Abstract
High tidal volume (HVT) ventilation causes pulmonary endothelial barrier dysfunction. HVT ventilation also increases lung nitric oxide (NO) and cGMP. NO contributes to HVT lung injury, but the role of cGMP is unknown. In the current study, ventilation of isolated C57BL/6 mouse lungs increased perfusate cGMP as a function of VT. Ventilation with 20 ml/kg VT for 80 min increased the filtration coefficient (Kf), an index of vascular permeability. The increased cGMP and Kf caused by HVT were attenuated by nitric oxide synthase (NOS) inhibition and in lungs from endothelial NOS knockout mice. Inhibition of soluble guanylyl cyclase (sGC) in wild-type lungs (10 μM ODQ) also blocked cGMP generation and inhibited the increase in Kf, suggesting an injurious role for sGC-derived cGMP. sGC inhibition also attenuated lung Evans blue dye albumin extravasation and wet-to-dry weight ratio in an anesthetized mouse model of HVT injury. Additional activation of sGC (1.5 μM BAY 41-2272) in isolated lungs at 40 min increased cGMP production and Kf in lungs ventilated with 15 ml/kg VT. HVT endothelial barrier dysfunction was attenuated with a nonspecific phosphodiesterase (PDE) inhibitor (100 μM IBMX) as well as an inhibitor (10 μM BAY 60-7550) specific for the cGMP-stimulated PDE2A. Concordantly, we found a VT-dependent increase in lung cAMP hydrolytic activity and PDE2A protein expression with a decrease in lung cAMP concentration that was blocked by BAY 60-7550. We conclude that HVT-induced endothelial barrier dysfunction resulted from a simultaneous increase in NO/sGC-derived cGMP and PDE2A expression causing decreased cAMP.
Keywords: nitric oxide; guanosine 3′,5′-cyclic monophosphate; phosphodiesterase 2A; adenosine 3′,5′-cyclic monophosphate; atrial natriuretic peptide; endothelial permeability
mechanical ventilation is necessary to support patients with the acute respiratory distress syndrome. However, mechanical ventilation also contributes to lung injury by a mechanism that directly correlates with tidal volume (VT) (39). High VT (HVT) ventilation causes pulmonary endothelial barrier dysfunction and edema, leading to ventilator-induced lung injury (VILI) (42). The mechanisms behind this injury are complex, but it is evident that HVT-induced changes in intracellular signaling play a significant role (12).
Recent evidence from animal models of VILI implicates nitric oxide (NO) in the pathogenesis of HVT endothelial barrier dysfunction (4, 6, 29). NO is synthesized by three isoforms of nitric oxide synthase (NOS): endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS. Ventilatory lung stretch stimulates pulmonary microvascular NO production by an increase in eNOS expression (6), phosphorylation of eNOS by phosphatidylinositol 3-kinase-induced protein kinase B activation (22), or induction of iNOS expression (13, 29). Once produced by NOS, NO reacts with reactive oxygen species (ROS) to generate peroxynitrite, a toxic free radical that contributes to VILI (29). NO can also stimulate endothelial soluble guanylyl cyclase (sGC) to generate cGMP. In fact, HVT ventilation of anesthetized rats (19) and isolated perfused rat lungs (2) caused VT-dependent increases in lung and perfusate cGMP concentrations, respectively. The effect of HVT-induced cGMP production on VILI, however, is unknown.
cGMP has several intracellular targets including cyclic nucleotide-gated ion channels, cGMP-sensitive phosphodiesterases (PDEs), and protein kinase G (PKGI). We (24) and others (9) have reported cGMP/PKGI-mediated protection against ROS-induced endothelial barrier dysfunction. Conversely, the effect of endothelial cGMP on barrier function was postulated to depend on the profile of PDE expression. In the presence of increased PDE2A, increased cGMP was shown to stimulate PDE2A-mediated hydrolysis of cAMP (35, 37). A decrease in endothelial cAMP, a barrier-protective second-messenger, is known to cause increased endothelial permeability (18).
The goal of the present study was to examine the effect of ventilatory stretch on pulmonary cGMP production and determine the role of cGMP on HVT endothelial barrier dysfunction. In isolated, perfused mouse lungs, we found that ventilatory stretch increased perfusate cGMP by generation of NO and consequent stimulation of sGC. This sGC-generated cGMP significantly contributed to HVT ventilatory stretch-induced endothelial barrier dysfunction in both isolated mouse lungs and an in vivo mouse model of VILI. The injurious effect of cGMP was only noted with sGC activation after the onset of HVT ventilation. A role for PDE2A was suggested by 1) the barrier-protective effect of PDE2A inhibition, 2) a VT-dependent increase in lung PDE2A expression, 3) a VT-dependent increase in cAMP hydrolytic activity, and 4) a VT-dependent decrease in lung cAMP content that was reversed by PDE2A inhibition.
METHODS
Animals and Reagents
Male C57BL/6 wild-type (WT) and eNOS knockout (−/−) mice (20–30 g, 8–12 wk old) were purchased from The Jackson Laboratories (Bar Harbor, ME) and maintained in a pathogen-free environment supervised by the Division of Comparative Medicine of The Johns Hopkins University. All animal protocols in this study were approved by The Johns Hopkins Institutions Animal Care and Use Committee.
Brinster's BMOC-3 media and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA). nitro-l-arginine methyl ester (l-NAME) was purchased from Calbiochem (San Diego, CA). 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ), 3-(4-amino-5-cyclopropylpyrimidin-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine (BAY 41-2272), and 2-(3,4-dimethoxybenzyl)-7-[(1R)-1-[(1R)-1-hydroxyethyl]-4-phenylbutyl]-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one (BAY 60-7550) were purchased from Alexis (Lausen, Switzerland). The cGMP analog 8-(4-chlorophenylthio)guanosine-3′,5′-cyclic monophosphate (8-pCPT-cGMP) was purchased from BIOLOG Life Science Institute (Bremen, Germany). Atrial natriuretic peptide (ANP) was purchased from Phoenix Pharmaceuticals (Burlingame, CA). [3H]l-arginine was purchased from Amersham Biosciences (Piscataway, NJ). Anti-PDE2A-112AP and anti-PDE3A rabbit antibodies were purchased from FabGennix International (Frisco, TX). Anti-iNOS antibody was purchased from Transduction Laboratories (Rockville, MD). Anti-nitrotyrosine antibody was purchased from Cayman Chemical (Ann Arbor, MI). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO). IBMX and ANP were dissolved in BMOC-3; all other drugs were dissolved in DMSO and later diluted to a final DMSO concentration of 0.2%.
Isolated, Perfused Mouse Lung Model
The ex vivo, in situ mouse lung isolation was performed as previously described (8), using the IL-1 Isolated Perfused Mouse Lung System (Harvard Apparatus, Holliston, MA). After anesthesia with intraperitoneal ketamine (150 mg/kg) and acetylpromazine (15 mg/kg), a tracheostomy was performed. The mouse was ventilated with 6 ml/kg VT with room air at 120 breaths/min with a volume-controlled ventilator (MiniVent Mouse Ventilator Type 845; Harvard Apparatus). Positive end-expiratory pressure was maintained at 3 mmHg. After sternotomy, cannulas were inserted and secured into the pulmonary artery and left atrium. The inspired gas was changed to 21% O2-5% CO2. Lungs were perfused with a constant flow of recirculating, warmed (37°C) BMOC-3 media supplemented with 3% Bovine Serum Albumin Fraction V. Perfusate flow was increased to 2.5 ml/min over 10 min. Left atrial pressure was maintained at 6 mmHg. At a point defined as time 0 of the ventilation protocol, VT was maintained at 6 ml/kg or set to 10, 15, or 20 ml/kg. For 15 and 20 ml/kg VT, ventilation was switched to a larger ventilator (Mouse Ventilator Model 687; Harvard Apparatus). Throughout the experiment, pulmonary artery pressure, left atrial pressure, airway pressure, and reservoir weight (the negative index of lung weight) were monitored and stored on a computer (PowerLab 4/30; ADInstruments, Colorado Springs, CO).
Filtration Coefficient
At the end of 80 min of protocol ventilation (0 min in sham mice), mice were returned to 6 ml/kg VT ventilation. Perfusate flow was stopped, and the left atrial outflow was clamped. Over two successive 15-min periods, the reservoir was pressurized to 10 and 15 mmHg. The rate of lung edema formation was determined from the change in reservoir weight over the last 2 min of each period. The right lung was then excised, weighed, and placed in a drying oven for later determination of dry weight (DW). From this DW, an uninjured lung wet weight (WW) was calculated using the average lung wet-to-dry weight ratio (WW/DW) obtained from a group of normal C57BL/6 mice. The filtration coefficient (Kf) was calculated from the slope of the rate of edema formation vs. vascular pressure and expressed as g·min−1·mmHg−1·100 g of uninjured lung WW−1.
Measurement of Cyclic Nucleotides
Aliquots of perfusate (500 μl) were sampled directly from the left atrial outflow and immediately frozen in liquid nitrogen. Isolated lungs were also rapidly frozen in liquid nitrogen for later analysis of cyclic nucleotide concentrations. Perfusate and lung cyclic nucleotide measurements were performed by ELISA as described in a commercially available kit (Assay Designs, Ann Arbor, MI).
In Vivo Mouse VILI Model
An intact mouse model of VILI was modified from Peng et al. (29). Briefly, 8- to 10-wk-old C57BL/6 mice (20–25 g) were anesthetized and intubated, as previously described (29). Mice were then exposed to 7 or 20 ml/kg VT at 160 breaths/min for 4 h on room air, using a volume-controlled ventilator (Inspira Advanced Safety Ventilator 557058; Harvard Apparatus). One hour before the end of the experiment, 20 mg/kg Evans blue dye albumin (EBD) was injected into the jugular vein. At the completion of ventilation, mice were killed via rapid exsanguination. Lung EBD extravasation and WW/DW were determined as previously described (29). Sham mice were anesthetized and injected with EBD without mechanical ventilation. The lungs of these mice were extracted 1 h later for determination of EBD extravasation and WW/DW.
NOS Activity Assay
Total NOS activity was measured in mouse lung homogenates from the conversion of [3H]l-arginine to [3H]l-citrulline, using a commercially available assay (Calbiochem Nitric Oxide Synthase Assay Kit; EMD Biosciences, San Diego, CA). [3H]l-citrulline concentration was measured with a liquid scintillation counter (LS 6000 SC; Beckman Coulter, Fullerton, CA). NOS activity was expressed in pmol of citrulline·min−1·g of protein−1 (20).
Gel Electrophoresis and Immunoblot Analysis
The immunoblots for PDE2A, PDE3A, iNOS, and nitrotyrosine were performed as previously described (8). Briefly, lungs were homogenized in 500 μl of cell lysis buffer (cat. 9803; Cell Signaling, Danvers, MA) with added 100× protease inhibitor cocktail (cat. P8340; Sigma-Aldrich), sodium vanadate (1 mM), and PMSF (1 mM; Sigma-Aldrich). After centrifugation, the protein concentration of the supernatant was determined (BCA protein assay kit; Pierce, Rockford, IL). For measurement of PDE2A, PDE3A, and iNOS expression, 100 μg of protein was subjected to SDS-PAGE on a 7.5% gel. For measurement of nitrotyrosine, 50 μg of protein was run on a 9% gel. The separated proteins were transferred to nitrocellulose membrane (BA-S 83, 0.2 μm), and the membrane was probed with primary antibody at a 1:500 (anti-PDE2A, anti-PDE3A, anti-iNOS) or 1:1,000 (anti-nitrotyrosine) dilution. The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin (Bio-Rad, Hercules, CA) used at 1:3,000 dilution. Bands were visualized using enhanced chemiluminescence (Amersham Biosciences) reagents and exposing the blot to X-ray film (Kodak BioMax MR). The gels were then stripped and reprobed for GAPDH to confirm equal loading. Densitometric quantification was performed using UN-SCAN-IT gel automated digitizing system software version 5.1 (Silk Scientific, Orem, UT).
Lung cAMP Hydrolytic Activity
PDE-mediated cAMP hydrolysis was measured in lung homogenates using a protocol modified from Zhu et al. (47). Snap-frozen lungs were homogenized at 4°C in a buffer containing TME (20 mM Tris·HCl, 5 mM magnesium acetate, 0.5 mM EDTA, pH 7.4), Triton X-100 (1%), ethylene glycol (20%), and protease inhibitors [10 μM Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), 2 μM leupeptin, 2 μM pepstatin A, 5 mM benzamidine, 2,000 units of aprotinin/ml]. The homogenate was centrifuged at 16,000 g for 20 min at 4°C. Supernatant cAMP-degrading PDE activity was then measured with 2 μM [3H]cAMP as substrate, as previously described (41, 47).
Protocols
Effect of VT on cGMP production.
To determine whether lung ventilatory stretch increased cGMP generation, we ventilated three groups of isolated perfused lungs for 80 min with a VT of 6, 15, or 20 ml/kg. PDE was inhibited before ventilation by the addition of IBMX (100 μM) to the perfusate. Perfusate samples were collected at 0, 40, and 80 min for measurement of cGMP concentration. To determine whether perfusate cGMP originated from NO-stimulated sGC, we performed additional experiments in isolated lungs ventilated with 15 ml/kg VT. This VT was chosen because it was intermediate on the dose-response curve of ventilatory stretch-induced cGMP production. We first examined the effects of inhibiting NO production with the NOS inhibitor l-NAME (100 μM). The involvement of eNOS in VT-mediated cGMP production was assessed by studying a group of eNOS −/− mouse lungs. The role of sGC was examined by adding the sGC inhibitor ODQ (10 μM) to WT mouse lung perfusate. This concentration of ODQ was chosen to avoid nonspecific NOS inhibition reported at higher concentrations (11).
In two other groups of lungs, we examined the consequences of further stimulating cGMP production with the direct sGC activator BAY 41-2272 (1.5 μM). In these experiments, we compared the effect of administering BAY 41-2272 10 min before initiating 15 ml/kg VT ventilation to that of adding BAY 41-2272 40 min after the start of the VT protocol. To determine the effect of particulate guanylyl cyclase (pGC) stimulation on perfusate cGMP, we administered 2.5 nM ANP to the perfusate of isolated, 15 ml/kg VT-ventilated lungs either 10 min before or 40 min after the onset of ventilation.
Influence of VT and ventilatory stretch-induced cGMP production on Kf.
The development of HVT pulmonary endothelial barrier dysfunction was determined by measurement of Kf after 0 (sham) or 80 min of ventilation with 6, 10, 15, or 20 ml/kg VT. The contribution of NOS activity to HVT endothelial barrier dysfunction was examined by the measurement of Kf after 80 min of 20 ml/kg VT ventilation in eNOS −/− lungs or WT lungs treated with l-NAME (100 μM). In some of these lungs, nitrotyrosine content was determined as an index of peroxynitrite formation. An additional group of WT lungs was treated with the inactive stereoisomer NG-nitro-d-arginine methyl ester HCl (d-NAME; 100 μM) to control for nonspecific l-NAME effects. The 20 ml/kg VT eNOS −/− Kf measurements were also compared with a group of eNOS −/− mice ventilated for 80 min with 6 ml/kg VT. The role of sGC activity on 20 ml/kg VT barrier dysfunction was determined by the administration of 10 μM ODQ to the perfusate of WT lungs. To examine the barrier effect of further augmentation of ventilatory stretch-induced cGMP production, we administered the direct sGC activator BAY 41-2272 (1.5 μM) either 10 min before or 40 min after the onset of ventilation with 6, 10, 15, or 20 ml/kg VT. To confirm that the effect of BAY 41-2272 on Kf was mediated through sGC activation, we administered BAY 41-2272 to lungs pretreated with 10 μM ODQ and ventilated with 15 ml/kg VT.
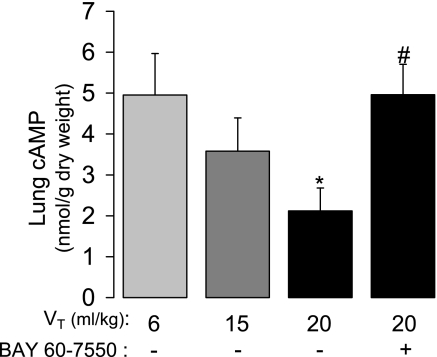
The role of PDE in HVT endothelial barrier dysfunction was determined first by the administration of a nonselective PDE inhibitor, IBMX. Kf was measured in 100 μM IBMX-perfused lungs ventilated for 80 min with either 20 ml/kg VT alone or 15 ml/kg VT with 1.5 μM BAY 41-2272 added to the perfusate at 40 min. PDE2A involvement was tested by measuring Kf in lungs ventilated for 80 min with 20 ml/kg VT after pretreatment with a PDE2A-selective inhibitor, BAY 60-7550 (10 μM) (37). To determine whether augmenting cAMP would prevent the increase in Kf caused by ventilation with 20 ml/kg VT, an additional group of lungs was pretreated with the membrane-permeable cAMP analog 8-bromo-cAMP (8-Br-cAMP; 100 μM).
To examine PDE-independent cGMP mechanisms of permeability change, we treated isolated lungs with 8-pCPT-cGMP (100 μM). This cGMP analog can activate PKGI or cGMP-gated ion channels but is incapable of either stimulating or inhibiting cyclic nucleotide-sensitive PDEs (37, 40). 8-pCPT-cGMP was given either 10 min before or 40 min after the onset of 15 ml/kg VT ventilation, mimicking the timing of the BAY 41-2272 experiments.
Effect of VT on lung PDE expression and cyclic nucleotide concentrations.
Changes in lung PDE2A and PDE3A protein expression were determined in isolated lungs subjected to 0 (sham), 40, or 80 min of ventilation with 6, 15, or 20 ml/kg VT. Lung cAMP and cGMP concentrations were measured in separate experiments after 80 min of ventilation with 6, 15, or 20 ml/kg VT. To determine whether changes in cAMP concentration resulted from PDE2A activity, cAMP was measured in an additional group of BAY 60-7550-treated lungs after 80 min of 20 ml/kg VT ventilation.
Effect of ODQ on EBD extravasation and WW/DW in intact, HVT-ventilated mice.
Intact C57BL/6 mice were subjected to 4 h of ventilation with 7 or 20 ml/kg VT. One hour before ventilation, mice received either 10 mg/kg ip ODQ or an equal volume (50 μl) of DMSO diluent. All EBD extravasation measurements were normalized to sham mice studied on the same day.
To ensure that the dose of ODQ used in our experiments did not inhibit NOS in intact mice (11), we compared NOS activity in lung homogenates from spontaneously breathing mice given either DMSO, 10 mg/kg ODQ, or 20 mg/kg ODQ. The mice were killed, and the lungs were harvested for measurement of NOS activity 30 min after drug administration.
Statistical Analysis
All time course measurements were analyzed with a two-factor (group, time) ANOVA. The Kf, lung EBD, WW/DW, lung NOS activity, lung nitrotyrosine, lung cAMP hydrolytic activity, and cyclic nucleotide content were analyzed using a randomized one-factor ANOVA, with the exception of the analysis of Kf in the early vs. late BAY 41-2272 administration comparison, which used a two-factor (group, VT) ANOVA. Least significant difference post hoc testing was used to compare individual measurements. All nonnormal data were log-converted before using the appropriate parametric test. For the comparison of experimental data collected at different times or with different diluents, data were normalized to their appropriate, contemporaneous controls. Values presented in the text are means ± SE. Differences were considered significant when P ≤ 0.05.
RESULTS
Ventilatory Stretch and Perfusate cGMP Concentration
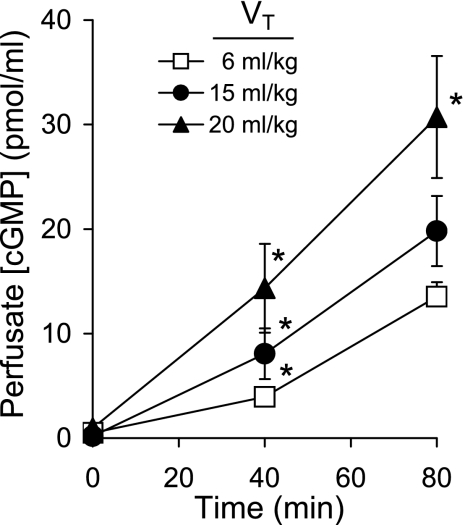
Ventilation of isolated, PDE-inhibited lungs for 80 min produced a time-dependent increase in perfusate cGMP concentration (Fig. 1). Perfusate cGMP was proportional to the magnitude of ventilatory stretch: lungs ventilated with 20 ml/kg VT had significantly (P < 0.03) greater cGMP concentrations at 40 and 80 min compared with lungs ventilated with the two lower (6, 15 ml/kg) VT. The perfusate cGMP concentrations differed between 6 and 15 ml/kg VT lungs at 40 min of ventilation (P < 0.03), but this difference was lost at 80 min.
Fig. 1.
The time course of perfusate cGMP concentration in isolated mouse lungs during 80 min of perfusion and ventilation with tidal volume (VT) of 6, 15, or 20 ml/kg (n = 8–9). Values are means ± SE. *P < 0.03 vs. cGMP concentrations from other VT groups at the same time.
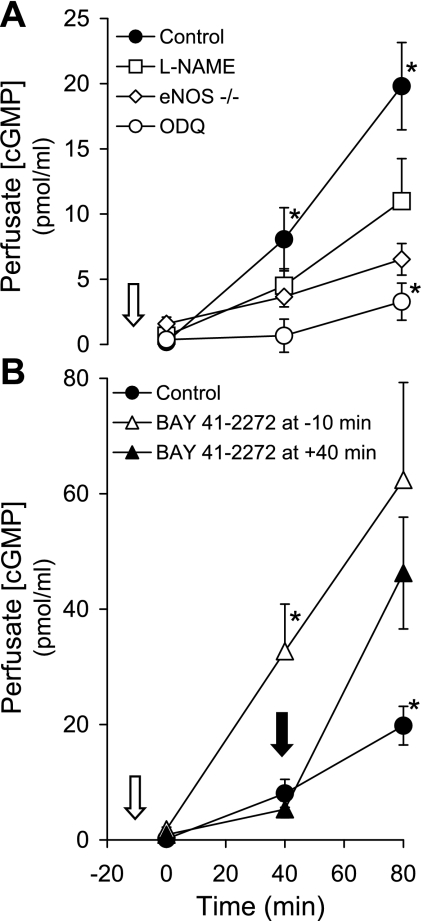
Pretreatment of isolated lungs with the NOS inhibitor l-NAME (100 μM) decreased perfusate cGMP concentration in 15 ml/kg VT-ventilated lungs (P < 0.003; Fig. 2A). Perfusate cGMP was also significantly decreased with 15 ml/kg VT ventilation of eNOS −/− lungs. Inhibition of sGC with 10 μM ODQ markedly attenuated the increase in perfusate cGMP (P < 0.003).
Fig. 2.
A: the time course of perfusate cGMP concentration in isolated lungs from wild-type (WT) and endothelial nitric oxide synthase (eNOS) knockout (−/−) mice ventilated with a VT of 15 ml/kg (n = 5–8). The WT lungs were perfused following the addition of DMSO diluent (control), nitro-l-arginine methyl ester (l-NAME; 100 μM), or 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ; 10 μM) 10 min before the initiation of the VT protocol (white arrow). Values are means ± SE. *P < 0.003 vs. cGMP concentrations from all other groups at the same time. B: the effect of adding BAY 41-2272 (1.5 μM) 10 min before (white arrow) or 40 min after (black arrow) initiation of 15 ml/kg VT ventilation on the time course of perfusate cGMP concentration in isolated mouse lungs (n = 8–10). Values are means ± SE. *P < 0.0005 vs. cGMP concentrations from other groups at the same time.
To corroborate the importance of sGC activity on ventilatory stretch-induced cGMP production, we examined the effect of the sGC activator BAY 41-2272 during ventilation with 15 ml/kg VT (Fig. 2B). Administration of 1.5 μM BAY 41-2272 10 min before HVT ventilation led to an increase in perfusate cGMP (P < 0.0005) by 40 min of ventilation. Administration of 1.5 μM BAY 41-2272 40 min after initiation of HVT ventilation resulted in a similarly increased perfusate cGMP at 80 min.
HVT Pulmonary Endothelial Barrier Dysfunction
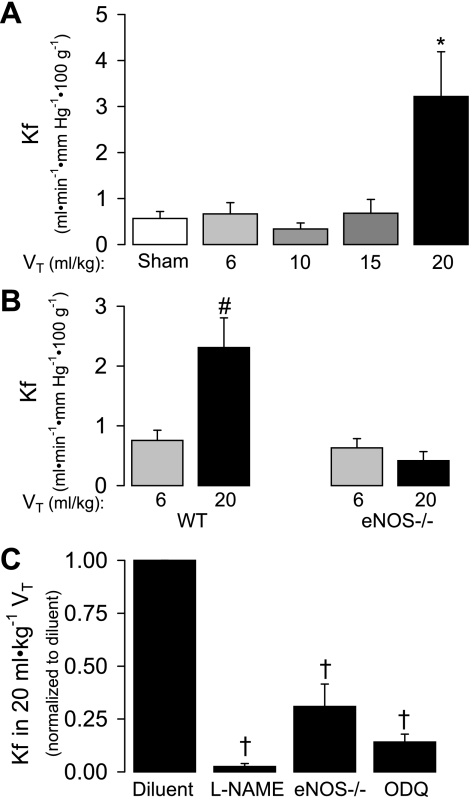
Ventilation of isolated, perfused mouse lungs with 6, 10, or 15 ml/kg VT for 80 min failed to alter Kf compared with sham lungs (Fig. 3A). Ventilation with 20 ml/kg VT, however, significantly increased Kf by 5.7-fold compared with sham (P < 0.006). We also compared the Kf in a separate group of WT mouse lungs subjected to 6 or 20 ml/kg VT to eNOS −/− lungs subjected to the same ventilation protocols. Ventilation with 20 ml/kg VT increased Kf in WT mouse lungs (P < 0.01) but not in eNOS −/− lungs (Fig. 3B). Importantly, the Kf following 6 ml/kg VT ventilation was nearly identical in WT and eNOS −/− lungs. As shown in Fig. 3C, the increased Kf in 20 ml/kg VT lungs was attenuated by inhibition of NOS with l-NAME (but not d-NAME; data not shown). The reduction of Kf of eNOS −/− lungs ventilated with 20 ml/kg VT is shown for comparison. Inhibition of sGC with ODQ also significantly decreased Kf in WT 20 ml/kg VT lungs at this time (P < 0.05; Fig. 3C).
Fig. 3.
A: the effect of VT on filtration coefficient (Kf) after 80 min of ventilation of isolated mouse lungs. A group of sham lungs, in which Kf was determined at time 0, are included for comparison (n = 4–5). Values are means ± SE. *P < 0.006 vs. all other groups. B: the effect of 6 vs. 20 ml/kg VT ventilation on Kf after 80 min in WT and eNOS −/− mouse lungs (n = 5–13). Values are means ± SE. #P < 0.01 vs. 20 ml/kg in eNOS −/− by ANOVA interaction. C: the effect of l-NAME (100 μM) or ODQ (10 μM) pretreatment on Kf in WT mouse lungs ventilated for 80 min with 20 ml/kg VT. The effect of these pharmacological inhibitors are compared with a group of diluent-treated, 20 ml/kg VT-ventilated eNOS −/− lungs (n = 4–13). Kf measurements are normalized to concurrent WT 20 ml/kg VT diluent control. Values are means ± SE. †P < 0.05 vs. diluent.
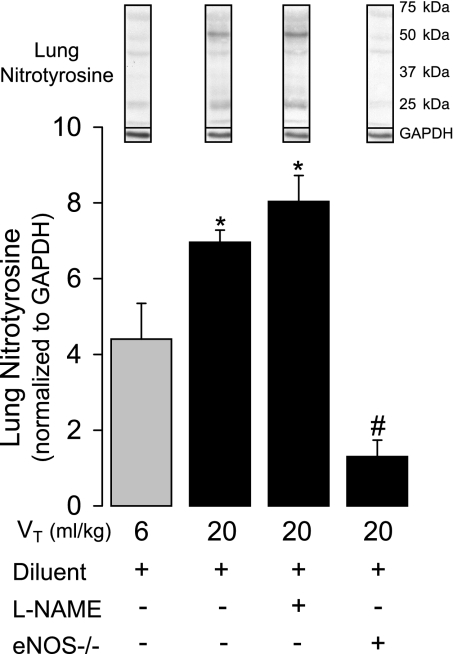
To assess the possible presence of peroxynitrite formation in the isolated lung model, we measured lung nitrotyrosine content (29) by Western blotting in lungs subjected to 6 and 20 ml/kg VT ventilation and compared the effects of l-NAME treatment in WT mice vs. lungs from eNOS −/−mice. Figure 4 shows that nitrotyrosine content significantly increased (P < 0.001) as a function of VT. l-NAME pretreatment, using the same dosage that significantly attenuated Kf, had no effect on nitrotyrosine content in 20 ml/kg VT lungs, whereas nitrotyrosine content was markedly decreased in lungs from eNOS −/−. Consistent with the significant contribution of eNOS to the nitrotyrosine signal, we found no evidence of iNOS expression by Western blot in any of the WT isolated mouse lungs (data not shown).
Fig. 4.
The effect of 6 (n = 4) vs. 20 ml/kg VT (n = 4) ventilation on lung nitrotyrosine content by Western blotting after 80 min in WT mouse lungs pretreated with diluent or l-NAME (100 μM; n = 3) and in eNOS −/− lungs (n = 3) pretreated with the same diluent. Example nitrotyrosine blots are shown with GAPDH controls. Values are means ± SE. *P < 0.003 vs. 6 ml/kg VT and eNOS −/−. #P < 0.003 vs. all other groups.
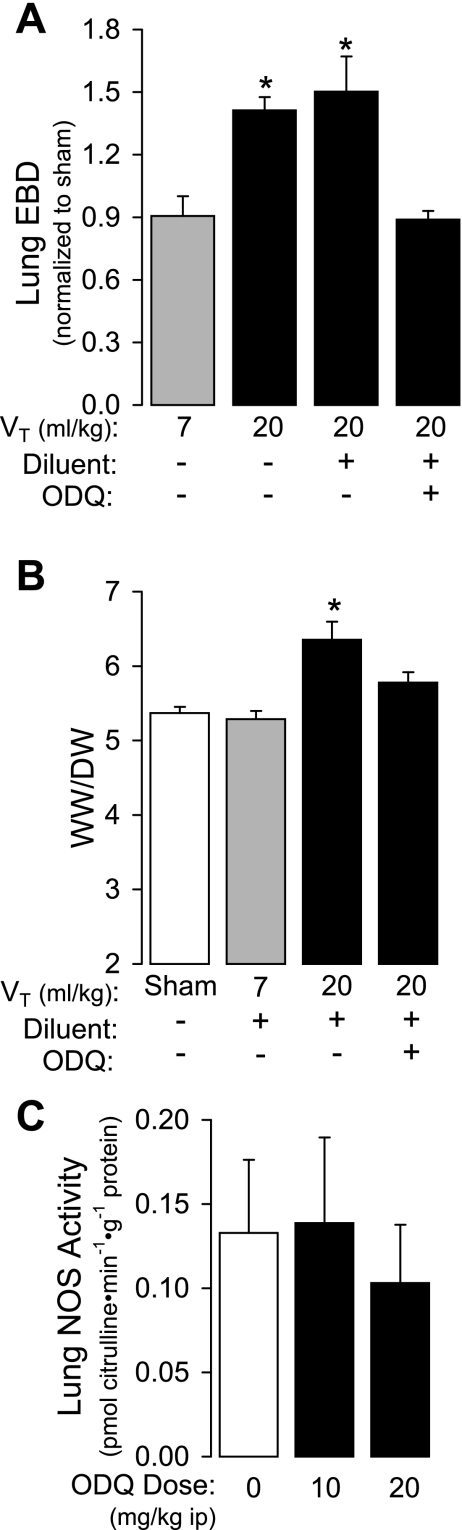
We next examined the effect of sGC inhibition in an intact mouse model of VILI. Ventilation of anesthetized mice with 20 ml/kg VT for 4 h increased EBD extravasation by 56% compared with mice ventilated with 7 ml/kg VT (P < 0.05; Fig. 5A). This increase in EBD extravasation was completely (P < 0.005) attenuated by the administration of 10 mg/kg ip ODQ 1 h before the onset of HVT ventilation. ODQ treatment also blocked the increase in lung WW/DW observed after 4 h of 20 ml/kg VT ventilation (Fig. 5B; P < 0.001). Neither 10 nor 20 mg/kg ODQ dosages altered lung NOS activity compared with diluent control mice (Fig. 5C).
Fig. 5.
A: the effect of ventilation with 7 (n = 4) or 20 ml/kg VT for 4 h without (n = 5) and with (n = 5) ODQ pretreatment (10 mg/kg ip) on lung Evans blue dye albumin (EBD) content in anesthetized C57BL/6 mice. Values are means ± SE. *P < 0.005 vs. 7 ml/kg VT and ODQ-treated 20 ml/kg VT. B: the effect of ventilation with 7 or 20 ml/kg VT (n = 5) for 4 h on wet-to-dry weight ratio (WW/DW) compared with sham-ventilated mice (n = 10). A separate group of 20 ml/kg VT lungs (n = 5) was pretreated with ODQ (10 mg/kg ip). Values are means ± SE. *P < 0.001. C: the effect of increasing ODQ dose in anesthetized mice on lung homogenate nitric oxide synthase (NOS) activity (n = 3).
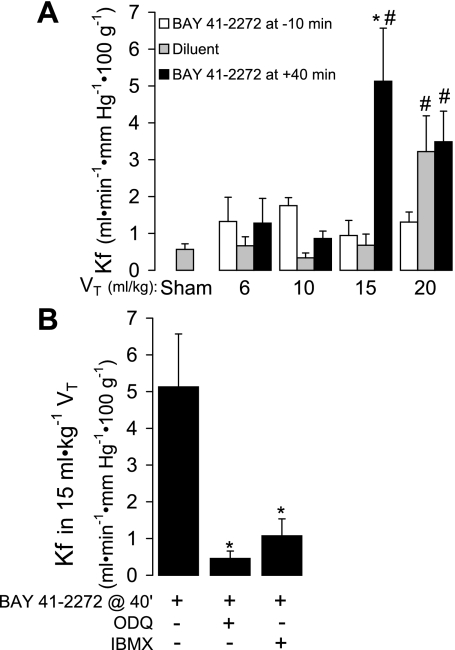
Consistent with the protective effect of NOS and sGC inhibition in HVT-ventilated lungs, additional sGC activation with 1.5 μM BAY 41-2272 after 40 min of ventilation decreased the VT threshold for barrier dysfunction (Fig. 6A). Specifically, a significant increase in Kf following 80 min of ventilation occurred in 15 ml/kg VT BAY 41-2272-treated lungs, a VT that was not injurious in diluent-treated lungs (Fig. 6A). The injurious effect of BAY 41-2772 was prevented by sGC or PDE inhibition (Fig. 6B). Interestingly, this barrier-harmful effect of sGC activation only occurred if BAY 41-2272 was added to the perfusate 40 min after the onset of ventilation. The addition of BAY 41-2272 10 min before time 0 did not cause a decrease in the VT threshold for injury. In fact, there was a trend toward endothelial barrier protection in lungs ventilated with 20 ml/kg VT and pretreated with BAY 41-2272.
Fig. 6.
A: the effect of adding BAY 41-2272 (1.5 μM) 10 min before or 40 min after initiation of 15 ml/kg VT ventilation on the Kf measured following 80 min of protocol ventilation. Sham lungs, in which Kf was measured at time 0 of ventilation, are included for comparison (n = 4–5). Values are means ± SE. *P < 0.005 vs. other 15 ml/kg VT groups; #P < 0.02 vs. corresponding values in 6 and 10 ml/kg groups. B: the effect of soluble guanylyl cyclase (sGC) inhibition with ODQ (10 μM) or phosphodiesterase (PDE) inhibition with IBMX (100 μM) on the Kf measured at 80 min in lungs treated with BAY 41-2272 (1.5 μM) 40 min (@ 40') after initiation of 15 ml/kg VT (n = 4–5). Values are means ± SE. *P < 0.006 vs. BAY 41-2272 treatment alone.
PDE and sGC-Mediated Endothelial Barrier Dysfunction
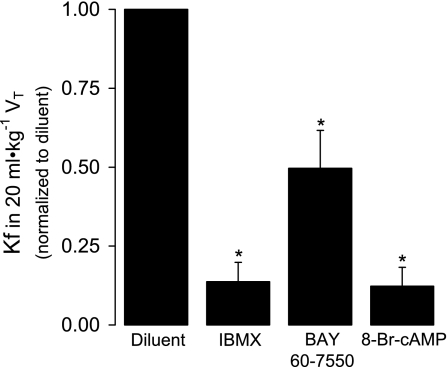
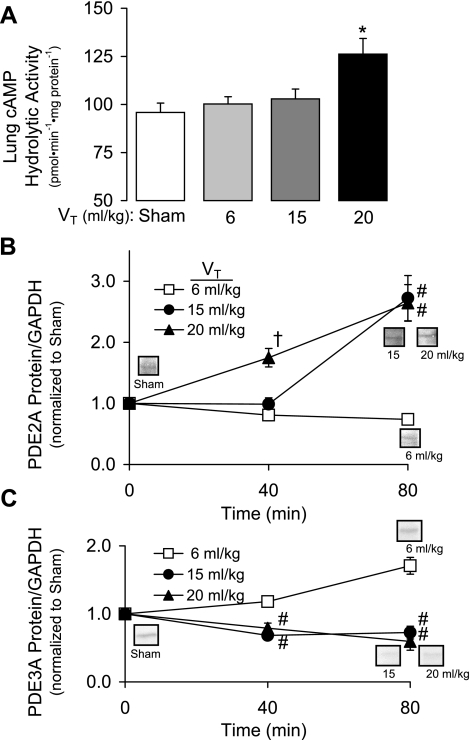
Because PDE inhibition with IBMX attenuated the injurious effect of sGC stimulation by BAY 41-2272 in 15 ml/kg VT lungs, we evaluated the effects of IBMX (100 μM) and the PDE2A inhibitor BAY 60-7550 (10 μM) on 20 ml/kg VT barrier dysfunction. We hypothesized that the protective effects of PDE inhibition would result from prevention of cAMP degradation. We therefore also examined the effect of a membrane-permeable cAMP analog on the increased Kf caused by injurious ventilation. As shown in Fig. 7, pretreatment with IBMX, BAY 60-7550, or 8-Br-cAMP (100 μM) significantly attenuated the increase in Kf (P < 0.05), suggesting a role for PDE2A and cAMP. Consistent with this, we found that increasing VT to 20 ml/kg significantly increased lung cAMP hydrolytic activity by 32% (Fig. 8A; P < 0.02). In a separate set of experiments, we demonstrated a significant VT-dependent increase in lung PDE2A protein expression (Fig. 8B; P < 0.01). Specifically, lung PDE2A expression was greater in 20 ml/kg VT lungs at 40 min compared with all other groups. By 80 min, both 15 and 20 ml/kg VT PDE2A levels were increased compared with 6 ml/kg VT lungs, which did not change compared with sham lungs. In contrast, PDE3A protein expression significantly decreased (P < 0.005) at 40 and 80 min in lungs ventilated with 15 or 20 ml/kg VT compared with 6 ml/kg lungs (Fig. 8C).
Fig. 7.
The effect of the nonspecific PDE inhibitor IBMX (100 μM), the PDE2A inhibitor BAY 60-7550 (10 μM), and the membrane permeable 8-bromo-cAMP (8-Br-cAMP; 100 μM) on Kf after 80 min of 20 ml/kg VT ventilation normalized to diluent control (n = 4–6). Values are means ± SE. *P < 0.05 vs. diluent control.
Fig. 8.
A: lung cAMP hydrolytic activity measured in lung homogenates from isolated mouse lungs ventilated with 6, 15, and 20 ml/kg VT for 80 min compared with sham-ventilated lungs (n = 4). Values are means ± SE. *P < 0.02. B: the time course of PDE2A protein expression in lungs ventilated with 6, 15, or 20 ml/kg VT for either 40 (n = 3 for each VT) or 80 min (n = 4 for each VT). PDE2A densitometry was divided by GAPDH loading control and then normalized to a group of sham lungs run on the same gel (n = 3–7). Values are means ± SE. †P < 0.01 vs. 6 and 15 ml/kg VT; #P < 0.01 vs. 6 ml/kg VT. C: the time course of PDE3A protein expression in lungs ventilated with 6, 15, or 20 ml/kg VT for either 40 (n = 3–5 for each VT) or 80 min (n = 4–6 for each VT). PDE3A densitometry was divided by GAPDH loading control and then normalized to a group of sham lungs run on the same gel (n = 3–7). Values are means ± SE. #P < 0.02 vs. 6 ml/kg VT.
To determine the impact of the VT-dependent changes in lung cAMP hydrolytic activity and PDE expression on lung cyclic nucleotide content, we measured lung cAMP and cGMP in the absence of PDE inhibition in separate experiments from those described in Fig. 8. Lung cAMP concentration significantly decreased as a function of VT, such that 20 ml/kg VT caused a 53% decrease at 80 min compared with 6 ml/kg lungs (Fig. 9). This decrease was completely blocked by inhibition of PDE2A with BAY 60-7550 administered before HVT ventilation. In the absence of PDE inhibition, lung cGMP concentrations did not significantly differ as a function of VT (6 ml/kg VT = 1.35 ± 0.48 nmol/g DW; 20 ml/kg VT = 1.15 ± 0.34 nmol/g DW; P = 0.77, n = 5–6).
Fig. 9.
The effect of VT on lung cAMP concentration after 80 min of ventilation of isolated mouse lungs without and with pretreatment with 10 μM BAY 60-7550 (n = 5–7). Values are means ± SE. *P < 0.05 vs. 6 ml/kg VT; #P < 0.05 vs. 20 ml/kg VT without BAY 60-7550 pretreatment.
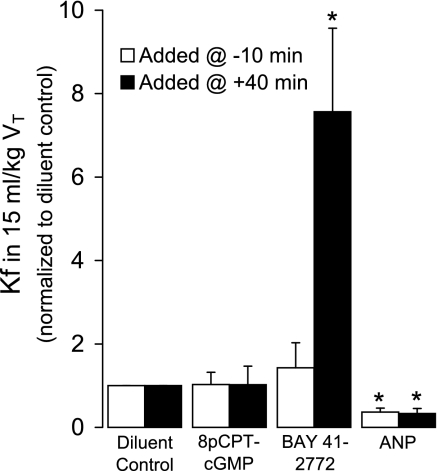
To further examine the possible role of PDE in mediating the cGMP-dependent effects of HVT ventilation, we increased endothelial cGMP with 8-pCPT-cGMP, administered 10 min before or 40 min after initiating 15 ml/kg VT ventilation. 8-pCPT-cGMP had no effect on Kf in 15 ml/kg VT ventilation regardless of the timing of administration (Fig. 10), whereas BAY 41-2272 markedly increased Kf if given at 40 min.
Fig. 10.
Effect of increasing endothelial cGMP on the 80-min Kf by adding 8-(4-chlorophenylthio)guanosine-3′,5′-cyclic monophosphate (8-pCPT-cGMP; 100 μM), BAY 41-2272 (1.5 μM), or atrial natriuretic peptide (ANP; 2.5 nM) either 10 min before or 40 min after onset of 15 ml/kg VT in isolated mouse lungs. All values in the drug-treated groups are normalized to the mean Kf observed after the addition of the appropriate diluent control (n = 4–5). Values are means ± SE. *P < 0.05 vs. all other groups at either administration time.
Interestingly, the injurious effect of sGC stimulation was not reproduced by stimulating pGC with ANP. Similar to BAY 41-2272, both early and late ANP administration markedly increased perfusate cGMP concentration in the presence of IBMX (data not shown). Unlike BAY 41-2272, however, ANP decreased Kf in 15 ml/kg VT lungs compared with diluent-treated control lungs, regardless of the timing of administration (Fig. 10; P < 0.05).
Pulmonary Artery Pressure and Perfusate Reservoir Weight Change During Perfusion
The average pulmonary artery pressure and final rate of perfusate reservoir weight loss (last 20 min) before Kf in lungs ventilated with 6, 10, 15, and 20 ml/kg VT did not differ (P > 0.05). For example, the pulmonary artery pressure and rate of reservoir weight loss in the 6 ml/kg VT group averaged 11.2 ± 0.9 mmHg and 9.1 ± 3.5 mg/min, respectively, compared with 11.0 ± 0.9 mmHg and 15.0 ± 10.2 mg/min in the 20 ml/kg VT lungs (P > 0.50; data not shown). Similarly, there were no statistically significant differences in these variables between lungs from any of the above-described treatment groups.
DISCUSSION
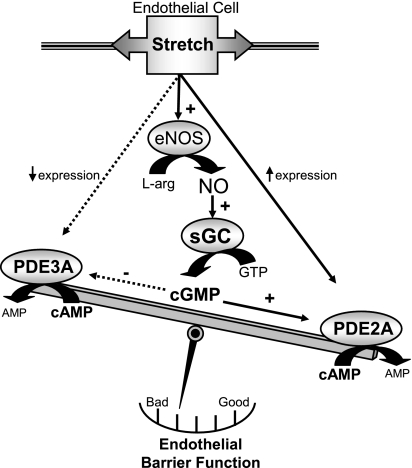
The major finding of this study was that ventilatory stretch-induced, eNOS/sGC-mediated cGMP production contributed to HVT endothelial barrier dysfunction. Additional enhancement of cGMP production by direct sGC activation decreased the VT threshold for endothelial barrier dysfunction. This injurious effect of sGC activation, however, only occurred if lungs were first subjected to 40 min of HVT ventilation. Our data suggest that HVT ventilation increased lung PDE2A, a cGMP-stimulated PDE that preferentially hydrolyzes barrier-protective cAMP, while simultaneously decreasing PDE3A, a cGMP-inhibited PDE isoform. Thus we propose that HVT simultaneously increased cGMP and PDE2A, resulting in endothelial barrier dysfunction through a PDE2A-induced decrease in barrier-protective cAMP (Fig. 11).
Fig. 11.
Proposed mechanism of high VT (HVT)-induced, cGMP-mediated endothelial barrier dysfunction. Solid arrows indicate either increased protein expression (where indicated) or activation. Dotted arrows indicate either decreased protein expression (where indicated) or inhibition. HVT ventilation increases eNOS/sGC-mediated cGMP production. At the same time, HVT increases PDE2A expression and decreases PDE3A expression. Under these circumstances, the effect of the increased cGMP from sGC is to activate PDE2A resulting in net loss of cAMP and worsened endothelial barrier function. L-arg, l-arginine; NO, nitric oxide.
Our results are compatible with recent studies that demonstrated a protective effect of NOS inhibition on the endothelial barrier dysfunction caused by HVT ventilation (4, 6, 29). The source and mechanism of NO production in VILI remains controversial. Similar to our findings, eNOS was implicated as the source for NO in an intact rat model of VILI (6). Moreover, Kuebler et al. (22) directly visualized HVT-induced capillary endothelial NO generation from eNOS in isolated mouse lungs, although the impact of eNOS on barrier function was not examined. Alternatively, increased iNOS expression was shown to contribute to NO-mediated injury in intact rat (13) and mouse (29) models of VILI. The reason for these differences is unclear, but it is likely that both NOS isoforms play a role depending on model-specific conditions. We did not detect iNOS protein expression in HVT-ventilated isolated mouse lungs over an 80-min time course, suggesting that iNOS played no role in our model of HVT-induced endothelial barrier dysfunction. We did find a significant VT-induced increase in lung nitrotyrosine by Western blotting (Fig. 4) suggesting an increase in lung peroxynitrite formation in our preparation. This signal was markedly attenuated in eNOS −/− mice further implicating eNOS as the major source of NO in this model.
The injurious effect of NO in previous studies was attributed to direct nitrosylation of proteins (13) or the production of the toxic peroxynitrite radical (29). Our study is the first to examine the role of sGC signaling in VILI, despite reports of ventilatory stretch-induced lung cGMP production (2, 19). Other studies have shown that production of endogenous endothelial cGMP or administration of membrane-permeable cGMP analogs attenuate the endothelial barrier dysfunction caused by insults other than ventilatory stretch (21, 24, 27, 28). Conversely, increased cGMP production worsened non-VILI endothelial barrier dysfunction in pulmonary (5, 32, 46) and systemic (7, 16–18) vascular beds.
To determine the role of increased lung cGMP production in VILI, we first characterized the effect of VT on cGMP generation and Kf in our isolated mouse lung model. The isolated lung preparation allowed us to directly examine these effects under controlled conditions with constant perfusate flow, temperature, and pH. Of note, we measured perfusate cGMP concentration in the presence of IBMX. cGMP is membrane-impermeable but is actively secreted by a family of membrane transporters (34). We therefore reasoned that a significant component of perfusate cGMP likely originated from the endothelial compartment. Moreover, in the presence of IBMX, secreted cGMP was shown to correlate directly with intracellular sGC activity (26, 45). Using this model, we confirmed a VT-dependent increase in lung cGMP generation (Fig. 1) consistent with previous results in intact (19) and isolated (2) rat lungs. We extended these observations by using eNOS −/− and NOS/sGC-inhibited WT mice to demonstrate that eNOS-derived NO was responsible for HVT-induced cGMP.
In the absence of IBMX, we found an increase in Kf in lungs ventilated with a VT of 20 ml/kg, suggesting increased pulmonary vascular permeability. When we treated isolated WT lungs with a NOS inhibitor or studied lungs from eNOS −/− mice, both the VT-dependent increase in cGMP concentration and Kf were attenuated (Figs. 2 and 3). These data suggested that eNOS-derived NO decreased endothelial barrier function by activating sGC to produce cGMP. Lung nitrotyrosine was also decreased in eNOS −/− (Fig. 4) suggesting a possible role for NO-derived peroxynitrite in the VT-dependent injury. However, l-NAME decreased cGMP and attenuated the increase in Kf without affecting nitrotyrosine content, indicating a primary injurious role for sGC activity in our model. This was confirmed by the attenuating effects of sGC inhibition with ODQ on cGMP and Kf in 20 ml/kg VT lungs (Fig. 3). In addition, we showed that ODQ provided protection in an intact mouse model of VILI similar to that used by Peng et al. (29), suggesting that NO in this more complex preparation was also capable of stimulating injurious cGMP production (Fig. 5, A and B). Moreover, the dose of ODQ we administered to intact mice did not affect lung NOS activity (11), supporting the specificity of ODQ as a sGC inhibitor in this model (Fig. 5C).
To provide additional support for an injurious role for sGC in our VILI model, we used the sGC activator BAY 41-2272. BAY 41-2272 directly activates sGC and strongly synergizes with NO through stabilization of the nitrosyl heme complex of sGC (10). If added 40 min after the onset of HVT ventilation, BAY 41-2272 shifted the threshold for barrier dysfunction downward to a lower VT (Fig. 6) but had no effect on the Kf in lungs ventilated with 6 or 10 ml/kg VT suggesting that this was not a nonspecific effect of the drug. This apparent endothelial barrier-harmful effect of cGMP differed from our previous data demonstrating cGMP-induced endothelial barrier protection (24, 27). In these studies, however, cGMP was increased before the induction of endothelial injury. Therefore, we wondered whether the effect of pretreatment with BAY 41-2272 10 min before initiating HVT ventilation would have a different effect on Kf. In fact, sGC activation before either 15 or 20 ml/kg VT ventilation had no injurious effect and suggested an endothelial barrier-protective trend in 20 ml/kg VT lungs (Fig. 6). The mechanism of the near-protective effect of stimulating sGC activity before the onset of injurious HVT ventilation is unknown. Our BAY 41-2272 pretreatment results are consistent with a recent report by Takenaka et al. (38) demonstrating resistance to HVT endothelial barrier dysfunction in mice overexpressing eNOS. These mice had increased lung cGMP concentrations before the initiation of HVT ventilation (38), similar to BAY 41-2272 pretreatment in our preparation.
The marked protection by IBMX and BAY 60-7550 on the adverse effects of cGMP in HVT-ventilated lungs strongly suggested a role for PDE2A. PDE2A is an endothelial PDE expressed in low levels in the normal human lung (33). It contains a regulatory binding site that, when bound with cGMP, results in stimulation of enzyme activity, leading to hydrolysis of cGMP or cAMP (3). PDE3A is also a dual-nucleotide specific endothelial PDE. In contrast to PDE2A, PDE3A-mediated hydrolysis favors cAMP and is competitively inhibited by cGMP (3). Importantly, changes in endothelial cAMP concentration are tightly linked to barrier integrity (23). Surapisitchat et al. (37) recently showed that the effect of cGMP on monolayer permeability in human umbilical vein endothelial cells (HUVEC) depended on both the concentration of cGMP and the relative amounts of PDE3A and PDE2A. Based on differences in kinetics, low concentrations of cGMP preferentially inhibited PDE3A and increased cAMP, whereas cGMP-mediated PDE2A stimulation dominated at higher cGMP levels with a resulting decrease in cAMP and barrier function. Importantly, TNF-α caused a simultaneous increase in PDE2A expression and decrease in PDE3A expression resulting in the loss of barrier protection mediated by the lower cGMP concentrations (37).
Based on this paradigm, the increased cGMP production and the observed changes in PDE2A and PDE3A protein expression in HVT-ventilated lungs (Fig. 8, B and C) likely produced conditions optimal for cGMP-mediated endothelial barrier dysfunction. These findings, coupled with the protective effect of PDE2A pharmacological inhibition in HVT lungs (Fig. 7), provide compelling evidence of PDE2A involvement in our model of VILI. Further support for this mechanism was provided by the observation of a VT-dependent increase in lung cAMP hydrolytic activity (Fig. 8A) accompanied by a decrease in lung cAMP content that could be prevented by PDE2A inhibition (Fig. 9). Of note, PDE2A inhibition returned lung cAMP concentration to baseline rather than supranormal levels suggesting that the protective effect of PDE inhibition was not due to a nonspecific increase in cyclic nucleotide levels. Indeed, the increased Kf in 20 ml/kg VT lungs was also blocked by a membrane-permeable cAMP analog (Fig. 7) providing additional evidence for the involvement of cAMP. The 8-Br-cAMP data are consistent with the protective effect of increasing cAMP with rolipram and isoproterenol in an isolated rat lung VILI model (25).
Similar to Bellamy and Tierney (2), we did not detect a VT-dependent increase in lung cGMP concentration in the absence of PDE inhibition. The constant lung cGMP concentration across VT in the face of a significant increase in PDE2A expression is consistent with the VT-dependent increase in cGMP production we found using PDE inhibition (Fig. 1). The lack of effect of the PDE-inactive 8-pCPT-cGMP on endothelial barrier function in 15 ml/kg HVT-ventilated lungs (Fig. 10) added additional support for a PDE-mediated mechanism rather than an injurious effect of PKGI (37, 40).
The mechanism of HVT-induced PDE2A upregulation is unknown at present. TNF-α increased PDE2A expression in HUVECs (35, 37) possibly through activation of p38 MAPK (35). Interestingly, VILI has been associated with both TNF-α production (44) and p38 MAPK activation (1), suggesting a possible explanation for PDE2A upregulation in HVT-ventilated lungs. The potential human use of BAY 41-2272 and other NO-independent stimulators of sGC has been proposed in several disease states (10), but our data suggest that these agents should be used with caution in conditions where PDE2A may be upregulated, such as acute lung injury requiring mechanical ventilation.
Finally, we found that the adverse effect of increased endogenous cGMP production in HVT lungs was specific for sGC. pGC stimulation resulted in barrier protection, regardless of the timing of ANP administration (Fig. 10). This suggested compartmentalization of cGMP signaling, an emerging theme in multiple cell types (14, 15, 30, 31, 36). The observation that 8-pCPT-cGMP did not mimic the effect of pGC activation suggested that a cGMP-sensitive PDE was again involved rather than PKGI. We speculate that pGC and PDE3A may reside within the same subcellular compartment in mouse pulmonary endothelial cells. Under these circumstances, increased cGMP production by pGC would result in increased cAMP and improved barrier function. A functional compartmentalization of pGC and PDE3A was suggested in atrial myocytes based on differential pGC-sGC signaling (43). Additional experiments using PDE3A inhibitors are planned to test this hypothesis.
In conclusion, we found that HVT ventilation increased cGMP production from NO-stimulated sGC, contributing to HVT endothelial barrier dysfunction. This injurious effect was associated with an increase in PDE2A protein expression and decrease in lung cAMP concentration. Both the increase in Kf and the decrease in cAMP were attenuated by specific PDE2A inhibition, suggesting a mechanism by which ventilatory stretch-induced cGMP production contributed to VILI.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-090296 (E. P. Schmidt), HL-67189 (D. B. Pearse), and HL-075236 (D. B. Pearse).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE, Kayyali US, Garcia JG, Tuder RM, Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291: L345–L353, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy PE, Tierney DF. Cyclic nucleotide concentrations in tissue and perfusate of isolated rat lung. Exp Lung Res 7: 67–76, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Broccard AF, Feihl F, Vannay C, Markert M, Hotchkiss J, Schaller MD. Effects of l-NAME and inhaled nitric oxide on ventilator-induced lung injury in isolated, perfused rabbit lungs. Crit Care Med 32: 1872–1878, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Chen LW, Hwang YC, Chen CJ, Wang JS, Chen JS, Hsu CM. Burn-induced lung damage in rat is mediated by a nitric oxide/cGMP system. Shock 20: 369–374, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med 167: 1627–1632, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Chong TJ, Victorino GP. Cyclic nucleotide second messengers (cAMP and cGMP) play a central role in signal transduction and regulation of mesenteric postcapillary fluid leak. J Trauma 59: 302–306, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Dodd-o JM, Hristopoulos ML, Kibler K, Gutkowska J, Mukaddam-Daher S, Gonzalez A, Welsh-Servinsky LE, Pearse DB. The role of natriuretic peptide receptor-A signaling in unilateral lung ischemia-reperfusion injury in the intact mouse. Am J Physiol Lung Cell Mol Physiol 294: L714–L723, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Draijer R, Atsma DE, van der LA, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res 76: 199–208, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5: 755–768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HH. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol Pharmacol 56: 243–253, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care 7: 233–241, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces NOS2 and impairs cAMP-dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol 284: L791–L798, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Green AK, Zolle O, Simpson AW. Atrial natriuretic peptide attenuates Ca2+ oscillations and modulates plasma membrane Ca2+ fluxes in rat hepatocytes. Gastroenterology 123: 1291–1303, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Hart CY, Hahn EL, Meyer DM, Burnett JC Jr, Redfield MM. Differential effects of natriuretic peptides and NO on LV function in heart failure and normal dogs. Am J Physiol Heart Circ Physiol 281: H146–H154, 2001. [DOI] [PubMed] [Google Scholar]
- 16.He P, Zeng M, Curry FE. Effect of nitric oxide synthase inhibitors on basal microvessel permeability and endothelial cell [Ca2+]i. Am J Physiol Heart Circ Physiol 273: H747–H755, 1997. [DOI] [PubMed] [Google Scholar]
- 17.He P, Zeng M, Curry FE. cGMP modulates basal and activated microvessel permeability independently of [Ca2+]i. Am J Physiol Heart Circ Physiol 274: H1865–H1874, 1998. [DOI] [PubMed] [Google Scholar]
- 18.He P, Zeng M, Curry FE. Dominant role of cAMP in regulation of microvessel permeability. Am J Physiol Heart Circ Physiol 278: H1124–H1133, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Klass DJ Lung tissue guanosine 3′,5′-monophosphate: effects of ventilation and anesthesia. J Appl Physiol 45: 487–494, 1978. [DOI] [PubMed] [Google Scholar]
- 20.Knowles RG, Salter M. Measurement of NOS activity by conversion of radiolabeled arginine to citrulline using ion-exchange separation. Methods Mol Biol 100: 67–73, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kubes P Nitric oxide affects microvascular permeability in the intact and inflamed vasculature. Microcirculation 2: 235–244, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Kuebler WM, Uhlig U, Goldmann T, Schael G, Kerem A, Exner K, Martin C, Vollmer E, Uhlig S. Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med 168: 1391–1398, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, Stephens RS, Verin AD, Tuder RM, Pearse DB. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290: L919–L930, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Parker JC Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol 89: 2241–2248, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Patel MJ, Wypij DM, Rose DA, Rimele TJ, Wiseman JS. Secretion of cyclic GMP by cultured epithelial and fibroblast cell lines in response to nitric oxide. J Pharmacol Exp Ther 273: 16–25, 1995. [PubMed] [Google Scholar]
- 27.Pearse DB, Shimoda LA, Verin AD, Bogatcheva N, Moon C, Ronnett GV, Welsh LE, Becker PM. Effect of cGMP on lung microvascular endothelial barrier dysfunction following hydrogen peroxide. Endothelium 10: 309–317, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Pearse DB, Wagner EM, Permutt S. Effect of ventilation on vascular permeability and cyclic nucleotide concentrations in ischemic sheep lungs. J Appl Physiol 86: 123–132, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JG, Hassoun PM. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med 172: 470–479, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rho EH, Perkins WJ, Lorenz RR, Warner DO, Jones KA. Differential effects of soluble and particulate guanylyl cyclase on Ca2+ sensitivity in airway smooth muscle. J Appl Physiol 92: 257–263, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Rivero-Vilches FJ, De Frutos S, Saura M, Rodriguez-Puyol D, Rodriguez-Puyol M. Differential relaxing responses to particulate or soluble guanylyl cyclase activation on endothelial cells: a mechanism dependent on PKG-Iα activation by NO/cGMP. Am J Physiol Cell Physiol 285: C891–C898, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest 115: 1666–1674, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadhu K, Hensley K, Florio VA, Wolda SL. Differential expression of the cyclic GMP-stimulated phosphodiesterase PDE2A in human venous and capillary endothelial cells. J Histochem Cytochem 47: 895–906, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Sager G Cyclic GMP transporters. Neurochem Int 45: 865–873, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Burger A, Hatzelmann A, Tenor H, Schudt C, Krull M, Schutte H, Hippenstiel S, Suttorp N. Tumor necrosis factor-α-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood 105: 3569–3576, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood) 230: 242–250, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res 101: 811–818, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Takenaka K, Nishimura Y, Nishiuma T, Sakashita A, Yamashita T, Kobayashi K, Satouchi M, Ishida T, Kawashima S, Yokoyama M. Ventilator-induced lung injury is reduced in transgenic mice that overexpress endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol 290: L1078–L1086, 2006. [DOI] [PubMed] [Google Scholar]
- 39.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung Injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Thomas MK, Francis SH, Beebe SJ, Gettys TW, Corbin JD. Partial mapping of cyclic nucleotide sites and studies of regulatory mechanisms of phosphodiesterases using cyclic nucleotide analogues. Adv Second Messenger Phosphoprotein Res 25: 45–53, 1992. [PubMed] [Google Scholar]
- 41.Thompson WJ, Terasaki WL, Epstein PM, Strada SJ. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res 10: 69–92, 1979. [PubMed] [Google Scholar]
- 42.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 110: 556–565, 1974. [DOI] [PubMed] [Google Scholar]
- 43.Wen JF, Cui X, Jin JY, Kim SM, Kim SZ, Kim SH, Lee HS, Cho KW. High and low gain switches for regulation of cAMP efflux concentration: distinct roles for particulate GC- and soluble GC-cGMP-PDE3 signaling in rabbit atria. Circ Res 94: 936–943, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Wilson MR, Choudhury S, Goddard ME, O'Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol 95: 1385–1393, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Woods M, Houslay MD. Desensitization of atriopeptin stimulated accumulation and extrusion of cyclic GMP from a kidney epithelial cell line (MDCK). Biochem Pharmacol 41: 385–394, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Zacharowski K, Berkels R, Olbrich A, Chatterjee PK, Cuzzocrea S, Foster SJ, Thiemermann C. The selective guanylate cyclase inhibitor ODQ reduces multiple organ injury in rodent models of Gram-positive and Gram-negative shock. Crit Care Med 29: 1599–1608, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Zhu B, Strada S, Stevens T. Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 289: L196–L206, 2005. [DOI] [PubMed] [Google Scholar]