Abstract
AMP-activated protein kinase (AMPK) responds to oxidative stress. Previous work has shown that ethanol treatment of cultured hepatoma cells and of mice inhibited the activity of AMPK and reduced the amount of AMPK protein. Ethanol generates oxidative stress in the liver. Since AMPK is activated by reactive oxygen species, it seems paradoxical that ethanol would inhibit AMPK in the hepatoma cells. In an attempt to understand the mechanism whereby ethanol inhibits AMPK, we studied the effect of ethanol on AMPK activation by exogenous hydrogen peroxide. The effects of ethanol, hydrogen peroxide, and inhibitors of protein phosphatase 2A (PP2A) [either okadaic acid or PP2A small interference RNA (siRNA)] on AMPK phosphorylation and activity were examined in rat hepatoma cells (H4IIEC3) and HeLa cells. In H4IIEC3 cells, hydrogen peroxide (H2O2, 1 mM) transiently increased the level of phospho-AMPK to 1.5-fold over control (P < 0.05). Similar findings were observed in HeLa cells, which do not express the upstream AMPK kinase, LKB1. H2O2 markedly increased the phosphorylation of LKB1 in H4IIEC3 cells. Ethanol significantly inhibited the phosphorylation of PKC-ζ, LKB1, and AMPK caused by exposure to H2O2. This inhibitory effect of ethanol required its metabolism. More importantly, the inhibitory effects of ethanol on H2O2-induced AMPK phosphorylation were attenuated by the presence of the PP2A inhibitor, okadaic acid, or PP2A siRNA. The inhibitory effect of ethanol on AMPK phosphorylation is exerted through the inhibition of PKC-ζ and LKB1 phosphorylation and the activation of PP2A.
Keywords: protein phosphatase
amp-activated protein kinase (AMPK), a heterotrimeric serine/threonine kinase, plays a key role in the regulation of energy homeostasis. Traditionally, elevation of intracellular AMP is considered the main activator of AMPK (9), acting through several mechanisms. First, AMP itself causes direct activation of AMPK (8, 9). Second, AMP also activates the upstream kinase, LKB1 (formerly known as AMPK kinase), which activates AMPK by phosphorylation of the α-subunit on Thr-172 (7, 10). Third, the binding of AMP to AMPK renders it a better substrate for the LKB1 and a worse substrate for protein phosphatase (2). The control of LKB1 activity is presently the focus of study. Sanders et al. (15) reported that LKB1 is constitutively active, on the basis of the activity of the recombinant proteins. AMP was thought to act indirectly on AMPK by inhibition of protein phosphatase 2 (PP2)C-α, thus protecting AMPK Thr-172 from dephosphorylation (15).
AMPK also responds to oxidative stress and reactive oxygen species (ROS). Exposure of endothelial cells to peroxynitrite increased phosphorylation of AMPK and its downstream target, acetyl-CoA carboxylase (ACC), without changing the cellular AMP/ATP ratio (24). Xie et al. (21) suggested that this was mediated by protein kinase C (PKC)-ζ, which phosphorylated LKB1 at Ser-428. More recently, this group (20) suggested that phosphorylation of LKB1 on Ser-428 promotes its export from the nucleus, resulting in activation of AMPK.
AMPK was activated by hydrogen peroxide in NIH 3T3 cells, and the effect was reversed by the hydroxyl radical scavenger dimethylsulfoxide (1). The mechanism of activation of AMPK by oxidative stress is poorly understood, and there may be several pathways involved. Transient increases in AMP levels were shown in some experimental models following hydrogen peroxide exposure (3). The tyrosine kinase inhibitor, genistein, was demonstrated to further stimulate hydrogen peroxide-induced AMPK activity without altering the AMP levels (1).
Among several functions of AMPK, it is an important sensor of the energy state and regulator of intermediary metabolism of lipid in the liver. AMPK inhibits sterol response element-binding protein (SREBP-1) and ACC (16). Activation of SREBP-1 induces a battery of enzymes involved in lipid synthesis, including ACC. Our laboratory has shown that ethanol treatment of cultured hepatoma cells and of mice activated SREBP-1 and ACC and, more importantly, inhibited the activity of the AMPK and reduced the amount of AMPK protein (22). Our findings raise an interesting question regarding the interaction of ethanol, oxidative stress, and the activity of AMPK. Ethanol generates oxidative stress in the liver, and several groups have shown that ethanol metabolism by isolated hepatocytes increases the generation of ROS (18). Since AMPK is activated by ROS, it seems paradoxical that ethanol would inhibit AMPK in the hepatoma cells.
In an attempt to understand the mechanism whereby ethanol inhibits AMPK, we studied the effect of ethanol on AMPK activation caused by exogenous hydrogen peroxide. Here, we report that hydrogen peroxide induced the phosphorylation of AMPK, and that ethanol inhibited this effect through the 1) inhibition of PKC-ζ and LKB1 phosphorylation and 2) increased PP2A activity.
MATERIALS AND METHODS
Materials
All chemicals unless specified were purchased from Sigma Chemical (St. Louis, MO). Trypsin and tissue culture media were purchased from Life Technologies (Rockville, MD), and rat hepatoma (H4IIEC3) and HeLa cell lines were from the American Type Culture Collection (Manassas, VA). SAMS peptide was from Upstate Biotechnology (Charlottesville, VA). All radioisotopes were purchased from American Radiolabeled Chemicals (St. Louis, MO). AMPK-α, phospho-AMPK-α (Thr-172), LKB1, phospho-LKB1 (Ser-428), PKC-ζ, phospho-PKC-ζ, phospho-ACC, ACC, and PP2A antibodies were purchased from Cell Signaling Technology (Beverly, MA). PP2A small interference RNA (siRNA) was from Ambion (Austin, TX).
Cell Culture and Treatment
All cells were grown in modified Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, and 63 μg/ml penicillin G. Serum starvation was achieved by incubating nearly confluent cells for 18 h in medium without FBS. Cells were then incubated with hydrogen peroxide for the indicated concentrations and time. In some experiments, the cells were exposed to ethanol for 24 h before the treatment with hydrogen peroxide. When ethanol was present, the cells were incubated in a chamber containing a beaker of 1.0 l of water containing ethanol at the same concentration to reduce the loss of ethanol from the cultures because of evaporation.
Measurement of AMPK Activity
The AMPK activity assay was carried out according to methods previously described (23). Briefly, H4IIEC3 cells were grown in MEM supplemented with 10% FBS, 100 μg/ml streptomycin, and 63 μg/ml penicillin G to 70–80% confluency. Cells were then cultured in serum-free MEM for 16 h, followed by treatment with different compounds. Twenty-four hours later, cells were collected in the lysis buffer (1× RIPA buffer, 1 mM NaVO3, 1 mM PMSF, complete protease inhibitor cocktail) and centrifuged to remove cell debris. AMPK activity assays were performed at 30°C in 25-μl reaction mixtures containing 4 μg protein in reaction buffer (40 mM HEPES, pH 7.0, 80 mM NaCl, 5 mM magnesium acetate, 1 mM DTT, 8% glycerol, 0.8 mM EDTA, 200 μM AMP and ATP, and 2 μCi [γ-32P]ATP) with or without 200 μM SAMS peptide. After 30-min incubation, 15-μl reaction mixtures were spotted on phosphocellulose filter paper (Whatman P81), and the filter papers were washed extensively with phosphoric acid. The radioactivity on the filter paper was measured in a scintillation counter, and the AMPK activity was expressed as picomoles of [32]P incorporation into the peptide per minute per microgram of protein.
HPLC Analyses of Nucleotides
H4IIEC3 cells were split into 10-cm plates. The day before the experiment, the cells were serum starved for 18 h in serum-free MEM. On the day of treatment, cells were treated with hydrogen peroxide and/or ethanol as indicated. At the end of treatment, cell culture medium was removed, and the cells were rinsed with ice-cold PBS. After the removal of PBS, 300 μl of ice-cold 10% TCA was added directly onto the 10-cm plates. Cells were then scraped with a rubber policeman, transferred to Eppendorf tubes, and chilled on ice for 10 min. Tubes were vortexed vigorously for 20 s at 4°C and centrifuged at 14,000 revolution/min at 4°C for 2 min. Supernatant was transferred to Eppendorf tubes already containing 0.6 ml of 0.5 M tri-n-octylamine in Freon, vortexed at 4°C for 20 s, and recentrifuged at 18,000 g at 4°C for 1 min. The bottom layer was carefully removed, leaving a clean top aqueous layer within the Eppendorf tubes. Tubes were frozen at −80°C until HPLC analysis. HPLC analysis for nucleotides was performed as previously published (14).
Measurement of Phosphatase Activity
The activity of PP2A was measured with the PP2A immunoprecipitation phosphatase assay kit (Millipore, Bedford, MA). Threonine phosphopeptide (K-R-Pt-I-R-R) was used as PP2A substrate. In brief, the cells were lysis buffered (0.5 M Tris·HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA, 1 mM PMSF, and protease inhibitors). Supernatants were incubated with anti-PP2A [C subunit, clone 1D6] and protein A agarose at 4°C for 2 h with constant rocking. The immunoprecipitates were then washed three times with Tris-buffered saline and diluted phosphopeptide (final concentration 750 μM), and Ser/Thr assay buffer were added. The mixtures were incubated for 10 min at 30°C in a shaking incubator and then briefly centrifuged, and 25 μl of mixtures were transferred to 96-well microtiter plate. PP2A activities were determined by the addition of the Malachite green phosphate detection solution into the mixtures and measuring the absorbance at 650 nm. The absorbance values of each sample were compared with negative controls containing no PP2A enzyme activity.
PP2A siRNA Construction and Transfection
Predesigned siRNA targeting the open reading frame for rat PP2A catalytic subunit-α (catalog no. S128253) and the scrambled sequence (negative control) were purchased from Ambion. Transfection of siRNA into hepatoma cells was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). H4IIEC3 cells were cultured in a six-well plate at 2 × 105 cells/well with MEM supplemented with 10% FBS without antibiotics to 40–50% confluency. The hepatoma cells were transfected with PP2A-specific siRNA or scrambled oligonucleotides at the final concentration of 40 nM in Lipofectamine 2000 complexes according to the manufacturer's protocol. PP2A protein expression was followed by immunoblots. We found that the maximal inhibition of PP2A was at 48 h after transfection. The hepatoma cells were treated at 72 h posttransfection.
Immunoblot Analysis
Immunoblot analyses were performed using 20 μg of whole cell extract separated by electrophoresis in a 10% or 6% SDS-polyacrylamide gel and transferred to nitrocellulose filters. Detection of the protein bands was performed using the ECL Plus Western Blotting Detection System Kit (Amersham Biosciences, Piscataway, NJ). The protein bands were then quantified on a PhosphoImager and ImageQuant (Amersham Biosciences) software analysis.
Immunoprecipitation
H4IIEC3 cells were grown in 10-cm plates and were treated with ethanol 50 mM for 24 h. Proteins were extracted with RIPA lysis buffer. Two hundred microliters of cell lysates (∼800 μg of protein lysate) were preincubated with 1 μg of rabbit IgG and 20 μl of 50% protein A/G Plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 60 min at 4°C. Precleared lysates were incubated with 2 μl of anti-AMPK antibody (Cell Signaling Technology) overnight at 4°C. Then, 20 μl of protein A/G plus agarose beads were added, and the complex was incubated at 4°C on a rocker platform for 2 h. Immunoprecipitates were subsequently collected by centrifugation at 2,500 revolution/min for 5 min at 4°C. The pellets were washed five times with 1 ml RIPA buffer. Equal volumes of 2× SDS-PAGE sample buffer were added to the washed agarose bead complex and boiled. After spinning, only the supernatants were saved for further analysis in the SDS-PAGE gel.
Data Analysis
All data are presented as the means ± SD. Statistical significance was calculated with the Student's t-test or one-way ANOVA analysis, followed by post hoc testing with least squares difference, when appropriate. P < 0.05 was considered statistically significant.
RESULTS
Effects of H2O2 on the Levels Of Phospho-AMPK and Its Activity In Hepatoma Cells
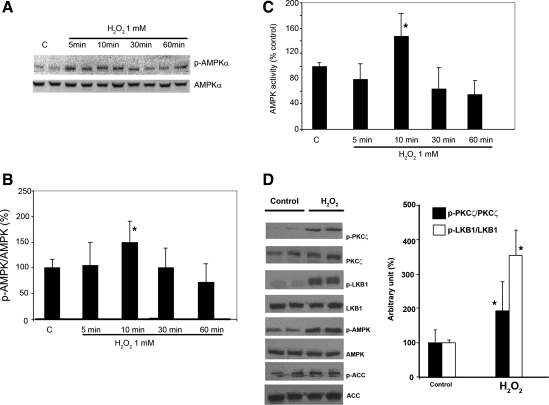
We first examined the time- and dose-dependent effects of H2O2 on the level of phospho-AMPK (p-AMPK). H4IIEC3 cells were serum starved for 18 h and then exposed to H2O2 at 0.5 mM and 1 mM for the indicated times. There was a transient, significant 1.5-fold induction in the level of phosphorylation of AMPK-α on Thr-172 without a significant change of AMPK-α protein expression level within the first 10 min of exposure to 1 mM H2O2 (Fig. 1, A and B). Similar effects were also observed with 0.5 mM H2O2 (data not shown). The levels of p-AMPK subsequently declined after 30- and 60-min treatment. The increase in AMPK phosphorylation was also correlated with its activity (Fig. 1C). Although Fig. 1A suggests activation as early as 5 min after addition of H2O2, on average the peak of AMPK phosphorylation and activity occurred after 10 min. To understand whether the induction of p-AMPK by H2O2 required the upstream kinases for AMPK (PKC-ζ and LKB1), we next measured levels of PKC-ζ (Thr-410/403, p-PKC-ζ) and LKB1 (Ser-428) phosphorylation (p-LKB1) in hepatoma cells treated with 1 mM H2O2 for 10 min. Ser-428 of LKB1 had been shown to be phosphorylated by PKC-ζ in response to peroxynitrite oxidative stress (21). As shown in Fig. 1D, treatment with H2O2 significantly increased the levels of p-PKC-ζ and p-LKB1 by two- and threefold, respectively compared with control (P < 0.05).
Fig. 1.
Effect of H2O2 on AMP-activated protein kinase (AMPK) phosphorylation and AMPK activity in rat hepatoma H4IIEC3 cells. A: immunoblot analysis. H4IIEC3 cells were maintained in modified Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS). Cells were then washed with PBS, refed with MEM supplemented with 10% dilapidated FBS, and, after incubation for 18 h, treated with H2O2 (1 mM) for the times indicated. Cell extracts (20 μg protein) were electrophoresed on a 10% SDS gel. Phospho-AMPK (p-AMPK) protein (Thr-172) was visualized by using anti-p-AMPK antibody. The effects of H2O2 on AMPK phosphorylation were rapid and transient. The peak effect occurred at 10 min after treatment (1.5-fold induction over control, P = 0.002). B: bar graph demonstrating quantification of the Western blot (A) by a PhosphorImager and ImageQuant software analysis. C: AMPK activity measured using a synthetic peptide substrate. D: immunoblot analysis demonstrated the effect of H2O2 (1 mM for 10 min) on protein kinase C (PKC)-ζ, LKB1 and its downstream proteins. H2O2 increased the expression of phospho-PKC-ζ and phospho-LKB1 by two and threefold, respectively, compared with control. Means ± SE (the blot is a representative of 3 blots from 3 individual experiments) are shown. *Significant difference vs. control; P < 0.05, by one-way ANOVA. ACC, acetyl-CoA carboxylase.
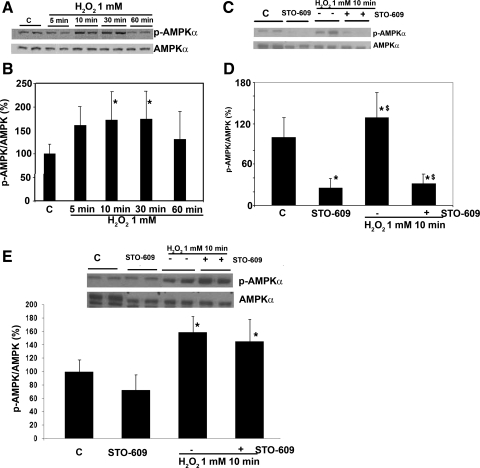
Activation of AMPK by Hydrogen Peroxide is Also Mediated Through a Non-LKB1 Pathway in HeLa Cells
Recently, several studies have shown that AMPK can also be activated by non-LKB1 kinases, namely Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) (17). In an effort to further identify the pathways through which H2O2 was acting, we next measured p-AMPK levels in HeLa cells, which lack LKB1 (6), confirmed by Western blotting (not shown). Similar to the findings with H4IIEC3 cells, the level of AMPK phosphorylation in HeLa cells was significantly increased after H2O2 treatment to 1.2-fold over control at 10 min (Fig. 2). We hypothesized that this was due to activation of CaMKK by H2O2. HeLa cells were incubated under basal conditions and were stimulated with H2O2 (1 mM for 10 min) in the presence of the CaMKK inhibitor, STO-609, at a concentration of 1 μg/ml. At baseline, STO-609 significantly inhibited AMPK phosphorylation by 75%, and it also prevented AMPK activation by H2O2 (Fig. 2C). These data suggest that phosphorylation of AMPK-α on Thr-172 by H2O2 can result from activation of either PKC-ζ/LKB1 or CaMKK depending on the cell type.
Fig. 2.
Effect of H2O2 on AMPK phosphorylation in HeLa cells. A and B: similar to the findings with H4IIEC3 cells, the levels of AMPK phosphorylation in HeLa cells were increased after H2O2 (1 mM) treatment to 1.2-fold over control at 10 min after treatment with 1 mM H2O2 (P < 0.05). C and D: AMPK phosphorylation in HeLa cells was affected by Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) inhibitor, STO-609 at a concentration of 1 μg/ml. At baseline, STO-609 significantly inhibits AMPK phosphorylation by 75%, and it also significantly prevents AMPK activation in response to H2O2. To further examine the major pathway for the activation of AMPK by H2O2, similar experiments were performed in H4IIEC3 cells in the presence of STO-609 (E). Contrary to the experiments in the HeLa cells, STO-609 did not significantly inhibit AMPK phosphorylation at baseline. The activation of AMPK by H2O2 persists despite the presence of STO-609. Means ± SE (the blot is a representative of 3 blots from 3 individual experiments) are shown. *Significant difference vs. control; P < 0.05, by one-way ANOVA; $significant difference compared with cells that were not treated with STO-609.
To further examine the dominant pathway for the activation of AMPK by H2O2 in H4IIEC3 cells, we performed similar experiments in the presence of STO-609 (Fig. 2E). Contrary to the experiments with the HeLa cells, STO-609 did not significantly inhibit AMPK phosphorylation at baseline, and the activation of AMPK by H2O2 persisted despite the presence of STO-609, suggesting that the PKC-ζ/LKB1 pathway is likely the main pathway of H2O2-induced AMPK phosphorylation in these cells.
Ethanol Inhibits H2O2-Induced AMPK Phosphorylation in Hepatoma Cells
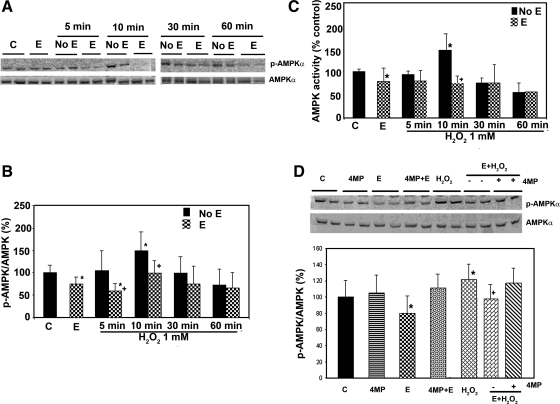
Ethanol generates oxidative stress in liver, as evidenced by the studies using fluorescent dyes. Since AMPK is activated by H2O2, it seems paradoxical that ethanol would inhibit AMPK (22). We therefore examined whether the effect of H2O2 was affected by the presence of ethanol (Fig. 3, A and B). After overnight serum starvation, H4IIEC3 cells were treated with ethanol (50 mM) for 24 h and then treated with H2O2 as indicated above. As previously reported, ethanol significantly reduced the basal level of p-AMPK by ∼25% in the H4IIEC3 cells compared with controls (Fig. 3). The overall effect of H2O2 treatment on AMPK phosphorylation was significantly reduced by 43% and 33%, respectively, at 5 and 10 min. The level of p-AMPK correlated well with its activity (Fig. 3C).
Fig. 3.
Effect of ethanol treatment on the activation of AMPK by oxidative stress. H4IIEC3 cells were grown as described for Fig. 1, followed by treatment with control (C) or ethanol (E) (50 mM); H2O2 1 mM (A) for the indicated duration of treatment, either with pretreatment with ethanol 24 h before H2O2 exposure (E) or without ethanol pretreatment (No E). Western blots were quantified by a PhosphorImager and ImageQuant software analysis (results shown as a bar graph, B). Ethanol treatment for 24 h decreased the p-AMPK/AMP ratio by 25% compared with control (P < 0.05). H2O2 treatment increased the expression of p-AMPK with peak effect occurring at 10 min after treatment (1.5-fold induction over control, P = 0.002). The effect of H2O2 at 5- and 10-min treatment on AMPK phosphorylation was reduced by 43% (P = 0.01) and 33% (P = 0.006), respectively, when the cells were pretreated with ethanol for 24 h. C: AMPK enzyme activity was analyzed with a synthetic peptide substrate, and it correlated well with the level of p-AMPK in B. D: H4IIEC3 cells were treated as indicated with control, 4-methylpyrazole (4MP) (0.1 mM), ethanol (50 mM), ethanol (50 mM) + 4-methylpyrazole (0.1 mM), H2O2 1 mM for 10 min, E + H2O2, pretreatment with ethanol (50 mM) followed by H2O2 1 mM for 10 min, pretreatment with ethanol (50 mM) + 4-methylpyrazole (0.1 mM) for 24 h followed by H2O2 1 mM for 10 min. 4MP did not significantly affect the phosphorylation level of AMPK. However, it abolished the inhibitory effect of ethanol on H2O2-induced AMPK phosphorylation. Means ± SD (the blot is representative of 3 blots from 3 individual experiments) are shown. *Significant difference vs. control; +significant difference compared with pretreatment with ethanol. P < 0.05 by one-way ANOVA.
H4IIEC3 cells have the ability to oxidize ethanol. We found that the alcohol oxidation rate by H4IIEC3 cells was 0.56 ± 0.10 mmol/h per 1.2 × 107 cells. We have previously shown that the inhibitory effect of ethanol on AMPK phosphorylation could be blocked in H4IIEC3 cells with 4-methylpyrazole, an inhibitor of class I alcohol dehydrogenase (ADH), indicating the need for ethanol to be metabolized (22). We next asked whether the ability of ethanol to block the activation of AMPK phosphorylation by H2O2 in H4IIEC3 cells required ethanol metabolism. Figure 3D showed that 4-methylpyrazole did not significantly affect the phosphorylation level of AMPK. However, 4-methylpyrazole nearly abrogated the inhibitory effect of ethanol on AMPK activation and also abolished the inhibitory effect of ethanol on H2O2-induced AMPK phosphorylation. HeLa cells do not express ADH (4), and we found that ethanol had no effect on basal AMPK activity and failed to inhibit H2O2-induced AMPK phosphorylation in HeLa cells (data not shown). These results suggest that metabolism of ethanol is required for it to block the ability of H2O2 to induce AMPK phosphorylation.
Mechanisms of the inhibition of AMPK by Ethanol
Effects on PKC-ζ and LKB1.
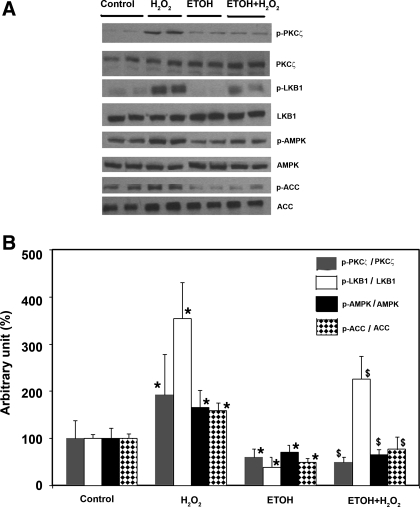
Because PKC-ζ/LKB1 are the upstream kinases of AMPK (20, 21), we next asked whether the inhibitory effect of ethanol on AMPK was mediated through these enzymes. As shown in Fig. 4, ethanol significantly reduced the level of p-PKC-ζ and p-LKB1 by ∼40% and ∼60%, respectively, in the H4IIEC3 cells compared with controls. When the cells were stimulated by H2O2, ethanol nearly completely blocked the phosphorylation of PKC-ζ but did not abolish the increase in phosphorylation of LKB1. This suggests that H2O2 may stimulate phosphorylation of LKB1 though other pathways not involving PKC-ζ.
Fig. 4.
Effect of ethanol (ETOH) and hydrogen peroxide on PKC-ζ/LKB1 and its downstream target proteins. H4IIEC3 cells were grown as described for Fig. 1 followed by treatment for times indicated with control, ethanol (50 mM) for 24 h, H2O2 1 mM for 10 min, and pretreatment with ethanol 24 h before H2O2 exposure. Western blots (A) were quantified by a PhosphorImager and ImageQuant software analysis (bar graph, B). Ethanol reduced the level of p-PKC-ζ and p-LKB1 by ∼40% and ∼60% in the H4IIEC3 cells compared with controls; on the other hand, H2O2 significantly increased its phosphorylation (by 3-fold) (P < 0.05). *Significant difference vs. control; $significant difference compared with pretreatment with ethanol.
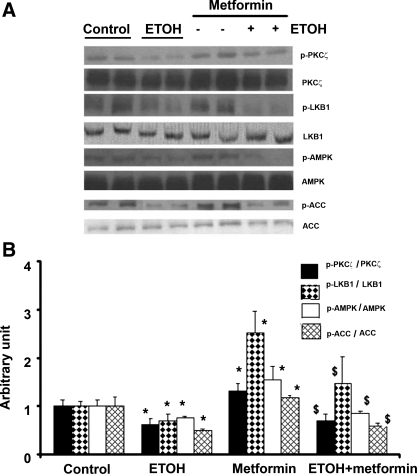
To further examine whether ethanol inhibited AMPK phosphorylation through the PKC-ζ/LKB1 pathway and whether ethanol could reduce AMPK activation by other activators, we next studied the effect of ethanol on metformin-induced AMPK phosphorylation. Metformin, a commonly used anti-diabetic agent, is reported to activate AMPK through the phosphorylation of PKC-ζ and LKB1 (20). In H4IIEC3 cells, the maximum stimulation for AMPK phosphorylation by metformin (2 mM) was at 2 h after treatment (data not shown). Similar to the experiments with H2O2, H4IIEC3 cells were treated with ethanol (50 mM) for 24 h followed by treatment with metformin (2 mM) for 2 h (Fig 5, A and B). Metformin significantly increased the phosphorylation of PKC-ζ, LKB1, and AMPK by 1.5-, 2.5-, and 1.8-fold, respectively. The effect of metformin on AMPK phosphorylation was markedly inhibited (approximately threefold reduction) in the presence of ethanol.
Fig. 5.
Effect of ethanol and metformin on PKC-ζ/LKB1 and its downstream target proteins. H4IIEC3 cells were grown as described for Fig. 1 followed by treatment for times indicated with control, ethanol (50 mM) for 24 h, metformin 2 mM for 2 h, and pretreatment with ethanol 24 h before metformin exposure. Western blots (A) were quantified by a PhosphorImager and ImageQuant software analysis (bar graph, B). Metformin significantly increased the phosphorylation of PKC-ζ, LKB1, and AMPK by 1.5-, 2.5-, and 1.8-fold, respectively. The effect of metformin on AMPK phosphorylation was markedly inhibited (∼3-fold reduction) in the presence of ethanol. The figure is representative of 3 blots from 3 individual experiments; *significant difference vs. control; $significant difference compared with no treatment with ethanol.
Effects on AMP:ATP ratio.
The intracellular level of AMP is a sensitive marker of the energy stores of the cell. Since the effects of AMP on AMPK are competitively opposed by ATP, the AMP/ATP ratio may in fact be the physiological signal sensed by AMPK. In fact, AMPK cascades have been shown to respond mainly to the intracellular level of AMP or AMP:ATP ratio (8). We hypothesized that ethanol, as a source of carbon and reducing equivalents, might inhibit the effect of H2O2-induced AMPK phosphorylation via increasing the energy charge of the cell. Therefore, we measured intracellular AMP and ATP levels when the cells were treated with ethanol and in the presence of H2O2 (Table 1). The baseline AMP:ATP ratio in H4IIEC3 cells was 0.03 ± 0.005, and it was not significantly different than the ratio in the cells that were treated with ethanol (50 mM) for 24 h (0.02 ± 0.007, P = 0.39). In each treatment condition, the AMP:ATP ratio at each time point was not significantly altered from baseline. Thus the effects of ethanol and H2O2 on AMPK phosphorylation in this cell line are likely mediated through an AMP-independent pathway.
Table 1.
Effect of ethanol and H2O2 on intracellular AMP-ATP ratio
| Treatment |
Treatment with H2O2, 1 mM |
||||
|---|---|---|---|---|---|
| 0 min | 5 min | 10 min | 30 min | 60 min | |
| Control | 0.027±0.005 | 0.027±0.013 | 0.028±0.014 | 0.019±0.013 | 0.019±0.024 |
| Ethanol 50 mM for 24 h | 0.019±0.007 | 0.013±0.005 | 0.011±0.006 | 0.008±0.002 | 0.009±0.006 |
The table shows the time course of the effect of H2O2 (1 mM) and ethanol (50 mM) on intracellular AMP-ATP ratio. The results are means ± SD (n = 3 replicate assays). The cells were treated as previously described. Intracellular AMP and ATP levels were measured by HPLC. The baseline AMP-ATP ratio in H4IIEC3 cells was 0.03 ± 0.005, and it is not significantly different than the ratio in the cells that were treated with ethanol (50 mM) for 24 h (0.02 ± 0.007, P = 0.39). In each treatment condition, the AMP-ATP ratio at each time point was not significantly altered from baseline.
Effects on PP2A.
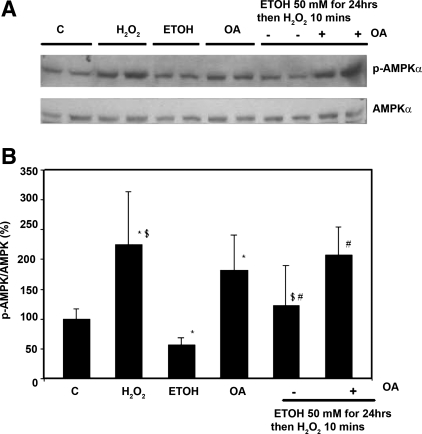
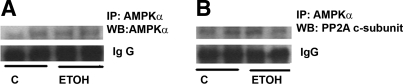
Dephosphorylation of AMPK has been attributed to PP2A (2) or to PP2C-α (15). Since PP2C-α activity is influenced by AMP, and that was not found to be changed by H2O2 or ethanol, we examined a role for PP2A. PP2A designates a family of mammalian serine/threonine phosphatases that is involved in the control of many cellular functions and signaling pathways (19). We examined the effect of an inhibitor of PP2A to determine whether the inhibitory effect of ethanol on AMPK phosphorylation involved effects of ethanol on PP2A. Okadaic acid is a potent inhibitor of protein phosphatases, inhibiting PP2A completely at 1–2 nM and PP1A at higher concentrations, 10–15 nM (5). After overnight serum starvation, H4IIEC3 cells were treated with ethanol 50 mM for 24 h followed by H2O2 (1 mM) for 10 min in the presence or absence of okadaic acid (2 nM). Okadaic acid treatment increased the phosphorylation of AMPK (Fig. 6, A and B); however, it had no effect on LKB1 phosphorylation (data not shown). H2O2 significantly increased AMPK phosphorylation (P < 0.05), and its effect was prevented when the cells were pretreated with ethanol for 24 h. Okadaic acid significantly attenuated the inhibitory effect of ethanol on H2O2-induced AMPK phosphorylation. We next determined the effects of ethanol on PP2A activity in immunoprecipitates of cell lysates. Ethanol increased PP2A activity by 30% (129 ± 5 vs. controls 100 ± 26, P < 0.05). We also attempted to demonstrate physical association of AMPK with PP2A. Immunoprecipitation experiments showed that PP2A could be coimmunoprecipitated with the α-subunit of AMPK (Fig. 7). However, we did not see a change in the amount of PP2A coimmunoprecipitated in cells treated with ethanol (Fig. 7).
Fig. 6.
Effect of okadaic acid on the ability of ethanol to inhibit H2O2-induced AMPK phosphorylation. A and B: H4IIEC3 cells were treated with ethanol (50 mM), H2O2 (1 mM 10 min), and okadaic acid (OA), as described in the text. At the times indicated, proteins were extracted and detected using the specific antibodies in Western blots. Ethanol (50 mM) inhibited the ability of H2O2 to induce AMPK phosphorylation by 1.8-fold ($P < 0.05). Okadaic acid blocked the inhibitory effect of ethanol on AMPK phosphorylation (#P < 0.05). *P < 0.05 compared with control.
Fig. 7.
Coimmunoprecipitation of AMPK and protein phosphatase 2A (PP2A). AMPK was immunoprecipitated (IP) from H4IIEC3 cells, and AMPK or PP2A was detected in Western blots (WB). A: Western blot analysis confirmed the presence of AMPK in the immunoprecipitates. B: AMPK and PP2A can be coimmunoprecipitated although we did not see an increased amount of PP2A associated with AMPK after ethanol treatment (see discussion in text). The blot is a representative of 5 blots from 5 independent experiments.
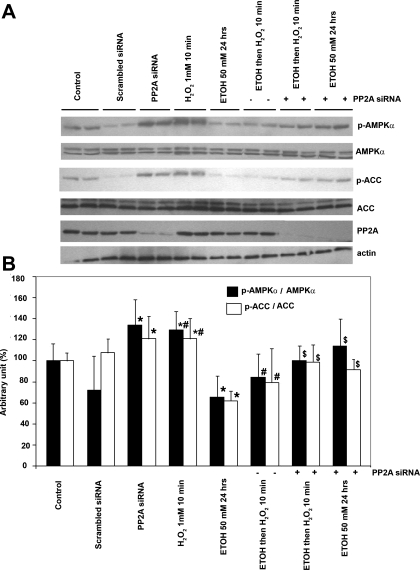
Because of the potential for nonspecific effects of okadaic acid, we further examined whether ethanol inhibited AMPK by activating PP2A in hepatoma cells by using PP2A siRNA (19). PP2A siRNA suppressed the expression of PP2A by 70% at 48 h after transfection (Fig. 8A). Suppression of PP2A significantly increased both basal AMPK and ACC phosphorylation by 33% and 21%, respectively, and it had no effect on LKB1 phosphorylation (data not shown). Importantly, when the cells were transfected with siRNA, the inhibitory effect of ethanol on AMPK and ACC phosphorylation (in the cells that were treated with or without H2O2) was attenuated; see Fig. 8. Taken together, our results implied that ethanol-induced AMPK inhibition in hepatoma cells is due to the activation of PP2A.
Fig. 8.
Effect of PP2A siRNA on the inhibitory effect of ethanol on AMPK phosphorylation. A: H4IIEC3 cells were transfected with PP2A siRNA, where indicated. They were then treated with ethanol (50 mM) and/or H2O2 (1 mM 10 min), as described in the text. At the times indicated, cellular extracts were blotted for the specific proteins. The basal AMPK and ACC phosphorylation were increased in hepatoma cells transfected with PP2A siRNA but not with scrambled siRNA (*P < 0.05). Inhibition of PP2A activity with siRNA abolished the inhibitory effect of ethanol on AMPK and ACC phosphorylation. B: summary data from 3 individual experiments, *P < 0.05, compared with control, #P < 0.05, compared with cells that were not pretreated with ethanol, $P < 0.05, compared with cells that were treated with ethanol alone.
DISCUSSION
This study provided evidence that AMPK can be activated by oxidative stress, using a model of exposure of cultured cells to H2O2, mediated by two of its upstream kinases, PKC-ζ/LKB1 and CaMKK. Ethanol inhibited the effect of H2O2 on AMPK phosphorylation in the hepatoma cells by 1) inhibition of PKC-ζ/LKB1 and 2) activation of PP2A, and this effect requires its metabolism by ADH.
The control of AMPK activity is complex. AMPK can be activated through LKB1 (formerly known as AMPK kinase) and LKB1-independent pathways. The best characterized LKB1-independent kinase was identified using HeLa cells, an LKB1-deficient cell line. 2-Deoxyglucose treatment activated AMPK by phosphorylation on Thr-172, which was significantly inhibited by the CaMKK inhibitor, STO-609 (11). The evidence that CaMKK is another upstream kinase for AMPK was strengthened by the observation that the phosphorylation of AMPK and its downstream target ACC was markedly decreased in HeLa cells transfected with siRNA specific for CaMKK (11). The present study suggests that AMPK activation in HeLa cells by oxidative stress was likely mediated through CaMKK. There was no effect of ethanol on this pathway, and therefore it was not further investigated.
Our laboratory had shown that ethanol treatment of cultured cells and ethanol feeding of mice for 4 wk inhibited the basal activity of AMPK and reduced the amount of AMPK protein (22). In this study, we found that ethanol blocked H2O2-induced AMPK phosphorylation in hepatoma cells. The effect of H2O2 in the hepatoma cells was not blocked by STO-609 (Fig. 2E), suggesting that LKB1 is the major kinase that phosphorylates AMPK in response to oxidative stress.
The role of LKB1 in the regulation of AMPK activity has been revised recently. Purified LKB1 is a trimer of LKB1, STRAD, and MO25. When expressed in vitro, the enzyme is constitutively active and can autophosphorylate (15). The purified enzyme is not sensitive to AMP. Previous reports of activation of LKB1 by AMP were thought to be the result of protein phosphatases (especially PP2C-α) present in the LKB1 preparations. Sanders (15) reported that AMP inhibited the phosphatases, resulting in increased phosphorylation of AMPK. The role of phosphorylation of LKB1 is incompletely understood; however, oxidative stress of peroxynitrite exposure activated PKC-ζ and resulted in LKB1 phosphorylation on serine 428 and phosphorylation of AMPK (21). Mutation of serine 428 abolished the effect of peroxynitrite, showing that this residue was essential for LKB1 activity (21). The recent study by Xie et al. (21) further showed that PKC-ζ acts as an upstream kinase for LKB1. The phosphorylation of LKB1 by this kinase results in export of LKB1 from the nucleus, leading to the activation of AMPK. H2O2 activated the phosphorylation of PKC-ζ and LKB1 in hepatoma cells, and this was inhibited by pretreatment of the cells with ethanol.
Since the hepatoma cells are capable of oxidizing ethanol, and the effect of ethanol was blocked by 4-methylpyrazole, we first considered that the effect of ethanol was due to an effect on cellular metabolism. It had been reported that ethanol increased liver AMP levels in the course of conversion of acetate to acetyl-CoA or as a consequence of inhibition of citric acid cycle activity (25). However, no significant change in the AMP/ATP ratio in the liver was observed with chronic ethanol feeding (12). Similarly, we failed to demonstrate a change in the AMP/ATP ratio in the H4IIEC3 cells cultured with ethanol for 24 h. The activation of AMPK by H2O2 did not appear to correlate with an increase in AMP or AMP:ATP ratios in the cells (Table 1). This finding differs from those reported by Choi et al. (1), who found that the AMP/ATP ratio was elevated in NIH-3T3 cells exposed to oxidative stress, suggesting a cell type-specific effect. Our data suggest that the effect of both H2O2 and ethanol on p-AMPK in the H4IIEC3 cells is not mediated by changes in AMP levels although we acknowledge that dynamic changes or changes limited to subcellular compartments might not have been detected by our experimental methods.
In light of new observations about the regulation of AMPK and LKB1, it seemed possible that the inhibition of AMPK phosphorylation by ethanol might result from activation of phosphatases or inhibition of yet other kinases (2). Ethanol is known to be involved in many signaling pathways such as PKC and MAP kinase (13). Ethanol inhibition of phosphorylation of LKB1 Ser-428 suggests that its effect on AMPK may be in part due to inhibition of LKB1 and of PKC-ζ (Fig. 4A) or activation of the phosphatase (not yet clearly identified), which dephosphorylates LKB1. Since ethanol reduced LKB1 phosphorylation below that of the control cells (Fig. 4), this effect may pertain to how ethanol inhibits AMPK under conditions other than oxidative stress.
We also found that the inhibitory effect of ethanol on AMPK involved PP2A. This conclusion is based on three lines of evidence: the presence of the PP2A inhibitor okadaic acid and PP2A siRNA prevented the inhibitory effect of ethanol on AMPK, and ethanol stimulated the activity of PP2A, suggesting the mechanism shown in Fig. 9. PP2A has been implicated in the inactivation of AMPK by others (19), and we show here for the first time that AMPK and PP2A can be coimmunoprecipitated. Although we could not see an increased amount of PP2A associating with AMPK after ethanol treatment, this may reflect the lack of sensitivity of this assay for small changes in PP2A activity. The mechanism by which ethanol metabolism is required to affect the activity of this phosphatase is not presently clear.
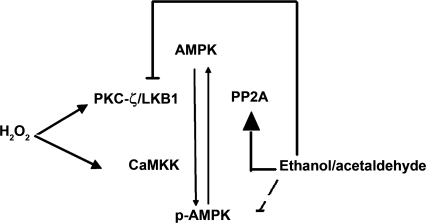
Fig. 9.
Proposed mechanism of H2O2 and ethanol effects on AMPK phosphorylation and activity. In this study, we found that H2O2 activates AMPK via LKB1 and CaMKK. We have previously shown that the inhibitory effect of ethanol on AMPK requires its metabolite, acetaldehyde (denoted by dashed line). We proposed that such effects of ethanol on AMPK phosphorylation are secondary to 1) inhibition of AMPK upstream kinase, LKB1, and 2) increasing AMPK dephosphorylation through PP2A (activation, inhibition).
In conclusion, the present study has several implications. The effect of ethanol on AMPK could be central to the net effects of ethanol on liver lipid metabolism. Given the ability of ethanol to block the effect of oxidative stress on AMPK, the balance between the rate of ROS formation (which induces AMPK phosphorylation and activity) and the ability of ethanol to inhibit AMPK is crucial. We previously postulated that AMPK activator (such as metformin) might alleviate alcohol-induced fatty liver; however, ethanol also blocked the action of metformin. On the basis of the results of this study, PP2A and the pathways that regulate its activity should be considered as other therapeutic targets that could prevent alcoholic fatty liver disease. Furthermore, these studies suggest ways by which ethanol could have synergistic effects with other hepatotoxins on lipid metabolism.
GRANTS
This study is supported by R01 AA15070 and P60 AA07611 from the NIAAA (D. Crabb), Veterans Administration Young Investigator Award/Indiana Institute for Medical Research (S. Liangpunsakul), K08 AA016570 (S. Liangpunsakul), and Veterans Administration Merit Review Award (H. Jayaram).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo KS, Ha J. The regulation of AMP-activated protein kinase by H2O2. Biochem Biophys Res Commun 287: 92–97, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett 377: 421–425, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Evans T, Jin H, Elkins N, Shapiro JI. Effect of acidosis on hydrogen peroxide injury to the isolated perfused rat heart. Am J Physiol Heart Circ Physiol 269: H308–H312, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Galli A, Price D, Crabb D. High-level expression of rat class I alcohol dehydrogenase is sufficient for ethanol-induced fat accumulation in transduced HeLa cells. Hepatology 29: 1164–1170, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez J, Cornejo A, Santos MR, Cordero EM, Gutierrez B, Porcile P, Mortara RA, Sagua H, Da Silveira JF, Araya JE. A novel protein phosphatase 2A (PP2A) is involved in the transformation of human protozoan parasite Trypanosoma cruzi. Biochem J 374: 647–656, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 282: 32549–32560, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardie DG New roles for the LKB1–AMPK pathway. Curr Opin Cell Biol 17: 167–173, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG Biochemistry. Balancing cellular energy. Science 315: 1671–1672, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG, Carling D, Halford N. Roles of the Snf1/Rkin1/AMP-activated protein kinase family in the response to environmental and nutritional stress. Semin Cell Biol 5: 409–416, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Hong SP, Momcilovic M, Carlson M. Function of mammalian LKB1 and Ca2+/calmodulin-dependent protein kinase kinase alpha as Snf1-activating kinases in yeast. J Biol Chem 280: 21804–21809, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280: 29060–29066, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Kono H, Bode JC, Martini GA. Effect of acute and chronic ethanol administration on the content of coenzymes linked to energy transfer in the liver of rats fed standard or low-protein diet. Gastroenterol Jpn 14: 226–232, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Morrow AL, Ferrani-Kile K, Davis MI, Shumilla JA, Kumar S, Maldve R, Pandey SC. Ethanol effects on cell signaling mechanisms. Alcohol Clin Exp Res 28: 217–227, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Paulik E, Jayaram HN, Weber G. Determination of NAD pyrophosphorylase activity in biological samples. Anal Biochem 197: 143–148, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomita K, Tamiya G, Ando S, Kitamura N, Koizumi H, Kato S, Horie Y, Kaneko T, Azuma T, Nagata H, Ishii H, Hibi T. AICAR, an AMPK activator, has protective effects on alcohol-induced fatty liver in rats. Alcohol Clin Exp Res 29: 240S–245S, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Wu D, Zhai Q, Shi X. Alcohol-induced oxidative stress and cell responses. J Gastroenterol Hepatol, 21 Suppl 3: S26–S29, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem 282: 9777–9788, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117: 952–962, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem 281: 6366–6375, 2006. [DOI] [PubMed] [Google Scholar]
- 22.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127: 1798–1808, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem 278: 34003–34010, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Zydowo MM, Smolenski RT, Swierczynski J. Acetate-induced changes of adenine nucleotide levels in rat liver. Metabolism 42: 644–648, 1993. [DOI] [PubMed] [Google Scholar]