Abstract
Migration of airway epithelial cells (AEC) is a necessary component of airway mucosal repair after injury. The cytokine IL-1β, present in airway inflammation, has protean effects on constituent cells within the mucosa, but its effects on epithelial repair are not known. We examined migration in differentiated primary human AEC grown in air-liquid interface culture for up to 3 wk and in the 16HBE14o− cell line. Wounds were created by mechanical abrasion and followed to closure using digital microscopy. Concurrent treatment with IL-1β (≤10 ng/ml) significantly accelerated migration in primary differentiated cells and in the 16HBE14o− cell line but did not accelerate migration in primary differentiated AEC collected from asthmatic donors. IL-1β treatment did not augment phosphorylation of stress-activated protein kinases normally activated by mechanical injury, such as heat shock protein 27, ERK1/2, and JNK, and did not elicit phosphorylation of signal transducer and activator of transcription-3. However, introduction of a silencing RNA to block expression of the p65 component of NF-κB blocked IL-1β-accelerated migration substantially. Our data demonstrate that IL-1β accelerates migration of normal, but not asthmatic, differentiated AEC by a mechanism that requires activation of the NF-κB signaling complex and suggests a trophic role for this cytokine in airway epithelial repair after injury.
Keywords: epithelium, repair, asthma, inflammation, mucosa, submucosa
the airway epithelium is a target of inflammatory and physical insults in asthma. Injury to the epithelium is a common finding in pathological studies of patients with asthma, even when the clinical state of the disease is mild (3, 33). Repair of the airway mucosa after injury begins quickly, with establishment of a provisional basement membrane made up of plasma proteins (14). Epithelial cells near the wound edge then shift their phenotype and migrate into the wound region (13, 14). Once the wound region is covered by new cells, the phenotype of these cells shifts to required, more differentiated, cells (27, 28). This repair process is regulated by constitutive and inflammatory cells within the airway mucosa and submucosa.
Migration of epithelial cells in airway injury frequently will close small wounds quickly without the need for cell proliferation (14). Proliferation requires >24 h, given the cell cycle time of airway epithelium, and may be more useful in repairing large areas. Although areas of mucosal damage may be extensive in severe or fatal asthma (6), epithelial injury, as defined by bronchoscopic biopsies, is more focal in mild-to-moderate asthma (26, 44). These sites are on a scale that would be predicted to heal primarily by cell spreading and migration. Because these focal injury sites persist in asthma, defects in repair processes, such as cell migration and spreading, may exist.
Repair and remodeling of the airway mucosa after injury require the integration of multiple external signals from growth factors, cytokines, and chemokines, as well as physical stimuli. Cytokines such as IL-1β are seen in increased abundance in asthmatic airways and have protean effects on constituent and inflammatory cells (for review see Refs. 30 and 42). Although little is known about its potential effects on airway epithelial cell (AEC) repair, some clues exist from work done in other inflammation models, such as atherosclerosis. IL-1β induces migration of vascular smooth muscle cells in culture (21) and may do so via induction of α5β1-integrin expression (2). IL-1β also induces migration of endothelial cells (31), perhaps by eliciting changes in matrix receptor composition (42).
Although knowledge of the function of IL-1β on AEC, such as the induction of MUC2 (29) and MUC5AC (18, 19) gene expression, regulation of tight junction assembly (22), and secretion of other proinflammatory cytokines (1, 38), has accumulated, little is known about the ability of IL-1β to regulate AEC migration after injury. One recent study suggests that IL-1β can induce expression of metalloproteinase genes and their regulators (8), thus suggesting a potential modulatory role in migration and repair.
To examine this issue, we used two-dimensional repair models of cell lines and primary differentiated cells to evaluate whether IL-1β could elicit migration of AEC. Our data demonstrate that IL-1β is a potent motogen for normal, but not asthmatic, AEC, an effect that is modulated by NF-κB. Our data suggest that IL-1β may have trophic effects on AEC repair after injury.
MATERIALS AND METHODS
Materials.
Mouse anti-human p38 MAPK, mouse monoclonal anti-human phosphorylated p38 MAPK (clone 28B10) that recognizes the Thr180/Thr182 residues in p38 MAPK, rabbit polyclonal anti-human JNK, rabbit polyclonal anti-human phosphorylated JNK that recognizes the Thr183/Thr185 residues in JNK, rabbit polyclonal anti-human ERK (p42/44 MAPK), mouse monoclonal anti-human phosphorylated ERK (clone E10) that recognizes the Thr202/Tyr204 residues in ERK, mouse monoclonal anti-human heat shock protein 27 (HSP27, clone G31), rabbit polyclonal anti-human phosphorylated HSP27 that recognizes the Ser82 residue in HSP27, and rabbit polyclonal anti-human phosphorylated c-Jun that recognizes the Ser63 residue in c-Jun were purchased from Cell Signaling (Beverly, MA); a goat polyclonal anti-human MUC5AC to label goblet epithelial cells (catalog no. sc-16910) and a mouse monoclonal anti-human cytokeratin 5 (CK5) to label basal epithelial cells (catalog no. sc-17090) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); a rabbit polyclonal anti-human β-tubulin to label ciliated epithelial cells (catalog no. ab6046) was purchased from Abcam (Cambridge, MA); and secondary anti-mouse and anti-goat antibodies for confocal microscopy were purchased from Invitrogen (Carlsbad, CA).
Purified IL-1β was purchased from R & D Systems (Minneapolis, MN); Super Signal West Fempto Maximum Sensitivity Substrate from Pierce (Rockford, IL); bronchial epithelial cell growth medium (BEGM) and supplements (BEGM Bullet Kit) from Lonza (Rockland, ME); DMEM from Cellgro (Manassas, VA); and the JAK2/STAT3 inhibitor AG-490 (7, 12, 40) from Calbiochem (San Diego, CA). All other reagents were obtained from Sigma (St. Louis, MO) and were of the highest quality available.
Culture media.
Medium A consisted of BEGM + BEGM Bullet Kit. Medium B consisted of medium A + 1.25 μg/ml amphotericin, 100 μg/ml ceftazidime, 80 μg/ml tobramycin, 100 g/ml vancomycin, 100 U/ml penicillin G, 100 μg/ml streptomycin, 100 U/ml nystatin, and 50 μg/ml gentamicin. Medium C consisted of DMEM supplemented with 10% FCS, 100 μg/ml streptomycin, and 100 U/ml penicillin G. Air-liquid interface (ALI) medium consisted of 1:1 medium A-medium C supplemented with 130 μg/ml bovine pituitary extract, 25 ng/ml epidermal growth factor, 50 nM retinoic acid, 0.5 mg/ml low-endotoxin BSA, and 20 U/ml nystatin.
Cell culture.
The use of primary human AEC was approved by the University of Chicago Institutional Review Board. Primary cells were obtained from two sources. Cells purchased from Lonza were grown, according to instructions from the supplier, on collagen IV-coated tissue culture dishes in medium A in a 5% CO2 atmosphere at 37°C.
Epithelial cells were also collected from lungs provided by the Regional Organ Bank of Illinois (Elmhurst, IL). The lungs had been collected for use in lung transplantation but were later discarded. Information provided by the organ bank declared that the donors did not have lung disease. Mucosal membranes from central airways were dissected free and incubated in 1% type IX protease at 37°C for 2 h. Epithelial cells then were resuspended by gentle pipetting in fresh medium A. Cell suspensions were centrifuged at 460 g for 3 min at 4°C. The cells were grown on collagen IV-coated tissue culture dishes in medium B for 1–3 days in a 5% CO2 atmosphere at 37°C. The concentration of antibiotics and antimycotics in medium B was reduced progressively every day to 80, 50, 25, and, finally, 0%.
Four lungs provided by the regional organ bank had been collected from individuals documented as having chronic, persistent asthma. No clinical information about the degree of airflow obstruction, airway responsiveness, number of exacerbations, or medication use was available. Cells were collected as described for normal lungs and were kept separate in all experiments and data analysis.
At 70% confluence, cells from both sources were collected and replated onto collagen IV-coated 12-mm Transwell membranes in submersion culture for 5–7 days in ALI medium to ∼75% confluence. Then cells were changed to ALI culture conditions for an additional 7–21 days and changed every other day on the basal side only. By day 21, cells in ALI culture displayed characteristics of differentiated epithelium, and ciliated cells could be identified on phase-contrast microscopy.
The cell line 16HBE14o− (obtained from Dieter Gruenert, California Pacific Medical Center, San Francisco, CA) was grown on collagen IV-coated 6- or 12-well tissue culture plates in medium C. All cells were used when 100% confluent, and all cell lines were used before passage 30.
Wound repair assay.
The wound repair assay is described in detail elsewhere (43, 46). Briefly, confluent monolayers were washed twice and placed in serum-free or defined medium appropriate for the cells being studied. Cells from at least two different donors were used for all studies. Mediators or control diluent (DMSO) was added as appropriate. In all experiments, the concentration of DMSO was <0.1% in culture wells. After 15 min, a small wound was made in the confluent monolayer with a rubber stylet: ∼1-mm2 ovoid wounds for the 16HBE14o− cell line and ∼0.8-mm-wide linear wounds for primary cells in ALI culture on Transwell membranes. All wounds were viewed immediately after creation by phase-contrast microscopy for signs of matrix removal within the wound; wounds with evidence of significant matrix removal were discarded. Wound closure was measured serially for 24 h starting immediately after wound creation. Microscope images were photographed using a digital camera attached to a Diaphot inverted-stage microscope (Nikon, Morton Grove, IL); image resolution was 0.04 μm at ×40 total magnification.
For ovoid wounds, perimeter length and area of the remaining wound in each image were analyzed using ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD). Values were normalized to time 0 values. Intraoperator variance was <1% for 1.5-mm2 wounds, and interoperator variance was <3%.
For linear wounds, each wound was photographed edge to edge, and images were assembled using Photoshop (Adobe). Measurements were made using ImageJ software. A 200 × 200 pixel grid was overlaid onto the panel. Approximately 15–20 perpendicular lines were drawn starting at the top edge of the wound where a grid intersected and ending at the bottom edge; the mean length represented average closure. Values were normalized to time 0 values. The coefficient of variation was <20% in each panel; operator variance was similar to that for submersion culture wounds.
Western blot analysis.
Confluent monolayers were wounded using a cell rake adapted from a metal lice comb. Monolayers were pretreated with inhibitor or sham 15 min before wounding and then incubated at 37°C for 5 min–6 h. Cell lysates were collected and blotting was carried out using techniques previously described (10, 46). Membranes were reprobed with an antibody for actin (Sigma) when appropriate to control for differences in protein loading.
Examination of kinase phosphorylation by kinase bead assay.
Phosphorylated stress-activated protein kinases and selected downstream effectors were detected in protein lysates using a Bio-Plex multiplex bead suspension array system (Bio-Rad, Hercules, CA). Multiplex bead identification numbers were selected to avoid overlap, and kit instructions were followed. Bead analysis was done using a Luminex Bio-Plex 2000 machine.
Confocal microscopy.
Primary AEC grown in submersion culture or in ALI culture for 1 or 3 wk were fixed using 4% paraformaldehyde for 15 min and then stored at 4°C in PBS until use. Then cells were washed, blocked with donkey serum as appropriate, and stained with a primary antibody directed against CK5 to mark basal cells (17, 45), β-tubulin to mark ciliated cells (32), or MUC5AC to mark mucoid cells (5, 37) at appropriate concentrations overnight at 4°C. Cells were washed, and secondary antibodies directed against each were then added at 20°C for 1 h. Cells were imaged using an Olympus DSU disk scanning confocal system on an IX81 microscope platform (Olympus America, Melville, NY).
Generation of silencing hairpin RNA for NF-κB components.
Silencing hairpin RNA (shRNA) for the p65 component of NF-κB was generated on the basis of previously published sequences: 5′-ACAAGGTGCAGAAAGAGGACA-3′ targeted the gene beginning at 729 (p65-NF-κB-shRNA-LV) (11). The sequence 5′-GGATATATCCCGAACTAGACA-3′ was used as a nonspecific (NS) control (NS-shRNA-LV).
Transduction and expression of an shRNA using lentiviral infection.
Biohazard safety level 2 containment practices were strictly observed at all times for the generation of lentivirus (LV). Constructs containing the shRNA of interest were ligated into the LV vector pLentilox 3.7 using appropriate restriction enzymes, with confirmation of orientation by sequencing. The LV vector has a green fluorescent protein (GFP) encoded downstream of the shRNA driven by a separate pCMV promoter; demonstration of GFP expression indicates successful infection.
LV vectors were cotransfected with psPAX2 (generous gift of Didier Trono, Swiss Institute of Technology, Lausanne, Switzerland) and envelope protein vector pHCMV-G into the HEK-293T cell line. Cells were transfected in 11 ml of DMEM containing a total of 20 μg of DNA and 40 μl of Mirus transfection reagent. Cells were incubated at 33°C in 5% CO2 for 48 h; then supernatants containing virus were passed through 0.45-μm filters and centrifuged at 27,000 g for 6 h. The LV pellet was resuspended in 4–6 ml of DMEM, divided into aliquots, and frozen until use. Expression and toxicity of all LV supernatants were determined in preliminary experiments by serial dilution using the 16HBE14o− cell line; the dilution combining the highest expression of GFP as determined by fluorescence microscopy and the least toxicity as measured by cell lifting was chosen for infection of primary cells.
16HBE14o− cells grown in 12-well plates were infected at ∼50% confluence. LV containing vectors for the shRNA of interest, an NS shRNA, or an empty vector (LV pLL3.7-null) were used at the same time. Sham-infected cells were treated in the same manner, except for the actual introduction of virus. Cells were infected with an appropriate dilution of LV suspended in 1 ml of medium A and 8 μg/ml polybrene for 5 h at 33°C. Then cells were washed and incubated in fresh medium for 3 additional days. At that time, cells were completely confluent and expressing GFP as demonstrated by fluorescence microscopy in >75% of all cells; cultures that failed to demonstrate appreciable GFP in ≥75% of cells were discarded. Additional cells in each experiment were sham infected with LV; these cells were otherwise treated in the same manner as infected cultures and examined side-by-side with the infected cultures as an additional control. In these sham-infected cells, no GFP expression could be demonstrated. LV- and sham-infected cells were used in wound repair experiments. Cells from separate wells infected at the same time were collected for examination of p65 abundance by Western blotting.
Assay of NF-κB components in nuclear proteins.
Nuclear proteins were collected at the conclusion of experiments using a nuclear extract kit (Active Motif, Carlsbad, CA) according to the manufacturer's instructions. The p65 and p52 components of the NF-κB heterodimer were assayed in nuclear protein extracts using TransAM NF-κB ELISA kits (Active Motif) according to the manufacturer's instructions. Each kit contained an immobilized oligonucleotide specific for the NF-κB consensus site (5′-GGGACTTCC-3′). Antibodies were used to recognize p52 or p65 specifically.
Statistical analysis.
Wound repair data were referenced to time 0 wound area for each wound and are expressed as means ± SE. To avoid confounding problems with multiple analyses along the time-response curve, differences were analyzed at 24 h. Multiplex bead assay data are expressed as fold change from time 0.
Differences were examined by analysis of variance; when significant differences were found, post hoc analysis was done using Fisher's protected least significant difference test. Differences between two groups were examined by t-test. Differences were considered significant when P < 0.05.
RESULTS
Wounds were created in primary AEC and in 16HBE14o− cell monolayers without difficulty. Starting width in primary cell experiments was 0.6–1.1 mm, and the coefficient of variation was always <30% within each series of experiments. Starting area in all 16HBE14o− cell experiments was ∼0.8–1.5 mm2, and the coefficient of variation was always <25% within each series of experiments.
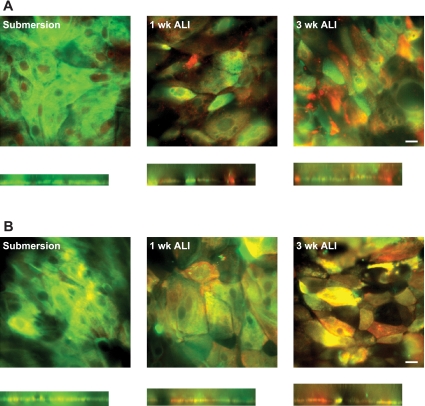
Primary cells grown in submersion culture stained positive for CK5 and almost always negative for β-tubulin and MUC5AC, as expected. As cells were grown in ALI culture over 3 wk, more cells labeling for MUC5AC or β-tubulin were seen (Fig. 1).
Fig. 1.
Confocal microscopy of cells grown in submersion culture or air-liquid interface (ALI) culture for 1 or 3 wk. Cells were labeled for the presence of cytokeratin 5 (green, basal epithelial cells; A and B), MUC5AC (red, goblet epithelial cells; A), or β-tubulin (red, ciliated epithelial cells; B). x,y Slices were taken from z stacks for each experiment. x,z Slices were reconstructed (below each x,y image); height of each x,z slice reflects relative height of the cell layer on the z plane. Note significant number of goblet and ciliated epithelial cells in cells grown for 3 wk in ALI culture. Images are representative of results from 3 experiments. Scale bars, 20 μm for all images.
Role of IL-1β in AEC migration.
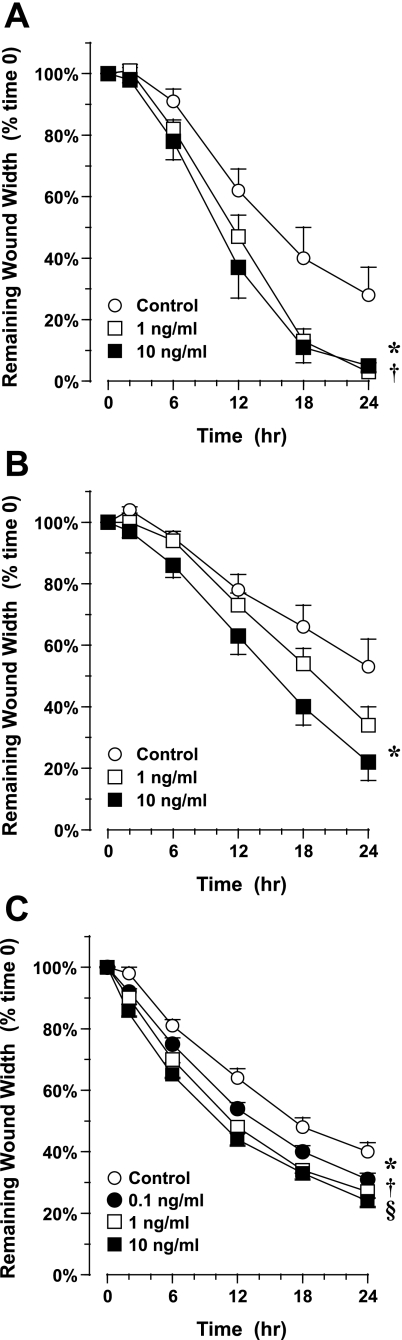
Primary AEC grown under ALI culture conditions for 1 wk migrated substantially in the first 24 h after wound generation (Fig. 2A). Treatment with 10 ng/ml IL-1β at the time of wound generation significantly accelerated migration and wound closure: after 24 h, wound width was 5 ± 2% of starting width vs. 28 ± 9% for control, untreated wounds (P = 0.02, n = 8–10 in each group). Similar results were obtained in primary AEC grown under ALI culture conditions for 3 wk, although migration to injury alone (baseline migration) was consistently slower than in 1-wk ALI cells. Treatment with 10 ng/ml IL-1β at the time of wound generation significantly accelerated migration and wound closure: after 24 h, wound width was 22 ± 6% of starting width vs. 53 ± 9% for control, untreated wounds (P = 0.007, n = 7–10 in each group; Fig. 2B).
Fig. 2.
Migration of airway epithelial cells (AEC) in response to IL-1β. Cells were injured using a rubber stylet, and migration was followed for 24 h by digital photomicroscopy. A: migration of primary AEC grown under ALI culture conditions for 1 wk. Treatment with 1 or 10 ng/ml IL-1β at the time of injury accelerated migration. Values are means ± SE (n = 8–10 experiments in cells collected from 3 normal donors). *P = 0.02; † P = 0.01 vs. control. B: migration of primary AEC grown under ALI culture conditions for 3 wk. Cells were injured and treated with 1 or 10 ng/ml IL-1β as described in A. Treatment with 10 ng/ml IL-1β at the time of injury accelerated migration. Values are means ± SE (n = 10 experiments in cells collected from 3 normal donors). *P = 0.01 vs. control. C: migration of 16HBE14o− cells in response to IL-1β. Confluent monolayers in submersion culture were injured with a rubber stylet. Treatment with 0.1–10 ng/ml IL-1β at the time of injury accelerated migration. Values are means ± SE (n = 8 experiments). *P = 0.01; †P = 0.0003; §P = 0.0001 vs. control.
A concentration response also was seen in the 16HBE14o− cell line after addition of IL-1β. In these experiments, concurrent treatment with 0.1–10 ng/ml IL-1β also accelerated closure significantly: after 24 h, wound area was 24 ± 2% of starting area for a wound treated with 10 ng/ml IL-1β vs. 40 ± 3% for control, untreated wounds (P < 0.0001, n = 8 in each group; Fig. 2C).
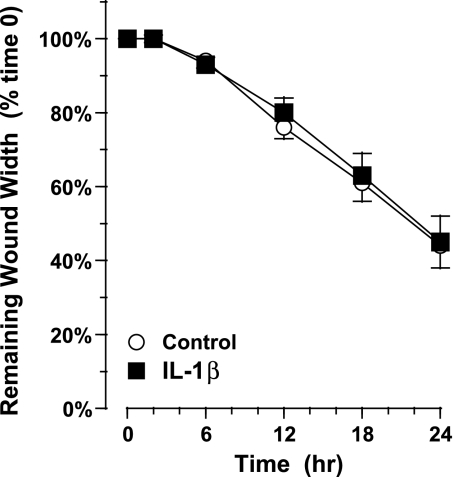
In contrast to normal, differentiated AEC, IL-1β did not accelerate migration in AEC obtained from four asthmatic subjects grown in ALI culture for 3 wk. Treatment with 10 ng/ml IL-1β at the time of wound generation did not accelerate migration and wound closure: after 24 h, wound width was 45 ± 7% of starting width (n = 18) vs. 44 ± 7% (n = 14) for control, untreated wounds [not significant; Fig. 3].
Fig. 3.
Migration of primary AEC collected from 4 asthmatic subjects and grown under ALI culture conditions for 3 wk. Treatment with 10 ng/ml IL-1β at the time of injury did not accelerate migration significantly compared with control. Values are means ± SE (n = 12 wounds for IL-1β treatment and 14 for control in cells collected from 4 asthmatic donors).
Phosphorylation of MAPKs in migrating cells after treatment with IL-1β.
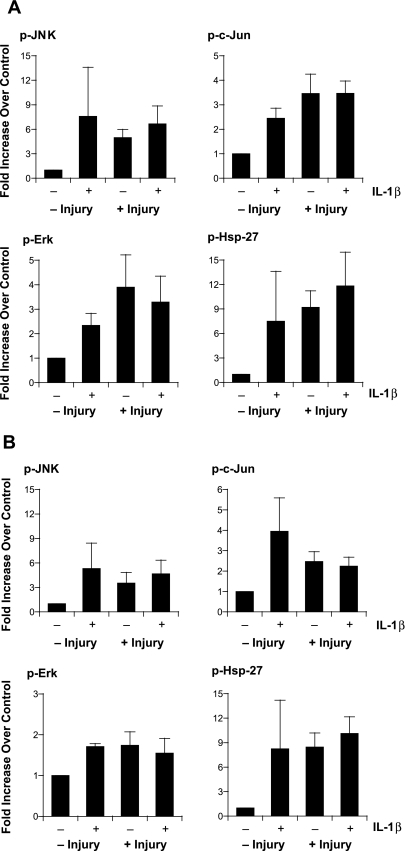
We previously demonstrated that several stress-activated protein kinases, including p38 MAPK, JNK, and ERK1/2, are phosphorylated within 15–30 min of injury in the 16HBE14o− cell line and that this leads to subsequent phosphorylation of HSP27 (46). Inasmuch as IL-1β can activate p38 (21), we asked whether phosphorylation of this kinase or others was augmented in the presence of IL-1β in the initial response to injury. In these experiments, multiple wound edges were generated in primary cells grown in ALI culture for 3 wk, and 10 ng/ml IL-1β was added immediately thereafter. Protein lysates were collected 15 or 30 min after wound generation, and the presence of phosphorylated p38 MAPK, JNK, and ERK1/2 was assessed by multiplex bead assay. Using this assay, we also examined phosphorylation of a downstream effector of p38 MAPK, HSP27, and of a downstream effector of JNK, c-Jun. In contrast to previous data from cell lines, phosphorylation of p38 MAPK could not be demonstrated after rake wound or with the addition of IL-1β (Fig. 4). However, substantial phosphorylation of HSP27, JNK, c-Jun, and ERK1/2 was noted 15 min (Fig. 4A) and 30 min (Fig. 4B) after injury alone and after treatment with IL-1β. The combination of injury and IL-1β did not lead to additive phosphorylation. These experiments suggested that accelerated migration elicited by IL-1β was not due to activation of any of the kinases examined.
Fig. 4.
Phosphorylation of mitogen-activated protein kinases in AEC after injury and treatment with IL-1β. Cells were injured and treated immediately with 10 ng/ml IL-1β or control vehicle only for 15 (A) or 30 (B) min. Cell lysates were collected and analyzed for the indicated kinase [phosphorylated c-JNK (p-JNK), c-Jun (p-c-Jun), ERK (p-ERK), and heat shock protein 27 (p-HSP27)] by multiplex bead assay. Values (means ± SE) are referenced to control (no injury) as fold increase (n = 5 in each group).
Activation of NF-κB in migrating cells after treatment with IL-1β.
One potential effector pathway for IL-1β is activation of NF-κB via a pathway that binds the interleukin-1 receptor subunit 1 to the myeloid differentiation factor 88 adapter protein, which recruits IL-1 receptor-associated kinase family members, with the eventual recruitment of the transforming growth factor-associated kinase-1 and phosphorylation of the IκB kinase complex (for review see Ref. 16).
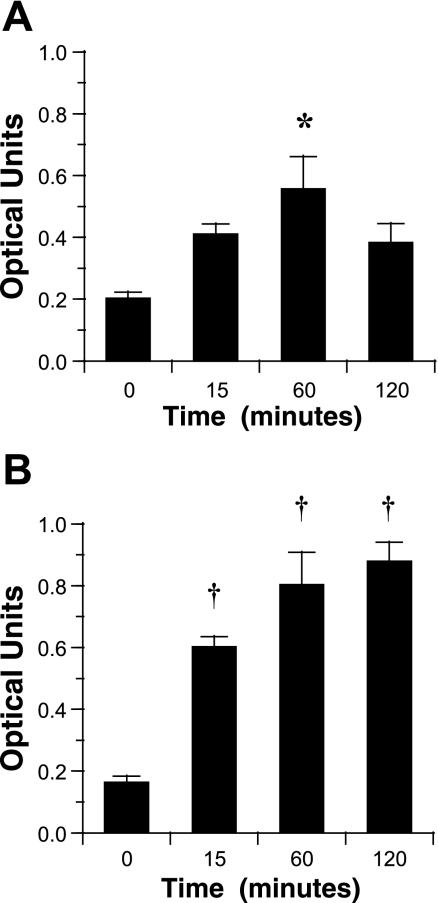
We first examined whether IL-1β treatment elicited NF-κB activation, as demonstrated by translocation of the p65 component, part of the classical pathway NF-κB heterodimer containing p50, and translocation of the p52 component that combines with p50 in the alternative pathway to the nucleus (16). In these experiments, primary cells grown in ALI culture for 3 wk (n = 3) or 16HBE14o− cells (n = 6) were treated with 10 ng/ml IL-1β for 15–120 min, and then nuclear protein extracts were collected for assay. Translocation of the p65-containing NF-κB to the nucleus could be demonstrated within 15 min of treatment and was maximal at 60–120 min for primary cells (Fig. 5A) and the 16HBE14o− cell line (Fig. 5B). In contrast, translocation of the p52-containing NF-κB could not be demonstrated after IL-1β treatment in primary cells or the cell line (data not shown). In additional experiments, wounding alone did not elicit significant translocation of p65-NF-κB over 2 h (data not shown).
Fig. 5.
Translocation of p65-containing NF-κB to the nucleus in response to IL-1β. Cells were treated with control or 10 ng/ml IL-1β for up to 120 min, and nuclear extracts were assayed for p65-containing NF-κB by ELISA. A: response in primary cells grown in ALI culture for 3 wk. Values are means ± SE (n = 3 collected from 3 normal donors). B: response in the 16HBE14o− cell line. Values are means ± SE (n = 6). *P = 0.05; † P = 0.01 vs. time 0.
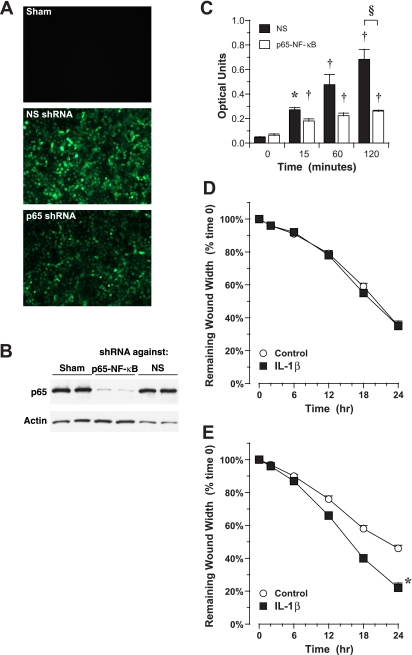
We then examined whether NF-κB was involved in transducing the migration signal elicited by IL-1β. To avoid the potential negative side effects of pharmacological inhibitors, we tested whether silencing the p65 component of NF-κB would block IL-1β-induced migration in epithelial cells. To avoid potential problems on growth and differentiation of long-term silencing of p65 in differentiated AEC in ALI culture over a 3-wk period, experiments were done using the 16HBE14o− cell line. Cells were infected with LV containing an shRNA directed against p65-NF-κB or a nonspecific shRNA and used 72 h later. In these cells, expression of GFP as a marker of successful LV infection was noted within 48 h (Fig. 6A ). Expression of p65-NF-κB protein in cells infected with p65-NF-κB-shRNA-LV was reduced by ∼90% compared with cells infected with NS-shRNA-LV or sham-infected cells (Fig. 6B). Similarly, translocation of p65-NF-κB to the nucleus was substantially reduced 120 min after treatment with 10 ng/ml IL-1β in cells infected with p65-NF-κB-shRNA-LV compared with cells infected with NS-shRNA-LV (Fig. 6C). Silencing p65 expression substantially attenuated the migration response to IL-1β in the 16HBE14o− cell line. In cells infected with p65-NF-κB-shRNA-LV, treatment with 10 ng/ml IL-1β did not accelerate migration (Fig. 6D), whereas similar treatment in cells infected with NS-shRNA-LV, accelerated migration substantially (Fig. 6E). These data demonstrated clearly the role of NF-κB in transducing the IL-1β signal in AEC migration.
Fig. 6.
Migration of 16HBE14o− cell line in response to IL-1β: effect of silencing p65-NF-κB. A: expression of green fluorescent protein (GFP) as a marker for successful infection with lentivirus (LV) containing silencing hairpin RNA (shRNA). Cells were infected with LV pLL3.7-shRNA-p65-NF-κB or nonspecific (NS) LV pLL3.7-shRNA-NS control or were sham infected. GFP fluorescence was examined 48 h later. Original magnification, ×40. B: knockdown of p65-NF-κB protein after expression of an shRNA directed against p65-NF-κB. Protein lysates collected from cells were resolved for total p65-NF-κB by Western blotting. Blots were reprobed for β-actin to demonstrate lane loading. C: translocation of p65-containing NF-κB to the nucleus in response to IL-1β after expression of an shRNA directed against p65-NF-κB. Cells were treated with 10 ng/ml IL-1β for up to 120 min, and nuclear extracts were assayed for p65-containing NF-κB by ELISA. Values are means ± SE (n = 5 for cells expressing an NS shRNA and 3 for cells expressing an shRNA directed against p65-NF-κB). *P = 0.05; †P = 0.01 vs. time 0. §P = 0.01 between groups. D: migration in response to IL-1β after expression of an shRNA directed against p65-NF-κB. Confluent monolayers in submersion culture were injured with a rubber stylet and then treated with 10 ng/ml IL-1β. Treatment with 10 ng/ml IL-1β at the time of injury did not accelerate migration significantly compared with control. Values are means ± SE (n = 12 experiments in each group). E: migration in response to IL-1β after expression of an NS shRNA. Confluent monolayers in submersion culture were injured with a rubber stylet and then treated with 10 ng/ml IL-1β. Treatment with 10 ng/ml IL-1β at the time of injury accelerated migration significantly compared with control. Values are means ± SE (n = 10–12 experiments in each group). *P = 0.0001 vs. time 0.
Activation of STAT3 in migrating cells after treatment with IL-1β.
One potential mechanism by which IL-1β could stimulate migration is through the activation of STAT3, one of the JAK/STAT signaling pathways that are involved in signaling responses of several cytokines, growth factors, and hormones. IL-1β decreases renin expression in As4.1 cells via a mechanism that involves tyrosine phosphorylation at position 705 and subsequent activation of STAT3 (36) and is involved in IL-1β-induced changes in cardiac myocyte morphology (39). We examined STAT3 phosphorylation at Y705 in primary cells grown in ALI culture for 3 wk. Multiple wound edges were generated in wells, and 10 ng/ml IL-1β was added immediately thereafter. Sham wounding with and without addition of IL-1β was used as a control. Protein lysates were collected 30 min after wound generation, and the presence of phosphorylated STAT3 was assessed by multiplex bead assay. Neither IL-1β treatment nor injury elicited STAT3 phosphorylation: 30 min after treatment with IL-1β alone, STAT3 phosphorylation was 119 ± 47% of control. In cell layers subjected to rake injury, STAT3 phosphorylation after injury alone was 110 ± 17% of control, and STAT3 phosphorylation after the combination of injury and treatment with IL-1β was 105 ± 21% of control (n = 8, not significantly different from control for all groups).
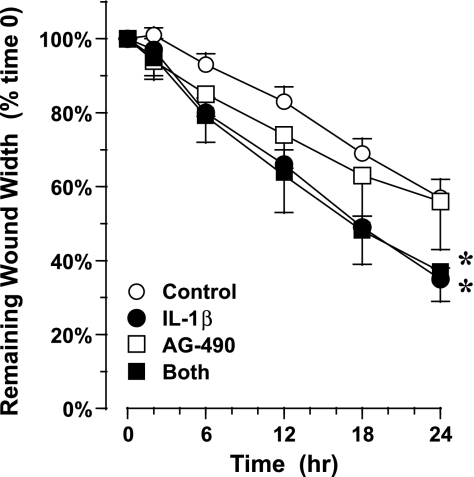
Next, we tested whether inhibition of phosphorylation of STAT3 with the JAK2-STAT3 inhibitor AG-490 blocked the response to IL-1β. After primary cells were grown in ALI culture for 3 wk and then treated with 50 μM AG-490 or vehicle control for 15 min, linear wounds were generated, and 10 ng/ml IL-1β or vehicle control was added. Treatment with 10 ng/ml IL-1β only at the time of wound generation significantly accelerated migration, as in previous experiments. Prior treatment with AG-490 did not block the response to IL-1β (Fig. 7). These data suggest that STAT3 activation was not required for AEC migration stimulated by IL-1β.
Fig. 7.
Migration of AEC in response to IL-1β: effect of the JAK2/STAT3 inhibitor AG-490. Cells grown in ALI culture for 3 wk were pretreated with 50 μM AG-490 or vehicle control for 15 min, injured using a rubber stylet, and then treated with 10 ng/ml IL-1β or control. Migration was followed for 24 h by digital photomicroscopy. Inhibition of STAT3 did not alter the response to IL-1β. Values are means ± SE (n = 6–8 experiments in cells collected from 2 normal donors). *P = 0.02 vs. control.
DISCUSSION
Repair of the airway mucosa after injury requires the integration of multiple external signals from growth factors, cytokines and chemokines, and physical stimuli. These integrated signals prompt spreading and migration of cells into the sites of injury, repair of defects in and changes in the composition of the underlying basement membrane, and phenotype shifting of cells to required cell subtypes. Perturbations in the repair process at multiple levels may leave the mucosa open to continued ingress of mediators and toxins within the airway lumen. Incomplete repair also may leave the epithelium in a phenotype that responds inappropriately to inflammatory stimuli.
IL-1β is produced by a variety of immune and structural cells, including T cells, macrophages, monocytes, and endothelial and epithelial cells. It has been demonstrated to elicit migration of endothelial cells (31) and vascular smooth muscle cells (2, 17) in culture. However, whether IL-1β would stimulate AEC migration was not known. To our knowledge, our data represent the first demonstration of the promigratory effect of IL-1β in airway epithelium. Accelerated migration was demonstrated in the 16HBE14o− cell line and in primary cells in differentiated, ALI culture, but was not seen in differentiated cells collected from asthmatic subjects. Because clinical information about these subjects was not available, we are unable to correlate issues concerning disease state, therapy, or disease severity with the responsiveness to IL-1β in culture. As one example of a potential difference, corticosteroid treatment may suppress (47) or increase (20) the presence of NF-κB in epithelial cells in bronchial biopsies collected from asthmatic subjects, which in the light of our present data might alter the response to IL-1β. We note that differentiated AEC collected from asthmatic subjects had a baseline migration to injury alone that was similar to that of normal cells. Our data regarding cells from asthmatic subjects should be viewed with caution but suggest that migration in these cells may be different after cytokine exposure.
IL-1β activates transcription factors such as NF-κB, which in turn governs expression of many genes that are involved in inflammatory and immune responses, as well as cell adhesion molecules, growth factors, and metalloproteinases (8), all of which may participate in the repair of a damaged airway mucosa. NF-κB is trapped in the cytoplasm by binding to an inhibitory IκB protein complex by masking nuclear localization sequences contained in the Rel homology domain (24); phosphorylation of the IκB complex by a kinase releases IκB and permits subsequent activation and translocation of NF-κB (13). In our experiments, silencing the p65 component of NF-κB blocked IL-1β-accelerated AEC migration completely, suggesting that this signaling pathway is responsible for the motogenic effect of this cytokine.
IL-1β also can activate stress-related kinases, such as p38 MAPK (21, 39), and, thus, downstream effectors, such as HSP27, which has several effects on actin skeleton and microfilament reorganization (4, 34). IL-1β also can activate JNK (41) and, thus, phosphorylate c-Jun and, subsequently, modulate activator protein-1 transcriptional activity. However, we could not demonstrate increased phosphorylation of HSP27, JNK, c-Jun, or ERK1/2 by multiplex bead assay in differentiated AEC after treatment with IL-1β following injury compared with injury alone. These data suggest that this cytokine is not eliciting phosphorylation of these stress-related kinases. Confirmation of these data by the use of inhibitors for each kinase is complicated by the fact that each kinase is activated by injury alone and required for AEC migration (46).
We examined the potential role of the JAK2/STAT3 signaling pathway in IL-1β-accelerated migration, first, by examining STAT3 phosphorylation after injury using multiplex bead assay and, then, by using the inhibitor AG-490. STAT3 phosphorylation was not demonstrated after injury or after IL-1β treatment. Furthermore, tyrphostin AG-490 did not slow IL-1β-accelerated migration. Our data suggest that this signaling pathway does not modulate significantly the motogenic effect of IL-1β.
IL-1β is expressed early in the repair response of alveolar (15), corneal (23), and colonic (9) epithelial cells in wound models and may mediate repair. Several mechanisms, including downregulation of adhesion proteins, such as focal adhesion kinase (25), degradation of matrix proteins (35), or expression of integrins (2), have been suggested. Although distal pathways that effect migration may be several and may be operative in a cell- and context-specific manner, each requires signaling from the IL-1β receptor. Our data clarify that, in airway epithelial cells, IL-1β-induced repair requires signaling from the NF-κB complex and the classical (p65-p50) pathway.
Cell migration requires the integration of multiple processes, including attachment and detachment of cells from the underlying matrix, actin reorganization, membrane ruffling, and lamellipodia extension. Assays measuring the passage of cells through a semipermeable membrane can assess the chemotactic potential of an agent but do not permit evaluation of signaling at different points. The two-dimensional migration assay used in our study permits repeated measurements of migration and evaluation of cell extensions. As with all in vitro migration assays, this assay has the major limitation that cells are studied under conditions not found in vivo. Furthermore, culture conditions vary between laboratories examining the functions of differentiated AEC. Changes in these conditions may elicit subsequent changes in the proportion of cell subtypes or responsiveness to various mediators and stimuli. Therefore, caution should be taken in extending our observations to repair in native airways.
In summary, we demonstrate that IL-1β modulates early steps in cell migration after mechanical injury in airway epithelial cells grown in submersion and differentiated ALI culture.
GRANTS
This work was supported by National Institutes of Health Grants AI-56352 (S. R. White), HL-080417 (S. R. White), and HL-081763 (B. M. Fischer).
Acknowledgments
We thank Mike Mitrovich for technical assistance.
This work was presented in part at the International Conference of the American Thoracic Society, San Diego, CA, 25 May 2005.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arima M, Plitt J, Stellato C, Bickel C, Motojima S, Makino S, Fukuda T, Schleimer RP. Expression of interleukin-1β by human epithelial cells. Inhibition by dexamethasone. Am J Respir Cell Mol Biol 21: 684–692, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Barillari G, Albonici L, Incerpi S, Bogetto L, Pistritto G, Volpi A, Ensoli B, Manzari V. Inflammatory cytokines stimulate vascular smooth muscle cell locomotion and growth by enhancing α5β1-integrin expression and function. Atherosclerosis 154: 377–385, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis 139: 806–817, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP27 abolish its actin polymerization-inhibiting activity. J Biol Chem 269: 20780–20784, 1994. [PubMed] [Google Scholar]
- 5.Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Am J Respir Cell Mol Biol 20: 595–604, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161: 1720–1745, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, Dalton WS, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10: 105–115, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Cooper P, Potter S, Mueck B, Yousefi S, Jarai G. Identification of genes induced by inflammatory cytokines in airway epithelium. Am J Physiol Lung Cell Mol Physiol 280: L841–L852, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Dieleman LA, Elson CO, Tennyson GS, Beagley KW. Kinetics of cytokine expression during healing of acute colitis in mice. Am J Physiol Gastrointest Liver Physiol 271: G130–G136, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Dorscheid DR, Wojcik KR, Sun S, Marroquin B, White SR. Apoptosis of airway epithelial cells induced by corticosteroids. Am J Respir Crit Care Med 164: 1939–1947, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dwarakanath RS, Sahar S, Reddy MA, Castanotto D, Rossi JJ, Natarajan R. Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-κB (NF-κB). J Mol Cell Cardiol 36: 585–595, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C, Nissen MH, Röpke C, Wasik MA, Odum N. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia 15: 787–793, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Erjefält J, Persson C, Sundler F. Epithelial barrier formation by airway basal cells. Thorax 52: 213–217, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erjefält JS, Erjefält I, Sundler F, Persson CGA. In vivo restitution of airway epithelium. Cell Tissue Res 281: 305–316, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1β augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol 279: L1184–L1190, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72: 1493–1505, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, Strieter RM. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol 176: 1916–1927, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, Burch L, Boucher R, Nettesheim P. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1 in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol 286: L320–L330, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gray T, Nettesheim P, Loftin C, Koo JS, Bonner J, Peddada S, Langenbach R. Interleukin-1β-induced mucin production in human airway epithelium is mediated by cyclooxygenase-2, prostaglandin E2 receptors, and cyclic AMP-protein kinase A signaling. Mol Pharmacol 66: 337–346, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hart L, Lim S, Adcock I, Barnes PJ, Chung KF. Effects of inhaled corticosteroid therapy on expression and DNA-binding activity of nuclear factor κB in asthma. Am J Respir Crit Care Med 161: 224–231. 2000. [DOI] [PubMed] [Google Scholar]
- 21.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38MAPK/HSP27 pathway in smooth muscle cell migration. J Biol Chem 274: 24211–24219, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Hermanns MI, Unger RE, Kehe K, Peters K, Kirkpatrick CJ. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab Invest 84: 736–752, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Hübner G, Brauchle M, Smola H, Madlener M, Fässler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 8: 548–556, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IκBα/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell 95: 759–770, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Iwaki K, Ohashi K, Ikeda M, Tsujioka K, Kajiya F, Kurimoto M. Decrease in the amount of focal adhesion kinase (p125FAK) in interleukin-1β-stimulated human umbilical vein endothelial cells by binding of human monocytic cell lines. J Biol Chem 272: 20665–20670, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma—an ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis 140: 1745–1753, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Keenan K, Combs JW, McDowell EM. Regeneration of hamster tracheal epithelium after mechanical injury. II. Multifocal lesions: stathmokinetic and autoradiographic studies of cell proliferation. Virchows Arch 41: 215–229, 1982. [DOI] [PubMed] [Google Scholar]
- 28.Keenan K, Combs JW, McDowell EM. Regeneration of hamster tracheal epithelium after mechanical injury. III. Large and small lesions: comparative stathmokinetic and single pulse and continuous thymidine labeling autoradiographic studies. Virchows Arch 41: 231–252, 1982. [PubMed] [Google Scholar]
- 29.Kim YD, Jeon JY, Woo HJ, Lee JC, Chung JH, Song SY, Yoon SK, Baek SH. Interleukin-1β induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J Korean Med Sci 17: 765–771, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kips JC, Pauwels RA. Are cytokines responsible for the asthmatic state of the airways? Monaldi Arch Chest Dis 51: 223–227, 1996. [PubMed] [Google Scholar]
- 31.Kohama Y, Oka H, Murayama N, Iida K, Itoh M, Itoh M, Ying X, Mimura T. Increase of migration of cultured endothelial cells by angiotensin-converting enzyme inhibitor derived from tuna muscle. J Pharmacobio-Dyn 15: 223–229, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Krunkosky TM, Jordan JL, Chambers E, Krause DC. Mycoplasma pneumoniae host-pathogen studies in an air-liquid culture of differentiated human airway epithelial cells. Microb Pathog 42: 98–103, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis 131: 599–606, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem 268: 24210–24214, 1993. [PubMed] [Google Scholar]
- 35.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM. Interleukin-1β induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-κB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol 211: 759–770, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Shi Q, Sigmund CD. Interleukin-1β attenuates renin gene expression via a mitogen-activated protein kinase kinase-extracellular signal-regulated kinase and signal transducer and activator of transcription 3-dependent mechanism in As4.1 cells. Endocrinology 147: 6011–6018, 2006. [DOI] [PubMed] [Google Scholar]
- 37.López-Ferrer A, Curull V, Barranco C, Garrido M, Lloreta J, Real FX, de Bolós C. Mucins and differentiation markers in bronchial epithelium. Squamous cell carcinoma and adenocarcinoma display similar expression patterns. Am J Respir Cell Mol Biol 24: 22–29, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Marini M, Soloperto M, Mezzetti M, Fasoli A, Mattoli S. Interleukin-1 binds to specific receptors on human bronchial epithelial cells and upregulates granulocyte/macrophage colony-stimulating factor synthesis and release. Am J Respir Cell Mol Biol 4: 519–524, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Ng DC, Long CS, Bogoyevitch MA. A role for the extracellular signal-regulated kinase and p38 mitogen-activated protein kinases in interleukin-1β-stimulated delayed signal transducer and activator of transcription 3 activation, atrial natriuretic factor expression, and cardiac myocyte morphology. J Biol Chem 276: 29490–29498, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen M, Kaltoft K, Nordahl M, Röpke C, Geisler C, Mustelin T, Dobson P, Svejgaard A, Odum N. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci USA 94: 6764–6769, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puddicombe SM, Davies DE. The role of MAP kinases in intracellular signal transduction in bronchial epithelium. Clin Exp Allergy 30: 7–11, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Rosenwasser LJ New immunopharmacologic approaches to asthma: role of cytokine antagonism. J Allergy Clin Immunol 105: S586–S589 2000. [DOI] [PubMed] [Google Scholar]
- 43.Sanghavi JN, Rabe KF, Kim JS, Magnussen H, Leff AR, White SR. Migration of human and guinea pig airway epithelial cells in response to calcitonin gene-related peptide. Am J Respir Cell Mol Biol 11: 181–187, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Vignola AM, Chanez P, Campbell AM, Fouques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med 157: 403–409, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 172: 1013–1018, 2005. [DOI] [PubMed] [Google Scholar]
- 46.White SR, Tse R, Marroquin BA. Stress activated protein kinases mediate cell migration in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 301–310, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Wilson SJ, Wallin A, Della-Cioppa G, Sandström T, Holgate ST. Effects of budesonide and formoterol on NF-κB, adhesion molecules, and cytokines in asthma. Am J Respir Crit Care Med 164: 1047–1052, 2001. [DOI] [PubMed] [Google Scholar]