Abstract
Pulmonary arterial hypertension (PAH) is a fatal disease associated with severe remodeling of the large and small pulmonary arteries. Increased accumulation of inflammatory cells and apoptosis-resistant cells are contributing factors. Proliferative apoptosis-resistant cells expressing CD133 are increased in the circulation of PAH patients. Circulating cells can contribute to tissue repair via cell fusion and heterokaryon formation. We therefore hypothesized that in the presence of increased leukocytes and CD133-positive (CD133pos) cells in PAH lung tissue, cell fusion and resulting genomic instability could account for abnormal cell proliferation and the genesis of vascular lesions. We performed analyses of CD45/CD133 localization, cell fusion, and proliferation during late-stage PAH in human lung tissue from control subjects and subjects with idiopathic (IPAH) and familial (FPAH) PAH. Localization, proliferation, and quantitation of cell populations in individual patients were performed by immunolocalization. The occurrence of cellular fusion in vascular lesions was analyzed in lung tissue by fluorescence in situ hybridization. We found the accumulation of CD45pos leukocytic cells in the tissue parenchyma and perivascular regions in PAH patients and less frequently observed myeloid cells (CD45/CD11b). CD133pos cells were detected in occlusive lesions and perivascular areas in those with PAH and were more numerous in those with IPAH lesions than in FPAH lesions. Cells coexpressing CD133 and smooth muscle α-actin were occasionally observed in occlusive lesions and perivascular areas. Proliferating cells were more prominent in IPAH lesions and colocalized with CD45 or CD133. We found no evidence of increased ploidy to suggest cell fusion. Taken together, these data suggest that abnormal lesion formation in PAH occurs in the absence of cell fusion.
Keywords: vascular remodeling, chromosomal aneusomy, chimerism, cell fusion
pulmonary arterial hypertension (PAH) is often characterized by progressive pulmonary vascular remodeling, a process that contributes to increased pulmonary vascular resistance and ultimately right ventricular failure (24, 40). The vascular remodeling includes intimal thickening and fibrosis, medial hypertrophy, and often adventitial fibroproliferative changes that can be observed at many points along the longitudinal axis of the pulmonary circulation (10, 32, 38, 45). The mechanisms contributing to the remodeling are complex and may vary depending on the specific vascular compartment or lesion and the potential stimuli involved in initiating or perpetuating the remodeling (8, 24, 29, 32, 38, 45, 58). However, it is increasingly appreciated that recruitment of inflammatory cells to the pulmonary artery wall occurs in the setting of PAH and that these cells likely play a pathogenic role in the remodeling process observed (18, 24, 32, 46, 50, 54). Despite this observation, a systemic analysis of cell accumulation in the remodeled vessels of idiopathic (IPAH) or familial (FPAH) PAH has not been performed.
Circulating peripheral blood cells have emerged as candidates for both endothelial and smooth muscle cell (SMC) accumulation in vascular diseases affecting both systemic and pulmonary circulations (1, 2, 6, 9, 18, 27, 43, 46–48, 50, 54). These circulating precursor cells, bone marrow-derived and hematopoietic in lineage, are capable of contributing to physiological tissue repair and/or pathogenic remodeling. Multipotent mesenchymal stem cells have been identified in peripheral blood (PBMSC) by the ability to form fibroblastic colonies (CFU-F) in culture (7, 21). Both PBMSC and endothelial progenitor cells share CD133 as a common marker (7, 21). They may be subfractionated based on CD45, CD34, α-smooth muscle actin (α-SMA), and CD11b expression. The fibrocyte, also a mesenchymal progenitor, forms CFU-F in culture and may be distinguished from the aforementioned populations by the simultaneous expression of leukocytic markers and α-SMA or type I collagen (1, 2, 9, 18, 54). How these cell lineages diverge or may be distinguished during disease when their programs are likely altered remains to be determined.
Infiltrating hematopoietic cells have been implicated in pulmonary vascular remodeling through mechanisms that are incompletely understood (7, 18, 54). Several possibilities exist, including recruitment of circulating cells that then engraft or localize in recipient tissues. These cells may also contribute to vascular remodeling in a paracrine or proangiogenic fashion by the release of soluble mediators. Still another possibility is fusion with resident cells that then affects cell and vascular function. Transdifferentiation and true plasticity of hematopoietic cells has been called into question due to earlier, less rigorous studies to detect fusion events (14, 17, 22, 23). Cell fusion by definition results in a heterokaryon with combined donor cell and recipient DNA. The polyploid cell often adopts the recipient cell characteristics as well as function (23, 51). Through cell fusion (as well as transdifferentiation), bone marrow-derived cells give rise to lung epithelium, cardiac myocytes, hepatocytes, Purkinje neurons, intestinal epithelium, and skeletal muscle (11, 16, 18, 22, 23, 26, 28, 30, 44, 51, 57, 60). Specifically, myeloid intermediates have been shown to fuse with skeletal muscle during regeneration (11). However, consequences of cell fusion include genetic instability, abnormal proliferation, and neoplastic transformation (28, 34). Interestingly, occlusive lesions in PAH have been likened to cancer originating from somatic mutation with disordered angiogenesis and monoclonal cell proliferation (32, 59, 61). These lesions have been noted to contain apoptosis-resistant cells. These cells have been identified not only in vascular lesions of patients with PAH, but in the circulation as well (32, 36, 61). Cytogenetic analysis to determine chromosomal content and the identification of vascular cell fusion with hematopoietic cells in lesions of human subjects with PAH has not been performed.
The purpose of this study was therefore to determine whether cells expressing cell surface markers compatible with progenitor cells accumulate in the vessels of patients with either IPAH or FPAH. Additionally, if such cells were identified, we sought to determine whether they might affect resident cell function or vascular remodeling through the process of cell fusion. Our approach was to analyze human IPAH or FPAH and non-PAH control lung tissue by analyzing small pulmonary arteries and small vessel occlusive lesions for the presence of leukocytes (CD45), CD133 cells, and a leukocytic subset, myeloid (CD45/CD11b) cells. We also performed cytogenetic analyses in lung tissue and aforementioned vessels to determine ploidy and sex-mismatched cellular chimerism. Proliferation was evaluated by quantitating Ki67 reactivity and colocalization with CD45 and CD133 or α-SMA.
METHODS
Human lung tissue specimens.
Human tissue was obtained from patients with FPAH or IPAH after approval from the Vanderbilt University and Cleveland Clinic institutional review boards. FPAH tissue specimens and their corresponding born morphogenic protein receptor 2 (BMPR2) mutation as well as IPAH specimens and patient data are presented in Table 1. These samples originated from autopsy specimens. The IPAH patients did not have genomic DNA available to screen BMPR2. Normal tissues were obtained from donor lungs not used for transplant of non-PAH lungs. Adjacent serial sections of 5 μm each were obtained from each patient for hematoxylin and eosin (H&E) staining, cytogenetic analysis, and immunostaining.
Table 1.
Patient history
| Patient | Mutation | Sex | Age, yr | Treatment |
|---|---|---|---|---|
| iPAH1 | Not tested | Female | 20 | Digoxin, Cardizem, Coumadin, and prostacyclin/Flolan |
| iPAH2 | Not tested | Female | 49 | No records available |
| iPAH3 | Not tested | Male | 34 | Prostacyclin/Flolan |
| iPAH4 | Not tested | Female | 32 | Calcium channel blockers and prostacyclin/Flolan |
| iPAH5 | Not tested | Male | 24 | Digoxin, aspirin, Coumadin, Remodulin (UT-15), and prostacyclin/Flolan with bosentan |
| fPAH1 | Exon 11 | Female | 12 | None |
| fPAH2 | Exon 3 | Female | 20 | None |
| fPAH3 | Exons 2-13 del | Female | 53 | No records available |
| fPAH4 | Exons 4 and 5 del | Female | 19 | Diltiazem, digoxin, and O2 |
| fPAH5 | Exon 8, C994T | Female | 30 | None |
| fPAH6 | Exon 3 | Female | 45 | No records available |
| fPAH7 | Exons 4 and 5 del | Female | 19 | No records available |
| fPAH8 | Exons 2-13 del | Female | 53 | None |
| Control 1 | Unk | Unknown | ||
| Control 2 | Male | Unknown/vascular remodeling | ||
| Control 3 | Female | Unknown | ||
| Control 4 | Unk | Unknown | ||
| Control 5 | Female | 61 | Tumor-free tissue | |
| Control 6 | Female | 58 | COPD/remodeling | |
| Control 7 | Male | 60 | Transplant–surgeon rejected | |
| Control 8 | Female | 36 | Transplant–surgeon rejected | |
| Control 9 | Male | 61 | COPD/remodeling |
iPAH, idiopathic pulmonary arterial hypertension; fPAH, familial pulmonary arterial hypertension; COPD, chronic obstructive pulmonary disease; Unk, unknown.
Immunochemical detection of inflammatory cells, CD45, CD133, smooth muscle, and proliferation.
Human lung tissue was evaluated by antibody staining for the presence of hematopoietic cells, identified by reactivity to the pan-leukocyte antigen CD45 (cat no. sc-25590; Santa Cruz Biotechnology, Santa Cruz, CA), vessels by staining for the presence of α-SMA (cat. no. ab5694; Abcam), myeloid cells by CD11b (cat no. 550282; BD Pharmingen), and progenitors by CD133 (cat no. sc-30219; Santa Cruz Biotechnology) or Ki67 (cat no. RM-9106-S; Lab Vision/NeoMarkers), the proliferation antigen. Controls for analyses included a secondary antibody only. Fluorescent-stained slides were photographed, quantitated, and restained with Ki67 using diaminobenzidine (DAB) detection to colocalize hematopoietic cells with proliferation. CD45, CD133, and proliferation were quantitated by scanning 35–50 random fields of view to identify a minimum of 10–12 vessels and occlusive lesions per specimen. Each was counted independently in a blinded manner using a ×40 or ×20 objective. Quantification of the parenchyma was performed using the ×40 objective. Data are presented as means ± SE. Statistical analysis was performed by conducting ANOVA followed by a t-test with Tukey adjustment using JMP Statistical software (version 5; SAS Institute, Cary, NC). Images were captured using a Nikon Eclipse 90i/DSFi-1 with NIS-Elements Imaging Software.
Cytogenetic assay and analysis of vascular cell ploidy.
Arterial lesions were identified as lesions consisting of a small, thickened, remodeled vessel associated with a terminal bronchiole. Occlusive lesions were identified as “fingerprint-like” occluded spiral vessels. Both lesion types consisted of smooth muscle, endothelial, and adventitial cells and fibroblasts. The histological lesions in each H&E section were numbered, and their microscope coordinate locations were identified. Next, each of these lesions was identified on the matched slide used for fluorescence in situ hybridization (FISH) assay. Complete analysis was conducted on 10 PAH specimens in 4–6 areas per specimen with 10–45 cells analyzed per area. In addition, 5 areas with histologically normal lung tissue and vasculature were selected and analyzed as controls for chromosomal gain and loss variability. In some areas, presence of collagen material in association with the thickened vessel walls and high level of nucleus overlapping within the lesions had decreased the number of cells that could be confidently analyzed. These problems especially affected the inner most cells of the occlusive lesions and the overlapping SMC commonly localized in the medial and adventitial regions.
To assess chromosomal aneuploidy in the lesion cells, not specific allelic gain or loss, formalin-fixed, paraffin-embedded tissue sections of pulmonary hypertension were subjected to FISH assay with the multicolor, four multitarget (4 specific loci on chromosomes 5–8) LAVysion probe (Vysis/Abbott Molecular) following a previously published protocol (28). The chromosome 6 probe is centromeric, uncoded sequences only used to assess chromosome copy number. Specific arterial and occlusive lesions were identified on the H&E-stained sections that were serial to the sections used for chromosomal analyses.
Any inconsistencies from diploid in the analysis of chromosomal markers presented may be due to simple technical limitations. In diploid cells, two copies of each target gene on specific chromosomes were expected. However, we (8) have previously shown that because the mean diameter of nuclei is greater than the sectioning width of 5 μm, truncation of the nuclei occurs and results in target copy numbers of less than two per cell even in normal samples. In these biological specimens, there were also cells undergoing division, and between growth 2 (G2) and anaphase stages of cell division, four copies of each target can be detected in a normal cell. In addition, poor hybridization efficiency can mimic loss of signals, whereas high background noise of the hybridization can mimic gain of signals. Overlapping cells and areas of fibrosis with matrix deposition were excluded from analysis due to overlapping signal or poor hybridization.
Cytogenetic assay of male cell chimerism in female PAH patient tissue.
Fluorescent in situ hybridization was performed to detect X and Y chromosomes and male-female chimerism. Formalin-fixed, paraffin-embedded, unstained sections of prostate control, three lung controls of unknown sex, three female FPAH patients with no male offspring, and three female PAH patients with male offspring were submitted to a FISH assay, as described for ploidy analysis, with the chromosomal enumeration DNA probe kit CEPX SO/CEPY SG (Abbott Molecular). The probe set consists of two DNA targets differentially labeled: satellite III DNA from Y chromosome labeled in green and α-satellite sequences of X chromosome labeled in red. Initial analysis was performed on the prostate control to verify the hybridization efficiency of the probe set, including the signal enumeration in 50 interphase nuclei. In the other specimens, first the pulmonary hypertension lesions were scanned for the presence of fluorescent signals indicating X and Y chromosomes. The entire hybridization areas were ultimately scanned for presence of Y chromosome signals in every lung specimen. Chromatin was counterstained with 4′,6′-diamidino-2-phenylindole (DAPI; 0.3 μg/ml in Vectashield Mounting Medium; Vector Laboratories). Analysis was performed on an epifluorescence microscope using single interference filters sets for blue (DAPI), green (FITC), and red (Texas red) band pass filters.
RESULTS
Localization of CD45-positive leukocyte, CD45-/CD11b-positive myeloid, and CD133-positive cells to remodeled vasculature in late-stage human PAH.
We analyzed lung tissue from human IPAH, FPAH, or control non-PAH patients using immunostaining to localize CD45, CD133, and α-SMA in the general parenchyma, in perivascular regions, and in the more general parenchyma. Quantification was performed on individual patients, and the results by group are summarized as means ± SE in Fig. 1. Results per individual patient were presented in Supplemental Tables 1 and 2 (available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). Control non-PAH tissue was analyzed as a reference for the presence of perivascular, vascular, and parenchymal CD45 or CD133. Of note, some control lung tissue was obtained from patients of advanced age with inflammatory diseases such as chronic obstructive pulmonary disease (COPD) that was remodeled and contained a high level of infiltrating cells, including erythrocytes. The data for individual patients including pathology, familial mutation, and treatment if available are presented in Table 1. Quantification of CD45 and CD133 relative to the vascular structures, identified by α-SMA to define SMC layers, was analyzed as mean ± SE and is presented in Fig. 1 and Supplemental Tables 1 and 2.
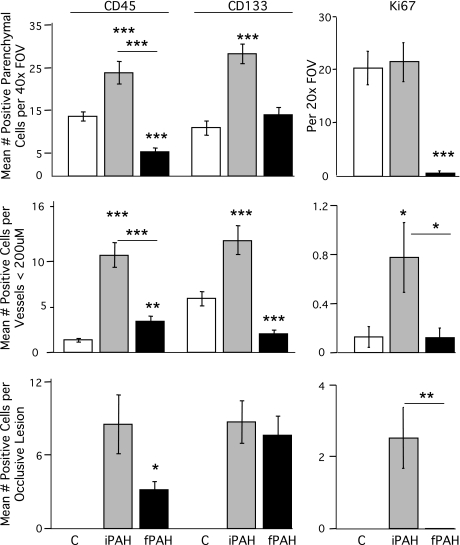
Fig. 1.
Quantification of CD45-, CD133-, and Ki67-positive cells in human pulmonary arterial hypertension (PAH) lung tissue. CD45, CD133, and Ki67 were localized in human lung tissue by antibody staining. Colocalization with vascular structures was performed by costaining with α-smooth muscle actin (α-SMA). 4′,6′-Diamidino-2-phenylindole (DAPI) was used as the nuclear stain. Thirty-five to fifty random fields of view (FOV) were scanned to identify a minimum of 10–12 vessels and occlusive lesions per specimen. Each FOV was counted in a blinded manner using a ×40 or ×20 objective. Data are presented as means ± SE counted by diagnosis. Groups were distinguished as: control (C), white; idiopathic PAH (iPAH), gray; and familial PAH (fPAH), black. *Indicates difference from control P < 0.05 unless indicated with a bar. **P < 0.01; ***P < 0.001.
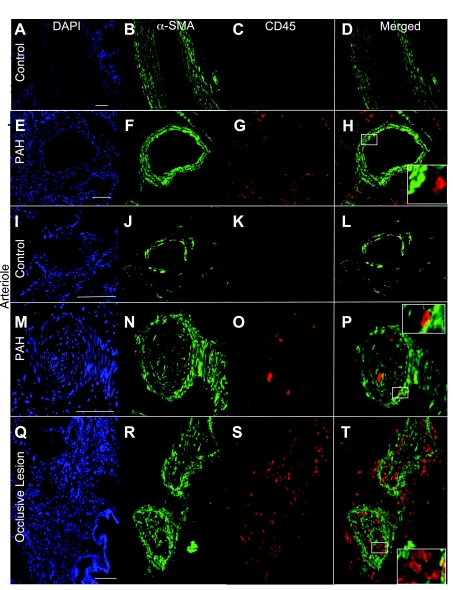
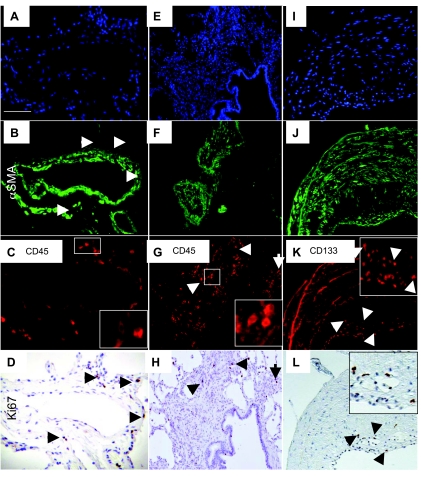
CD45 staining in control non-PAH lung tissue was localized to the tissue parenchyma and the adventitial layer of vessels (Fig. 2, A–D and I–L). Expression of CD45 in PAH patients varied depending on the vessel size and type of remodeling. In the PAH patients, CD45 staining was consistently present in the parenchyma and in perivascular areas. In the larger vessels (greater than 200 μm diameter), CD45 staining was present in the adventitia (Fig. 2, G and H). In the smaller caliber vessels (20–200 μm diameter), CD45 was present in the lumen, infrequently in the smooth muscle layers and in the adventitia (Fig. 2, M–P). Occlusive lesions often exhibited higher levels of CD45 expression than the small caliber vessels (Fig. 2, Q–T). IPAH tissue exhibited greater levels of CD45 immunolocalization than FPAH and control. Colocalization of CD45 and α-SMA was observed only as a rare event in the specimens analyzed (Fig. 2, D, H, L, P, and T). Myeloid cells were identified by colocalization of CD45 and CD11b (Supplemental Fig. 1) because they are known leukocytic subsets that participate in fusion as a means of tissue engraftment (11). Low levels of CD45-/CD11b-positive cells were detected in the large and small vessel adventitia of PAH vessels (Supplemental Fig. 1, A–H).
Fig. 2.
Leukocytes localized to occlusive lesions and areas of smooth muscle hypertrophy in human PAH lung tissue. Hematopoietic cells were identified in lung tissue by antibody staining to detect CD45 and vascular structures by α-SMA. DAPI was used as the nuclear stain. Large normal vessels and tissue (A–D) had less CD45 reactivity than the PAH vessels and parenchymal tissue (E–H). Arterioles from PAH tissue (M–P) had CD45 localized to the smooth muscle layers, whereas none was detected in the control (I–L). High levels of CD45 expression were detected in occlusive lesions (Q–T). Scale bars = 75 μm. Insets: enlarged panels of CD45 staining.
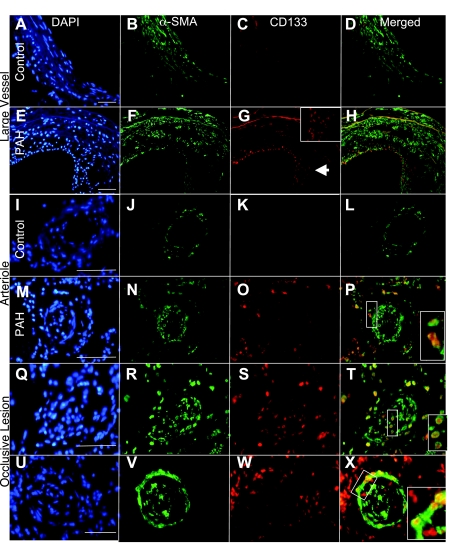
CD133-positive cells were detected at varying levels in control lung tissue (Figs. 1 and 3, A–D and I–L). In IPAH tissue, CD133 was consistently present in the lung parenchyma. In the larger vessels (greater than 200 μm diameter), CD133 staining was present in the intima of some vessels (Fig. 3, E–H). In the smaller caliber vessels (20–200 μm diameter), CD133 was present in all layers (Fig. 3, M–P) to varying degrees. Occlusive lesions exhibited high levels of CD133 expression within the lesion and in perivascular tissues (Fig. 3, Q–X). Overall, IPAH tissue had much higher levels of CD133 localization than FPAH subjects. Colocalization of CD133 and α-SMA was observed as a rare event in the specimens analyzed (Figs. 1 and 3, D, H, L, P, T, and X). These double positive cells were identified at low levels in the parenchyma, associated with small vessels and occlusive lesions in both IPAH and FPAH specimens. CD45 did not consistently colocalize with CD133 (Supplemental Fig. 2, G and H).
Fig. 3.
CD133-positive progenitor cells increased in PAH tissue. CD133-positive progenitor cells were identified in lung tissue by antibody staining to detect CD133 and vascular structures by α-SMA. DAPI was used as the nuclear stain. CD133 was not detected in association with large control vessels (A–D), arterioles (I–L), or parenchymal tissue. In contrast, large vessels from PAH patients displayed intimal remodeling and a high number of CD133-positive cells (E–H; G, inset, enlarged intimal area). Arterioles from PAH tissue (M–P) had CD133 localized both adjacent to and infiltrating the smooth muscle layers. High levels of CD133 expression were detected within and adjacent to occlusive lesions (Q–X). CD133 coexpression with α-SMA was detected and indicated by yellow in the merged panels. Scale bars = 75 μm.
Proliferation was variable in vascular lesions and associated cells.
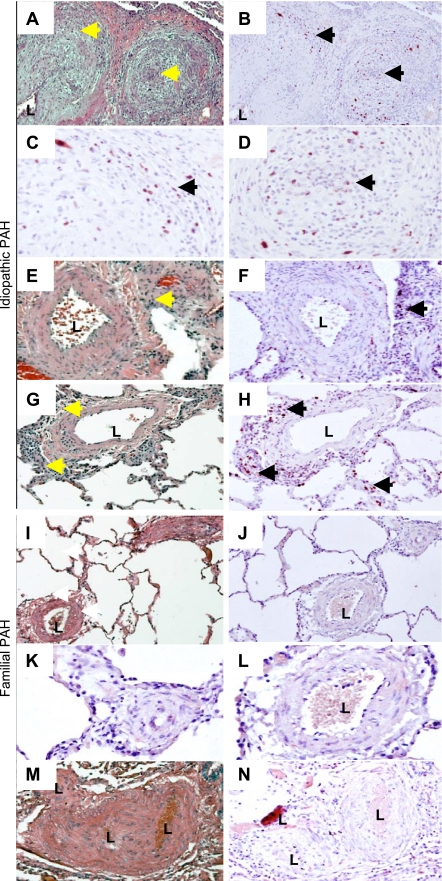
The presence of CD45- and CD133-positive cells in the PAH tissue were associated with changes in structure not present in the controls. We therefore analyzed whether the presence of these cells in the vessel adventitia affected resident cell proliferation. Abnormal proliferation of endothelial cells and SMC has been described as characteristic of PAH. Therefore, we analyzed proliferating cells in PAH and control lung tissue by immunoreactivity for Ki67 and summarized the results in Fig. 1. Ki67-positive cells were detectable in the adventitia and perivascular regions of small pulmonary arteries in IPAH tissue but largely absent in remodeled medial SMC layers (Fig. 4). The levels of proliferation varied among IPAH patients but were present in the parenchyma and both adventitial and intimal portion of lesions (Fig. 5). Very little proliferation was associated with occlusive lesions in FPAH patients. Proliferation was not a major component in the majority of vascular lesions in the PAH patients.
Fig. 4.
Proliferation was not uniformly present in PAH vascular lesions. Proliferating cells were identified in lung tissue by antibody staining to detect Ki67 with diaminobenzidine (DAB) detection (brown; arrows) with a hematoxylin counter stain (blue). Idiopathic (A–H) and familial specimens (I–N) are shown. Hematoxylin and eosin (H&E) stain of representative iPAH muscularized microvessels, intermediate sized (150 μm), and occlusive lesions (A, E, and G). Proliferation was detectable in and around iPAH occlusive lesions (A and B; C and D, enlarged), adjacent to muscularized microvessels (E and F), and adjacent to vessels and in the parenchyma (G and H). Arrows indicate similar areas between H&E and Ki67. No significant proliferation was detected in smooth muscle cell (SMC) layers of remodeled vessels less than 200 μm in diameter. I and M: H&E stain of representative fPAH muscularized microvessels and occlusive lesions. J–L and N: Ki67 immunostain. K and L: enlarged from J. L, lumen.
Fig. 5.
Ki67 reactivity associated with vascular lesions colocalized with CD45-positive cells. CD45-positive cells were identified in PAH lung tissue by antibody staining (C, G, and K), vascular structures by α-SMA (B, F, and J), and proliferating cells by Ki67 (D, H, and L). DAPI was used as the nuclear stain (A, E, and I). CD45 associated with vascular structures were photographed, and the sample was reanalyzed with Ki67 immunostain identified with DAB (brown). Proliferating cells in vascular structures and occlusive lesions colocalized with CD45 (C, D, G, and H) and in the intima of large vessels with CD133 (K and L). Insets: enlarged panels of CD45 staining.
Ploidy of cells in vascular lesions.
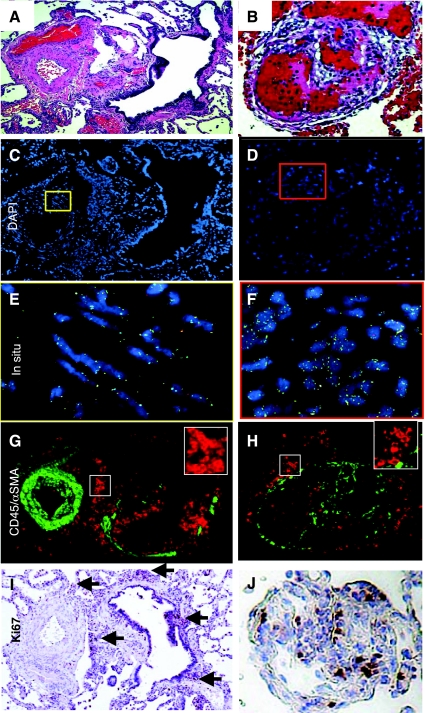
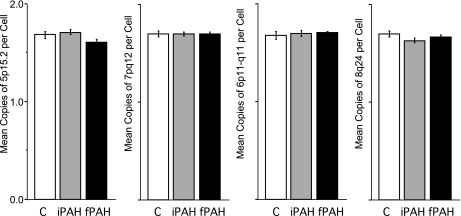
Cells can contribute to remodeling either by cell fusion as opposed to differentiation into an endothelial or smooth muscle-like cell. Fusion is detectable by measuring increased copy number of chromosomes in cells or tissue. Vascular lesions in human lung tissue specimens from control (n = 2), FPAH (n = 5), and IPAH (n = 5) were analyzed and quantified for increased chromosomal content, or ploidy, to determine whether cell fusion was present in the lesions (Fig. 7, Supplemental Tables 4 and 5). Serial sections from areas analyzed by FISH were stained with H&E (Fig. 6, A and B) or CD45 and α-SMA (Fig. 6, G and H). Representative images colocalize medial thickening (Fig. 6, C and E) or occlusion (Fig. 6, D and F) with the FISH probes illustrating the presence of diploid cells in the remodeled regions. Proliferation was not detected in the medial layers, however, many cells in the perivascular region were reactive for Ki67 (Fig. 6I, arrows). Occlusive intimal lesions contained proliferating cells, which localized to areas of CD45 expression (Fig. 6J). The statistical analysis of chromosomal ploidy for PAH patients were within the normal expected range of loss and gain determined by mean ± 2 SD. These findings indicated that cells in the arterial and occlusive lesions in specimens from all patients had normal chromosomal ploidy (Fig. 7).
Fig. 6.
Cells present in PAH vascular lesions had normal ploidy. Remodeled arterioles with SMC hypertrophy (A) and intimal occlusive lesions (B) were identified in human lung tissue specimens by H&E stain. Serial sections were then analyzed for ploidy using chromosome probe sets (C–F) and CD45 (red) and α-SMA (green) expression (G and H). Boxes in C and D were enlarged to E and F where fluorescence in situ hybridization probe sets may be visualized in the nuclei. Magnification, ×20. I and J: corresponding Ki67 stain on serial sections. Arrows point to areas of positive Ki67-stained nuclei.
Fig. 7.
Ploidy analysis of vascular lesions in human PAH lung Tissue. Specific arterial and occlusive lesions were identified on the H&E-stained sections that were serial to the sections used for chromosomal analyses. Fluorescence in situ hybridization (FISH) assay was performed with the multicolor, 4 multitarget LAVysion probes. Complete chromosomal analysis was conducted on 10 PAH specimens, 5 iPAH and 5 fPAH, in 4–6 areas per specimen with 10–45 cells analyzed per area. In addition, 5 areas with histologically “normal” lung tissue and vasculature were selected and analyzed as controls for chromosomal gain and loss variability. No significant change in ploidy content from 2 N was identified with any of the 4 probes (y-axis). Data are presented as means ± SE counted by diagnosis. Groups were distinguished as: control, white; iPAH, gray; and fPAH, black.
In diploid cells, two copies of each DNA target were expected. To investigate whether these deviations from diploidy in the PAH areas indicated chromosomal (or allelic) gain or loss, we analyzed statistical indices for each type of lesion, combining multiple lesions and distinct specimens (Supplemental Table 4). The highest and lowest mean were determined, and SD values for each target among all control and all PAH areas as well as the mean and SD for the combined control areas and the mean − 2 SD and the mean + 2 SD. Inconsistencies regarding the diploid content may occur due to technical artifacts such as nuclear truncation or overlapping or to cell cycle between G2 and anaphase stages of cell division.
Sex-mismatched chimerism in female PAH patients with male offspring.
To determine whether any cells in PAH vascular lesions were derived from circulating cells, we analyzed lung tissue of female FPAH patients who had given birth to male offspring (sex-mismatched transplant tissue would be ideal but is unavailable). The efficiency of Y chromosome detection in prostate tissue was 50–60%, within the range of detection for this technology (4). Female patients with systemic lupus erythematosus who have borne male offspring demonstrate protective chimerism in tissues undergoing remodeling and repair (31, 41). However, we did not detect any Y chromosome in the PAH tissue tested (Supplemental Fig. 3). No indication of chimerism for sex chromosomes was found.
DISCUSSION
In these studies, we sought to analyze the presence of circulating cells and ploidy of cells relative to vascular lesions in human PAH tissue to determine the likelihood that lesions had arisen as a result of progenitor fusion with resident vascular cells. We found that CD45- and CD133-positive progenitor cells were detected in greater numbers in occlusive lesions, in the adventitia of remodeled large and small arteries, and in the lung parenchyma of PAH tissue compared with control lung tissue. Our chromosomal analyses indicated that ploidy was diploid in all vascular lesions analyzed, supporting the conclusion that abnormal cell differentiation or proliferation, or secretion of paracrine factors but not cell fusion, contributes to remodeling in PAH. This is the first study to demonstrate that fusion is likely not an important contributor to pulmonary vascular remodeling in PAH.
The contribution of cells expressing CD45 to vascular lesions in PAH may derive from the circulation and/or those resident within the lung tissue. A small number of CD45-positive cells reside adjacent to the adventitial layer in the vessel wall as part of a vascular progenitor cell niche (62). An additional source of resident lung vascular endothelial progenitors with mesenchymal stem cell characteristics is the Hoechstlow lung side population of cells (25, 35). Apoptosis-resistant, proliferative circulating CD133 cells increase during human IPAH (7). As anticipated, the number of vascular-associated CD45/SMAneg cells indicative of inflammatory processes was greater in both IPAH and FPAH patients compared with control, with IPAH increased to a greater degree than FPAH. The most likely explanation for the increase in IPAH over FPAH patients was the difference in treatment regime; the FPAH patient samples were primarily from the pre-prostacyclin era, whereas the IPAH patients had almost all been treated with Flolan, which has been reported to strongly increase number of inflammatory cells (5). CD133 was detectable in PAH occlusive lesions and in the intima of large vessels. CD133/α-SMA cells were also identified and may represent a “transitional phenotype” indicative of injury or a transdifferentiation event. Transdifferentiating and migrating endothelial cells express α-SMA (14, 17). It is possible that the CD133 cells may also decrease expression of CD45 in tissue.
CD45 and CD133 cells may function in a paracrine manner regulating proliferation, inflammation, and vascular cell differentiation (3, 13, 19, 20, 49). We therefore analyzed proliferation in the PAH tissue and found Ki67 expression correlated with the numbers of CD45- and CD133-positive cells in occlusive lesions (P < 0.001 by correlation z-test) and trends toward correlation in the perivascular regions and parenchyma (P = 0.10 and 0.07, with correlations of 0.46 and 0.53, respectively). This observation may explain why the IPAH patients had the highest levels of proliferation. The aforementioned were in contrast to the α-SMA-positive SMC, which did not demonstrate any significant Ki67 expression. Because the lungs were obtained from end-stage patients with late-stage disease undergoing drug therapy, we may have missed early proliferation events contributing to the remodeling and occlusion of the vessel lumens. Along the same line, these patients with end-stage disease may be past the point where active proliferation is occurring and cells are all terminally differentiated. Alternatively, proliferation appears to localize to infiltrating cells, which may be inflammatory and influence migration or differentiation of vascular cells from other areas of tissue in the absence of significant vascular proliferation. Perhaps early-stage vascular remodeling in PAH is the result of a combination of both scenarios.
The PAH-associated increase in CD45 and CD133 cells suggested that cell-to-cell fusion events may be present (11, 16, 22, 23, 26, 28, 30, 44, 51, 57, 60) and contributing to the remodeling. We therefore analyzed ploidy in cells comprising vascular lesions in human PAH tissue because any cellular fusion event would result in at least twice the chromosomal content (4 N). Fusion can result in genetic changes that confer selective growth advantages as well as microsatellite instability, point mutations, allelic imbalance, and loss of heterozygosity (28, 33, 37–39, 42, 52, 53, 56, 61). The result of such changes are uncoupling from normal checkpoint apoptosis, germ-line mutation, or hypermethylation, which could in part explain why apoptosis-resistant cells exist in PAH. Following heterokaryon formation, chromosomal loss is random (22, 23). Therefore, the use of four unrelated chromosomal targets increased the detection efficiency for polyploidy as well as reductive division and a random chromosomal loss. We did not detect any amplification in IPAH or FPAH specimens of the genes analyzed. However, if fusion occurs, there are a few scenarios under which it may not be detected. These scenarios include loss of CD45, heterokaryon apoptosis while affecting inflammation via efferocytosis or lack thereof, and the possibility that heterokaryons may undergo reductive divisions and be therefore completely undetectable in the absence of a stable donor marker. Our studies demonstrated the chromosomal content of vascular lesions in PAH samples to be diploid suggesting any genetic instability (33, 37, 39, 42, 52, 53, 56), and proliferative advantage in the tissue was not the result of aneuploidy, which has also been demonstrated in cancer (12, 15). If a “neoplastic switch” has occurred, it did not involve genes typical to lung cancer (28, 55).
These studies do not distinguish whether the changes described are part of the central pathogenesis, a response to it, or simply a marker of disease. A more detailed characterization of the origin of cellular components in vascular lesions in PAH is necessary. Because of the complexity of the vascular lesions and limitations of studying human tissue from end-stage disease, our studies cannot determine the specific cellular mechanisms of PAH disease. Future studies using stable chimeric rodent or large animal models of PAH may provide insight into the role of hematopoietic cells and temporal proliferation in vascular lesions and are warranted to clarify this issue. Rodent models, which recapitulate vascular remodeling and intimal lesions present in human PAH disease, may be exploited as recipient hosts for labeled donor bone marrow. In parallel, human hematopoietic cells may be introduced into immunocompromised rodents, and their fates mapped. These models will provide insight into the hematopoietic lineage and paracrine roles of cells present in vascular lesions.
In conclusion, we have demonstrated that although CD45- and CD133-positive cells are present in and associated with vascular lesions in human PAH, the lesions themselves do not arise from fusion of existing vasculature with candidate infiltrating cells. The proliferation and differentiation states of the cells present in these lesions may arise as a consequence of abnormal vascular cell-to-cell interaction, transdifferentiation, as well as recruitment of circulating or local progenitors. Fusion may occur at low frequency but is not likely a contributing factor to the onset of vascular remodeling.
GRANTS
This work was funded by grants to S. Majka from the American Heart Association (SDG-0335052N), the University of Colorado Denver Department of Medicine, and the American Physiological Society Giles F. Filley Memorial Award and by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-82694 to J. West. Additional support was provided by NHLBI Grant PO1 072058 to J. E. Loyd.
Acknowledgments
Control Lung tissue was obtained from Suzy Comhair and Serpil Erzurum and the Pathobiology Lung Tissue and Cell Core (HL-081064) in the Lerner Research Institute of the Cleveland Clinic. We acknowledge technical assistance from the University of Colorado Cancer Center Cytogenetics Core [National Institutes of Health (NIH) 5 P30 CA 46934-15].
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe R, Donnelly S, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med 170: 1158–1163, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, Greer D, Pimentel J, Kolankiewicz LM, Puente N, Faradyan S, Ferland P, Bearer EL, Passero MA, Adedi M, Colvin GA, Quesenberry PJ. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells 25: 2245–2256, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anversa P, Nadal-Ginard B. Cardiac chimerism: methods matter. Circulation 106: e129–e131, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Achcar RO, Yung GL, Saffer H, Cool CD, Voelkel NF, Yi ES. Morphologic changes in explanted lungs after prostacyclin therapy for pulmonary hypertension. Eur J Med Res 11: 203–207, 2006. [PubMed] [Google Scholar]
- 6.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, Anand-Apte B, Yoder MC, Tuder RM, Erzurum SC. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 172: 615–627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berezuk M, West J, Varella-Garcia M, Franklin WA. Adjusting interphase FISH results in epithelial tissue sections to whole cell complement. Anal Quant Cytol Histol 23: 93–100, 2001. [PubMed] [Google Scholar]
- 9.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1: 71–81, 1994. [PMC free article] [PubMed] [Google Scholar]
- 10.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 109: 159–165, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 9: 1520–1527, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham JM, Shan A, Wick MJ, McDonnell SK, Schaid DJ, Tester DJ, Qian J, Takahashi S, Jenkins RB, Bostwick DG, Thibodeau SN. Allelic imbalance and microsatellite instability in prostatic adenocarcinoma. Cancer Res 56: 4475–4482, 1996. [PubMed] [Google Scholar]
- 13.Deregibus M, Cantaluppi V, Calogero R, Lolacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell-derived microvessicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110: 2440–2448, 2007. [DOI] [PubMed] [Google Scholar]
- 14.DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res 80: 444–451, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Duesberg P, Rausch C, Rasnick D, Hehlmann R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc Natl Acad Sci USA 95: 13692–13697, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunsmore S, Shapiro S. The bone marrow leaves its scar: new concepts in pulmonary fibrosis. J Clin Invest 113: 180–182, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frid M, Kale V, Stenmark K. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation. Circ Res 90: 1189–1196, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 70: 580–587, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855–1863, 2007. [DOI] [PubMed] [Google Scholar]
- 21.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells 25: 69–77, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Herzog EL, Krause DS. Engraftment of marrow-derived epithelial cells: the role of fusion. Proc Am Thorac Soc 3: 691–695, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog EL, Van Arnam J, Hu B, Zhang J, Chen Q, Haberman AM, Krause DS. Lung specific nuclear reprogramming is accompanied by heterokaryon formation and Y chromosome loss following bone marrow transplantation and secondary inflammation. FASEB J 21: 2592–2601, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13–24, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Irwin D, Helm K, Campbell N, Imamura M, Fagan K, Harral J, Carr M, Young KA, Klemm D, Gebb S, Dempsey EC, West J, Majka S. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol 293: L941–L951, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B, Shimoda K, Shimoda S, Kanemaru T, Nakamura K, Ito H, Kaji Y, Perry AC, Harada M. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J 20: 950–952, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Iwata H, Sata M. Potential contribution of bone marrow-derived precursors to vascular repair and lesion formation: lessons from animal models of vascular diseases. Front Biosci 12: 4157–4167, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen BM, Harrell JC, Jedlicka P, Borges VF, Varella-Garcia M, Horwitz KB. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res 66: 8274–8279, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Jeffery T, Wanstall J. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther 92: 1–20, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kocher A, Schuster M, Szabolcs S, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone marrow derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7: 430–436, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Kremer Hovinga IC, Koopmans M, Baelde HJ, de Heer E, Bruijn JA, Bajema IM. Tissue chimerism in systemic lupus erythematosus is related to injury. Ann Rheum Dis 66: 1568–1573, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 101: 927–934, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loyd JE Genetics and pulmonary hypertension. Chest 122, Suppl 6: 284S–286S, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Mantel CR, Guo Y, Lee MR, Kim MK, Han MK, Shibayama H, Fukuda S, Yoder MC, Pelus LM, Kim KS, Broxmeyer HE. Checkpoint-apoptosis uncoupling in human and mouse embryonic stem cells: a source of karyotpic instability. Blood 109: 4518–4527, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin J, Helm K, Ruegg P, Vaerlla-Garcia M, Burnham E, Majka S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy 10: 140–151, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548–L554, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Mei R, Galipeau PC, Prass C, Berno A, Ghandour G, Patil N, Wolff RK, Chee MS, Reid BJ, Lockhart DJ. Genome-wide detection of allelic imbalance using human SNPs and high-density DNA arrays. Genome Res 10: 1126–1137, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitani Y, Ueda M, Komatsu R, Maruyama K, Nagai R, Matsumura M, Sakurai M. Vascular smooth muscle cell phenotypes in primary pulmonary hypertension. Eur Respir J 17: 316–320, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Murname JP Telomeres and chromosomal instability. DNA Repair (Amst) 5: 1082–1092, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Nabel E New approaches to pulmonary hypertension: will therapies in mice work in humans? Circulation 101: 839–840, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Nelson JL Microchimerism: incidental byproduct of pregnancy or active participant in human health? Trends Mol Med 8: 109–113, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Niv Y Microsatellite instability and MLH1 promoter hypermethylation in colorectal cancer. World J Gastroenterol 13: 1767–1769, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peinado VI, Ramírez J, Roca J, Rodriguez-Roisin R, Barberà JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 34: 257–263, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA 103: 6321–6325, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 361: 1533–1544, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Sata M Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arterioscler Thromb Vasc Biol 26: 1008–1014, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone marrow cells are a source of donor intimal smooth muscle cells in murine aortic transplant arteriopathy. Nat Med 7: 738–741, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation 106: 1199–1204, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Takakura N, Wantanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell 102: 199–209, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka K, Sata M, Natori T, Kim-Kaneyama J, Nose K, Shibanuma M, Hirata Y, Nagai R. Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. FASEB J 22: 428–436, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416: 542–545, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Thomas AQ, Carneal J, Markin C, Lane KB, Phillips JA 3rd, Loyd JE. Allelic imbalance demonstrated by microsatellite analysis of lung samples from patients with familial pulmonary fibrosis. Chest 121, Suppl 3: 25S–26S, 2002. [PubMed] [Google Scholar]
- 53.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, Nichols WC, Morrell NW, Berg J, Manes A, McGaughran J, Pauciulo M, Wheeler L. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 345: 325–334, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Varcoe RL, Mikhail M, Guiffre AK, Pennings G, Vicaretti M, Hawthorne WJ, Fletcher JP, Medbury HJ. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost 4: 1125–1133, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Varella-Garcia M Stratification of non-small cell lung cancer patients for therapy with epidermal growth factor receptor inhibitors: the EGFR fluorescence in situ hybridization assay. Diagn Pathol 1: 1–10, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vassilakis DA, Sourvinos G, Markatos M, Psathakis K, Spandidos DA, Siafakas NM, Bouros D. Microsatellite DNA instability and loss of heterozygosity in pulmonary sarcoidosis. Am J Respir Crit Care Med 160: 1729–1733, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 422: 901–904, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Veyssier-Belot C, Cacoub P. Role of endothelial and smooth muscle cells in the pathophysiology and treatment management of pulmonary hypertension. Cardiovasc Res 44: 274–282, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 114, Suppl 3: 225–230, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422: 897–900, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Yeager ME, Heiko BS, Golpon A, Voelkel NF, Tuder RM. Microsatellite mutational analysis of endothelial cells within plexiform lesions from patients with familial, pediatric, and sporadic pulmonary hypertension. Chest 121, Suppl 3: 61S, 2002. [PubMed] [Google Scholar]
- 62.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133: 1543–1551, 2006. [DOI] [PubMed] [Google Scholar]