. 3Bc, P < 0.01). When the thrombin response is expressed as a ratio relative to the CPA-induced Ca2+ release, a significantly higher thrombin-mediated CPA-insensitive Ca2+ release is observed in PAEC (Fig. 3Bd, P < 0.001).
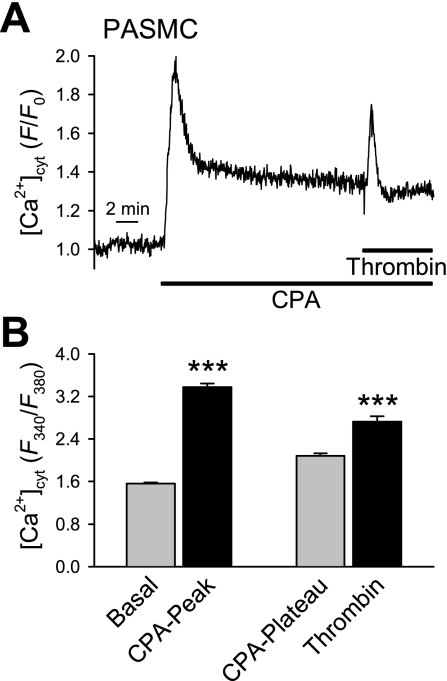
Upon reintroduction of Ca2+ to the perfusate in the presence of CPA, a biphasic capacitative Ca2+ entry (CCE) was observed. The peak and plateau amplitudes of this CCE were significantly greater in PASMC than in PAEC (Fig. 3, Be and Bf; P < 0.001). The data suggests that, in addition to causing Ca2+ influx through plasma membrane channels, thrombin-induced increases in [Ca2+]cyt occur by promoting Ca2+ release from different intracellular stores in PASMC and PAEC. To confirm that the thrombin-mediated signaling pathway, via G protein-coupled receptors (GPCR), was not uncoupled and that the PAR were not desensitized, the experiment in PASMC was repeated in the presence of extracellular Ca2+ (Fig. 4). As expected, with constant extracellular Ca2+ present, the CPA-mediated Ca2+ transient reached steady state; addition of thrombin consistently caused a significant Ca2+ transient, indicating that in the presence of extracellular Ca2+, thrombin still evokes an increase in [Ca2+]cyt and that CPA does not disassociate signaling pathways or desensitize receptors.
Fig. 4.
Thrombin signaling pathway remains intact after CPA pretreatment in human PASMC. A: representative trace (F/F0) of thrombin-mediated calcium release after CPA pretreatment in the presence of extracellular Ca2+. B: averaged data from 39 cells (means ± SE) indicating the increase in [Ca2+]cyt in PASMC after CPA and thrombin treatment in the presence of extracellular Ca2+ expressed as the F340/F380 ratio. Basal, the basal level of [Ca2+]cyt; CPA-Peak and CPA-Plateau, the amplitude of CPA-induced increases in [Ca2+]cyt (peak and plateau); Thrombin, the thrombin-induced increase in [Ca2+]cyt (on top of CPA-induced increase in [Ca2+]cyt). ***P < 0.001 vs. Basal or CPA-Plateau bar.
Caffeine- and ryanodine-sensitive stores are not thrombin-releasable stores in PAEC.
As shown in Figs. 2 and 3, thrombin clearly caused Ca2+ release in PAEC from an intracellular store that could not be depleted by CPA. We verified whether ryanodine-sensitive stores were modulated by thrombin in PAEC. Pretreatment of PAEC with a cocktail containing CPA (10 μM), caffeine (1 mM), and ryanodine (10 μM) caused a Ca2+ transient in Ca2+-free solutions (Fig. 5). Depletion of ryanodine- and caffeine-sensitive stores had no effect on the thrombin-induced increase in [Ca2+]cyt. These results suggest that thrombin stimulates Ca2+ mobilization from an intracellular store that is not sensitive to caffeine, ryanodine, or CPA.
Fig. 5.
Treatment with CPA, caffeine, and ryanodine fails to inhibit thrombin-induced Ca2+ release in PAEC. A: representative trace (denoted by the fluorescence ratio, F/F0) indicating thrombin (10 nM)-mediated Ca2+ release after pretreatment with CPA, caffeine (Caf), and ryanodine (Ry). B: averaged data (means ± SE) indicating the increase in [Ca2+]cyt in PAEC pretreated with a combination of CPA (10 μM), caffeine (1 mM), and ryanodine (10 μM) in the absence of extracellular Ca2+. Basal, the basal level of [Ca2+]cyt; CPA-Peak and CPA-End, the amplitude of [Ca2+]cyt increases at the beginning (CPA-Peak) of application of CPA, Caf, and Ry and at the time right before application of thrombin (CPA-End); Thrombin, the thrombin-induced increase in [Ca2+]cyt (on top of CPA/Caf/Ry-induced increase in [Ca2+]cyt). ***P < 0.001 vs. Basal or CPA-End bar.
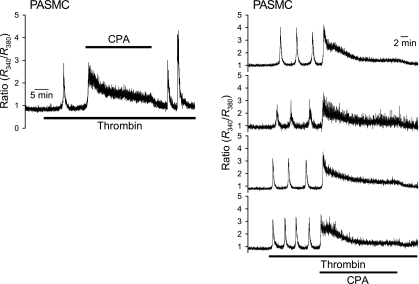
Thrombin causes rhythmic [Ca2+]cyt oscillations in PASMC.
In human PASMC, low-concentration thrombin (10 nM) induced [Ca2+]cyt oscillations with different frequency and amplitudes (Fig. 6). Removal of extracellular Ca2+ abolished thrombin-induced Ca2+ oscillations; this effect was reversible, i.e., restoration of extracellular Ca2+ restored thrombin-induced Ca2+ oscillations (Fig. 7, A and B). Upon removal of Ca2+, thrombin often triggered a single Ca2+ transient whose amplitude was comparable to that of oscillations observed in Ca2+-containing perfusate (Fig. 7C). Finally, CPA effectively blocked thrombin-induced Ca2+ oscillations (Fig. 8), again indicating that CPA-sensitive intracellular stores are also thrombin-sensitive stores in human PASMC.
Fig. 6.
Thrombin induces rhythmic Ca2+ oscillations in PASMC. The representative traces depict the Ca2+ oscillations induced by 10 nM thrombin in 3 separate PASMC, shown as higher (top, 0.0055 Hz), medium (middle, 0.0036 Hz), and lower (bottom, 0.00033 Hz) frequency.
Fig. 7.
Thrombin-induced Ca2+ oscillations require Ca2+ influx in PASMC. A: sample traces showing Ca2+ oscillations induced by thrombin (10 nM) in the presence or absence (0Ca) of external Ca2+ in the perfusate. B and C: bar graphs depict oscillation frequency (B) and oscillation amplitude (C) in PASMC (n = 8) exposed to thrombin before (white bars) and during (black bars) removal of external Ca2+, as well as upon return of Ca2+ to the perfusate (gray bars). ***P < 0.001 vs. thrombin.
Fig. 8.
CPA reversibly attenuates thrombin-induced Ca2+ oscillations in PASMC superfused with Ca2+-containing solution. Left: representative record showing 100 nM thrombin-induced [Ca2+]cyt changes (denoted by the fluorescence ratio, R340/R380) in PASMC before, during, and after treatment with 5 μM CPA. Right: individual traces show the effect of 5 μM CPA on the frequency and waveform of Ca2+ oscillations induced by 100 nM thrombin.
Together, the results suggest that thrombin-induced activation of PAR, in addition to calcium influx mechanisms, increases [Ca2+]cyt via Ca2+ release from CPA-sensitive SR/ER. The IP3-mediated store depletion then causes CCE via store-operated channels (SOC); however, the incoming Ca2+ is probably directly or rapidly sequestered into the SR/ER to refill Ca2+ into these intracellular stores. In other words, the thrombin-induced Ca2+ oscillations depend on the capacity or levels of Ca2+ in the SR/ER, whereas the store-operated Ca2+ influx plays an important role in refilling the stores so that a “normal” Ca2+ oscillation can occur.
DISCUSSION
In the lung, the formation of fibrotic clots due to the persistence of pulmonary embolisms can lead to vascular injury, and, particularly, to disruption of the endothelial barrier. Such occluding clot regions, typical of chronic thromboembolic pulmonary hypertension, are sites that can accumulate thrombin as well as vasoactive and mitogenic agents produced by circulating platelets or by endothelial cells themselves. In the endothelial monolayer, changes in microvascular permeability or vascular leakage are major downstream effects of altered [Ca2+]cyt. In vascular smooth muscle cells, increased [Ca2+]cyt can translate into contraction and/or proliferation. Thrombin is known to induce vascular injury predominantly via effects on endothelial cells leading to endothelial barrier dysfunction due to mobilization of Ca2+ and rearrangement of the cytoskeleton (12). In the current study, we evaluated the impact of thrombin on Ca2+ signaling in human PASMC and PAEC.
Many of the cellular effects of thrombin are consistent with a primary role in vessel wound healing and revascularization (10) and involve a multitude of cell types (i.e., platelets, endothelium, smooth muscle cells, neutrophils, leukocytes, neurons, glial cells) in the systemic response to vascular damage. Thrombin receptors, known as PAR, are members of the G protein superfamily of seven-transmembrane domain receptors. Through binding to PAR expressed on smooth muscle cells (PAR 1–4), thrombin has been shown to modulate Ca2+ signaling, in turn increasing cell proliferation and migration (2). We found that the mRNA expression of all four thrombin-specific PAR subtypes was similar in human PASMC and PAEC (Fig. 1), although there was a tendency for PASMC to express more PAR-2 and PAR-3 than PAEC, whereas PAEC expressed more PAR-4 than did PASMC. PAR-1 function has been linked to regulation of endothelial barrier function (19, 37). PAR-2 is also expressed in vascular tissue and highly vascularized organs, where it is purported to play a role in regulating vascular tone (endothelium-dependent relaxation) and the chronic response to vessel inflammation and wound healing (21). PAR-3, expressed in many cell types, may be involved in the vascular inflammatory response (29) and smooth muscle cell proliferation (7). Platelet PAR-3 deficiency can also protect against thrombosis in mice (41). Some have even suggested that PAR-3 may act as a cofactor for PAR-4 activation by thrombin, rather than as a functional receptor (28, 41). PAR-4 itself may have an important role in regulating vascular tone (16) and endothelial cell proliferation (13). We previously reported that mRNA expression of PAR-1 and PAR-3 was increased in PASMC from idiopathic pulmonary arterial hypertension (IPAH) patients compared with PASMC from normal subjects and normotensive patients (31). These results suggest that thrombin signaling, at least in PASMC, may be involved in triggering pulmonary vasoconstriction and vascular remodeling in IPAH patients.
In the current study, we showed that thrombin causes significant increases in [Ca2+]cyt in both PASMC and PAEC, which could be attributed to both Ca2+ influx and Ca2+ release from the sarcoplasmic/endoplasmic reticulum. Indeed, thrombin has previously been shown to stimulate Ca2+ influx mechanisms, particularly via the canonical transient receptor potential channels, TRPC1 and 6 (1, 33). Such thrombin-mediated TRPC-dependent elevations in [Ca2+]cyt represent an important mechanism of increased intracellular Ca2+. In addition, we have shown here that thrombin has specific effects on Ca2+ release from intracellular stores that appear to be different in PASMC and PAEC. These complementary studies into the regulation of thrombin-mediated elevations of [Ca2+]cyt may indicate an interaction of thrombin-sensitive stores with either different TRPCs or the same TRPCs activated via different mechanisms in the different cell types.
Our findings indicate a substantial thrombin-dependent release of Ca2+ from intracellular stores in both PASMC and PAEC; however, in PASMC this release is entirely CPA sensitive, indicating predominant Ca2+ release from IP3- and CPA-sensitive stores in the SR. Thrombin was able to still initiate a rise in intracellular Ca2+ in the presence of extracellular Ca2+ after store depletion by CPA, confirming that the GPCR-mediated signaling pathways were still intact and functional in these cells.
While CPA- and IP3-sensitive stores appeared to play major roles in the [Ca2+]cyt response to thrombin in PASMC, it was apparent that PAEC possessed another source of Ca2+. In PAEC, a significant thrombin-mediated Ca2+ release was still present even after pretreatment with a cocktail of CPA, caffeine, and ryanodine, implicating a substantial Ca2+ release from a different intracellular store. These data show that the thrombin-mediated Ca2+ release in PAEC may involve sources in addition to an IP3-sensitive ER that can be depleted by CPA: for example, 1) ER stores that cannot be depleted by CPA, and 2) non-ER-based stores. Although we have yet to identify the source, this finding is similar to one we previously described in human PAEC treated with histamine where pretreatment with caffeine, ryanodine, bafilomycin, and FCCP did not abolish histamine-induced Ca2+ transients in PAEC (23). While the ER/SR represent the largest intracellular store in most excitable and nonexcitable cells (including PASMC and PAEC), mitochondria, Golgi apparatus, lysosomes, peroxisomes, and endosomes all represent other potential Ca2+ sources (25). We did observe equivalent results in response to thrombin using a similar cocktail (i.e., caffeine, ryanodine, bafilomycin, and FCCP) (Agange and Yuan, unpublished observations). The presence of this additional source of intracellular Ca2+ specific to PAEC and stimulated by two different agonists, histamine and thrombin, may have a significant role in vascular injury in both inflammatory and thromboembolic pulmonary vascular diseases. The activation of such stores by distinct GPCR signaling pathways (histamine Gq coupled, PAR either Gαi, Gα12/13, or Gq coupled) therefore indicate that this Ca2+ release from a novel intracellular store in PAEC may be important in understanding mechanisms of endothelial dysfunction in pulmonary vascular disease. The data in the current manuscript are supportive of previously published work from our laboratory describing a similar “histamine-releasable store” specific to PAEC. We are continuing to determine the precise nature of this store and to ascertain if these thrombin- and histamine-inducible stores are indeed activated via the same specific mechanism in PAEC. Thrombin and histamine have been shown to contribute in different manners to vascular endothelial barrier dysfunction, histamine in a physiological Ca2+-calmodulin dependent pathway and thrombin by a Ca2+ sensitization, potentially involving protein tyrosine phosphorylation and RhoA activation (39, 40).
Whole cell Ca2+ oscillations are generally associated with rhythmic cell contraction and vasomotion (24). In our experiments, we observed Ca2+ oscillations in response to thrombin in several PASMC studied (Figs. 6–8). The oscillations varied dramatically in frequency, but not significantly in amplitude. Oscillations could be abolished by removal of extracellular Ca2+ and by treatment with CPA, indicating that their underlying mechanism involved primarily Ca2+ influx from the extracellular space, and that their occurrence is highly regulated by reuptake of Ca2+ into the ER/SR by CPA-sensitive Ca2+-ATPase pumps. The putative link between Ca2+ influx and ER/SR refilling also indicates that store-operated Ca2+ influx via TRPC is involved. As the oscillations were observed in so few PASMC, and not in PAEC, the physiological role of the Ca2+ oscillations is unclear at this time.
Together, our results suggest that the mechanisms underlying thrombin-mediated calcium release are different in PASMC and PAEC. Thrombin causes Ca2+ release from CPA-sensitive IP3-modulated stores and Ca2+ influx from the extracellular space potentially via store-operated Ca2+ channels in PASMC; however, in PAEC, the thrombin-mediated Ca2+ release is predominantly via intracellular stores insensitive to CPA, caffeine, and ryanodine. Finally, we demonstrate that only PASMC exhibit Ca2+ oscillations in response to thrombin, ostensibly due to release-reuptake cycling within the SR. The CPA-, caffeine-, and ryanodine-insensitive Ca2+ release in the PAEC may contribute substantially to thrombin-mediated vascular injury and plays a significant role in endothelial barrier dysfunction in pulmonary diseases such as CTEPH.
GRANTS
This work is supported in part by grants from National Heart, Lung, and Blood Institute Grants HL-54043, HL-064945, and HL-66012.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem 279: 20941–20949, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bachhuber BG, Sarembock IJ, Gimple LW, McNamara CA, Owens GK. Thrombin-induced mitogenesis in cultured aortic smooth muscle cells requires prolonged thrombin exposure. Am J Physiol Cell Physiol 268: C1141–C1147, 1995. [DOI] [PubMed] [Google Scholar]
- 3.BelAiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, Hess J, Görlach A. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ Res 98: 828–836, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Belham CM, Scott PH, Twomey DP, Gould GW, Wadsworth RM, Plevin R. Evidence that thrombin-stimulated DNA synthesis in pulmonary arterial fibroblasts involves phosphatidylinositol 3-kinase-dependent p70 ribosomal S6 kinase activation. Cell Signal 9: 109–116, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Bogatcheva NV, Wang P, Birukova AA, Verin AD, Garcia JGN. Mechanism of fluoride-induced MAP kinase activation in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290: L1139–L1145, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Borbiev T, Verin AD, Shi S, Liu F, Garcia JGN. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol 280: L983–L990, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bretschneider E, Spanbroek R, Lötzer K, Habenicht AJR, Schrör K. Evidence for functionally active protease-activated receptor-3 (PAR-3) in human vascular smooth muscle cells. Thromb Haemost 90: 704–709, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bunnett NW Protease-activated receptors: how proteases signal to cells to cause inflammation and pain. Semin Thromb Hemost 32: 39–48, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Carbajal JM, Gratrix ML, Yu CH, Schaeffer RC Jr. ROCK mediates thrombin's endothelial barrier dysfunction. Am J Physiol Cell Physiol 279: C195–C204, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Carney DH, Mann R, Redin WR, Pernia SD, Berry D, Heggers JP, Hayward PG, Robson MC, Christie J, Annable C, Fenton JW, Glenn KC. Enhancement of incisional wound-healing and neovascularisation in normal rats by thrombin and synthetic thrombin receptor activating peptides. J Clin Invest 89: 1469–1477, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DMF, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267–1278, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis CA, Malik AB, Gilchrist A, Hamm H, Sandoval R, Voyno-Yasenetskaya T, Tiruppathi C. Thrombin induces proteinase-activated receptor-1 gene expression in endothelial cells via activation of Gi-linked Ras/mitogen-activated protein kinase pathway. J Biol Chem 274: 13718–13727, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara M, Jin E, Ghazizadeh M, Kawanami O. Differential expression of protease-activated receptors 1, 2, and 4 on human endothelial cells from different vascular sites. Pathobiology 71: 52–58, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW 2nd, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol 128: 96–104, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Goncharova EA, Billington CK, Irani C, Vorotnikov AV, Tkachuk VA, Penn RB, Krymskaya VP, Panettieri RA Jr. Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am J Respir Cell Mol Biol 29: 19–27, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton JR, Moffatt JD, Frauman AG, Cocks TM. Protease-activated receptor (PAR) 1 but not PAR2 or PAR4 mediates endothelium-dependent relaxation to thrombin and trypsin in human pulmonary arteries. J Cardiovasc Pharmacol 38: 108–119, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Hernández-Rodríguez NA, Cambrey AD, Chambers RC, Gray AJ, McAnulty RJ, Laurent GJ, Harrison NK, Southcott AM, duBois RM, Black CM, Scully MF. Role of thrombin in pulmonary fibrosis. Lancet 346: 1071–1073, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Johns A, Lategan TW, Lodge NJ, Ryan US, van Breemen C, Adams DJ. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell 19: 733–745, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, Griffin C, Coughlin SR. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood 102: 3224–3231, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Krymskaya VP, Penn RB, Orsini MJ, Scott PH, Plevin RJ, Walker TR, Eszterhas AJ, Amrani Y, Chilvers ER, Panettieri RA Jr. Phosphatidylinositol 3-kinase mediates mitogen-induced human airway smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 277: L65–L78, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin GD. Proteinase-activated receptors. Pharmacol Rev 53: 245–282, 2001. [PubMed] [Google Scholar]
- 22.Mathas S, Hinz M, Anagnostopoulos I, Krappmann D, Lietz A, Jundt F, Bommert K, Mechta-Grigoriou F, Stein H, Dörken B, Scheidereit C. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-κB. EMBO J 21: 4104–4113, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauban JRH, Wilkinson K, Schach C, Yuan JXJ. Histamine-mediated increase in cytosolic [Ca2+] involves different mechanisms in human pulmonary artery smooth muscle and endothelial cells. Am J Physiol Cell Physiol 290: C325–C336, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng F, To W, Kirkman-Brown J, Kumar P, Gu Y. Calcium oscillations induced by ATP in human umbilical cord smooth muscle cells. J Cell Physiol 213: 79–87, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Curr Opin Cell Biol 17: 135–140, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Moore TM, Norwood NR, Creighton JR, Babal P, Brough GH, Shasby DM, Stevens T. Receptor-dependent activation of store-operated calcium entry increases endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 279: L691–L698, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Morel NM, Petruzzo PP, Hechtman HB, Shepro D. Inflammatory agonists that increase microvascular permeability in vivo stimulate cultured pulmonary microvessel endothelial cell contraction. Inflammation 14: 571–583, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature 404: 609–613, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Ostrowska E, Reiser G. The protease-activated receptor-3 (PAR-3) can signal autonomously to induce interleukin-8 release. Cell Mol Life Sci 65: 970–981, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-α-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol 287: L1303–L1313, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Sacks RS, Agange N, Ogawa A, Yuan JXJ. Role of protease-activated receptors in pulmonary vascular remodeling. Am J Respir Crit Care Med 175: A291, 2007. [Google Scholar]
- 32.Sandoval R, Malik AB, Naqvi T, Mehta D, Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 280: L239–L247, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem 282: 7833–7843, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Sokolova E, Grishina Z, Bühling F, Welte T, Reiser G. The protease-activated receptor-1 (PAR-1) in human lung fibroblasts mediates a negative feedback downregulation via prostaglandin E2. Am J Physiol Lung Cell Mol Physiol 288: L793–L802, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Strukova SM Thrombin as a regulator of inflammation and reparative processes in tissues. Biochemistry 66: 8–18, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Tiruppathi C, Ahmmed G, Vogel S, Malik A. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 13: 693–708, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ Res 91: 70–76, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol 39: 173–185, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Van Nieuw Amerongen GP, Musters RJ, Eringa EC, Sipkema P, van Hinsbergh VW. Thrombin-induced endothelial barrier disruption in intact microvessels: role of RhoA/Rho kinase-myosin phosphatase axis. Am J Physiol Cell Physiol 294: C1234–C1241, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res 83: 1115–1123, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Weiss EJ, Hamilton JR, Lease KE, Coughlin SR. Protection against thrombosis in mice lacking PAR3. Blood 100: 3240–3244, 2002. [DOI] [PubMed] [Google Scholar]