Abstract
The transcription factor NF-κB plays a critical role in inflammatory and cell death responses during acute pancreatitis. Previous studies in our laboratory demonstrated that protein kinase C (PKC) isoforms PKCδ and ɛ are key regulators of NF-κB activation induced by cholecystokinin-8 (CCK-8), tumor necrosis factor-α, and ethanol. However, the downstream participants in regulating NF-κB activation in exocrine pancreas remain poorly understood. Here, we demonstrate that protein kinase D1 (PKD1) is a key downstream target of PKCδ and PKCɛ in pancreatic acinar cells stimulated by two major secretagogues, CCK-8 and the cholinergic agonist carbachol (CCh), and that PKD1 is necessary for NF-κB activation induced by CCK-8 and CCh. Both CCK-8 and CCh dose dependently induced a rapid and striking activation of PKD1 in rat pancreatic acinar cells, as measured by in vitro kinase assay and by phosphorylation at PKD1 activation loop (Ser744/748) or autophosphorylation site (Ser916). The phosphorylation and activation of PKD1 correlated with NF-κB activity stimulated by CCK-8 or CCh, as measured by NF-κB DNA binding. Either inhibition of PKCδ or ɛ by isoform-specific inhibitory peptides, genetic deletion of PKCδ and ɛ in pancreatic acinar cells, or knockdown of PKD1 by using small interfering RNAs in AR42J cells resulted in a marked decrease in PKD1 and NF-κB activation stimulated by CCK-8 or CCh. Conversely, overexpression of PKD1 resulted in augmentation of CCK-8- and CCh-stimulated NF-κB activation. Finally, the kinetics of PKD1 and NF-κB activation during cerulein-induced rat pancreatitis showed that both PKD1 and NF-κB activation were early events during acute pancreatitis and that their time courses of response were similar. Our results identify PKD1 as a novel early convergent point for PKCδ and ɛ in the signaling pathways mediating NF-κB activation in pancreatitis.
Keywords: PKC delta, PKC epsilon, pancreatitis, carbachol, cerulein
pancreatitis is characterized by inflammation and necrosis of the parenchymal (i.e., acinar and ductal) cells in the pancreas, and a substantial body of evidence has indicated that the inflammatory responses play a central role in the mechanism of pancreatitis (10, 28, 29, 47). The nuclear transcription factor NF-κB is one of several key signaling systems that mediate the production of proinflammatory cytokines, chemokines, immune receptors, and other inflammatory molecules (10, 28, 29, 44, 47), which subsequently lead to the severe systemic inflammatory complications of this disease (10, 28, 44). In vivo studies in experimental pancreatitis demonstrated that NF-κB activation in acinar cells is one of the earliest events in pancreatitis, and the inhibition of NF-κB activation attenuates the severity of pancreatitis (10, 37, 47). Furthermore, the direct activation of NF-κB within the pancreas by adenovirus-mediated gene transfer is sufficient for the initiation of pancreatic and systemic inflammatory responses (5). Therefore, elucidating the signaling mechanisms underlying NF-κB activation is of critical importance for understanding the pathophysiology of pancreatitis.
Cholecystokinin-8 (CCK-8) is an agonist of pancreatic digestive enzyme secretion at physiological doses. However, supramaximally stimulating doses of CCK-8 cause the inflammatory and cell death responses that are features of human pancreatitis (10, 51). In vivo and in vitro experiments using pancreatic acini demonstrated that supramaximal stimulation with CCK-8 activates NF-κB through IκB degradation (10, 11). In addition to CCK, the cholinergic system also mediates exocrine pancreas functions and pancreatitis responses. Accumulated evidence in both animal and human studies indicates that the pancreatic response to low and physiological doses of CCK is due to CCK's ability to activate neural pathways and the cholinergic receptors on the acinar cell (1, 26). The cholinergic agonist carbachol (CCh) has been demonstrated to stimulate the NF-κB pathway in rat pancreatic acinar cells (19, 56) and other cell types (18, 31). Furthermore, cholinergic hyperstimulation of the pancreas has been implicated as a mechanism of alcohol-induced pancreatitis responses including NF-κB activation (19). Thus it is necessary to further define the intracellular signaling mechanisms by which CCK-8 and CCh stimulate NF-κB activation.
PKCs are a family of serine/threonine kinases comprising 10 isoforms, namely conventional PKC isoforms (α, βI, βII, and γ), novel PKC isoforms (δ, ɛ, η, and θ), and atypical PKC isoforms (ζ and λ/ι) (24). Each PKC isoform can be activated independently by specific stimuli and mediates distinct biological functions (25). PKCs have been shown to stimulate NF-κB activation in various cell types and thus mediate cell survival as well as proinflammatory signaling (16, 27, 38, 55). In pancreatic acinar cell, four PKC isoforms, α, δ, ɛ, and ζ, have been detected (3). Although we demonstrated that the novel PKC isoforms PKCδ and ɛ are key regulators of NF-κB activation induced by CCK-8 (38), the downstream signaling targets of these PKCs within the NF-κB pathway in pancreatitis remain to be identified.
Protein kinase D1 (PKD1, also known initially as PKCμ), a serine/threonine protein kinase with structural, enzymological, and regulatory properties different from the PKC family members, has recently emerged as a major target in the signal transduction pathways initiated by diacylglycerol (DAG) and PKC in a variety of cell types (46). PKD1 is the founding member of the protein kinase D family, which includes PKD1, PKD2, and PKD3 (reviewed in Ref. 35). PKD1 can be activated in intact cells by multiple stimuli, including tumor promoting phorbol esters, G protein-coupled receptor (GPCR) agonists, growth factors and antigen-receptor engagement (35, 52, 57). In many cases, rapid PKD1 activation is mediated by PKC-dependent phosphorylation of Ser-744 and Ser-748 in PKD1 activation loop (32, 48, 50). Particularly, the novel isoforms of PKC including δ, ɛ, η, and θ have been identified as upstream kinases of PKD1 (42, 48, 50, 54). PKD family members are increasingly implicated in the regulation of multiple cellular functions including protein secretion (7, 17), phosphorylation of heat shock protein 27 (53) and histone deacetylase (22), cell proliferation (35), and apoptosis (45). Recent studies revealed that PKDs play a critical role in induction of gene transcription through activation of nuclear transcription factors c-jun (12, 49), the cAMP-response element-binding protein (14), and, particularly, NF-κB (6, 23, 41–43). For example, in Hela cells, PKCδ-dependent PKD1 activation loop phosphorylation regulates NF-κB activation in response to oxidative stress (41–43). PKD2 mediates NF-κB activation by Bcr-Abl in myeloid leukemia cells (23). PKD2 is also involved in lysophosphatidic acid-induced and PKC-dependent NF-κB activation and interleukin-8 production in colonic epithelial cells (6).
More recently, PKD1 was reported to be regulated by CCK in pancreatic acinar cells through predominantly PKCδ-dependent pathway (4). The roles of PKD1 in acinar cells, and specifically in NF-κB activation in pancreatitis, have not been explored. Thus in the present study we addressed the possibility that PKD1 lies downstream of PKCs in a signal transduction pathway activated by exocrine pancreas secretagogues CCK-8 and CCh and mediates NF-κB activation. Our results identify PKD1 as a novel early convergent point of PKCδ and ɛ in the signaling pathways triggered through CCK-8 or cholinergic receptor and demonstrate, for the first time, that PKD1 mediates NF-κB activation in pancreatitis.
MATERIALS AND METHODS
Reagents.
CCK-8 was from American Peptide (Sunnyvale, CA); TPA (12-O-tetradecanoylphorbol-13-acetate) was from Sigma-Aldrich (St. Louis, MO). Medium 199 was from GIBCO (Grand Island, NY). Medium F-12K was from the American Type Culture Collection (ATCC) (Manassas, VA). ATP and [γ-32P]ATP were from Amersham (Piscataway, NJ) and Perkin Elmer (Torrance, CA). Nitrocellulose membranes were from Schleicher and Schuell BioScience. Carbachol, GF1 (also known as GF 109203X or bisindolylmaleimide I), and PKD3 polyclonal antibody were from Calbiochem (La Jolla, CA). Antibodies against PKD C-20, PKCɛ, PKCδ, or IκB-α were from Santa Cruz Biotechnology (Santa Cruz, CA). PKD2 polyclonal antibody was obtained from Bethyl Laboratories (Montgomery, TX) and Upstate (Lake Placid, NY). Phosphoserine 744/748 PKD antibody that detects primarily the phosphorylated state of Ser744 (13), phosphoserine 916 PKD antibody, and phosphoserine 32/36 IκB-α antibody were obtained from Cell Signaling Technology (Beverly, MA). Protein-A-agarose was from Roche Applied Science (Mannheim, Germany) and PKD substrate, syntide-2, was from Bachem (Chicago, IL). The specific PKC inhibitors and PKCɛ activator peptides were synthesized as described previously (38, 36). Other items were from standard suppliers or as indicated in text.
Preparation and treatments of dispersed pancreatic acini.
Pancreatic acinar cells were prepared from Sprague-Dawley rats (75–100 g) by use of a collagenase digestion method as described previously (10) and then incubated in medium 199 supplemented with 0.01% trypsin inhibitor (wt/vol), penicillin (100 U/ml), and streptomycin (0.1 mg/ml) at 37°C in a 5% CO2-humidified atmosphere. For experimental purposes, the acinar cells were preincubated in the medium 199 for 3 h at 37°C with or without inhibitors as described previously (38) and then treated further with or without agonists. Acinar cells were collected and washed with PBS. Aliquots of the acinar cells were lysed and sonicated in lysis buffer as described previously (38). After centrifugation at 15,000 g for 10 min at 4°C, the protein concentration in supernatants was measured by use of the Bio-Rad protein assay reagent.
Western blot analysis, PKD immunoprecipitation, and in vitro kinase assay.
Western blot analyses were performed as described previously (38). PKD1 in pancreatic acinar cell or pancreatic tissue lysates was immunoprecipitated at 4°C for 3 h with the PKD C-20 antibody (1:100) and protein-A-agarose as previously described (52, 54). Exogenous substrate syntide-2 phosphorylation by immunoprecipitated PKD1 was carried out by mixing 20 μl of the washed immunocomplexes with 10 μl of a phosphorylation mixture containing 100 μM ATP (including [γ-32P]ATP at 2 μCi/assay or with specific activity, 400–600 cpm/pmol) and 2.5 mg/ml syntide-2 (PLARTLSVAGLPGKK) in kinase buffer. After 10 min of incubation at 30°C, the reaction was stopped by adding 100 μl of 75 mM H3PO4, and 75 μl of the supernatant was spotted on P-81 phosphocellulose paper. Free [γ-32P]ATP was separated from the labeled substrate by washing the P-81 paper four times for 5 min in 75 mM H3PO4. The papers were dried, and the radioactivity was incorporated into syntide-2 was determined by Cerenkov counting.
Preparation of nuclear extracts and NF-κB DNA binding activity measurement.
Nuclear protein extracts were prepared using ActiveMotif nuclear extract kit (Carlsbad, CA) following the manufactory instructions. NF-κB DNA binding activities were measured with electrophoretic mobility shift assay (EMSA) as described previously (10) or with ELISA (for pancreas tissue) using ActiveMotif NF-κB p65 Transcription factor assay kit following the manufacturer's instructions.
PKCδ−/− mice and PKCɛ−/− mice.
PKCδ−/− mice have been described previously (40) and were obtained from Dr. Keiichi I. Nakayama (Kyushu University, Fukuoka, Japan). PKCɛ−/− mice, as described in Ref. 15, were obtained from Dr. Robert O. Messing (University of California, San Francisco, CA). Genetic deletions of PKCδ and PKCɛ were confirmed by PCR and Western blot. Our animal experiments for this study were approved by IACUC at both University of Southern California and VA Los Angeles Health Care System.
Cell culture and transient transfection.
The rat pancreatic acinar AR42J cells were obtained from ATCC and cultured in F-12K medium supplemented with 20% fetal bovine serum at 37°C with a humidified atmosphere containing 5% CO2. Transfection of AR42J cells with cDNA plasmids or small interfering RNAs (siRNAs) was done using Nucleofector II (Amaxa, Gaithersburg, MD) according to the manufacturer's protocol. The plasmid constructs encoding the green fluorescent protein (GFP)-PKD2, GFP-PKD3, and PKD1, described previously (33, 46), or pcDNA3 were applied in the transfection with equivalent amounts (6 μg/6-cm dish containing ∼8 × 106 cells). For siRNA transfection, PKD1 siRNAs pool (pool of 4 duplexes) from Dharmacon (Chicago, IL) were used. The sequences of the four mouse PKD1 siRNAs (sense strands) are as follows: GAAGAGAUGUAGCUAUUAA; GAAAGAGUGUUUGUU-GUUA; CAUAAGAGAUGUGCAUUUA; and CAGCGAAUGUAGUGUAUUA. siCONTROL nontargeting siRNA pool (pool of 4 duplexes) from Dharmacon was used as a control. Forty-eight hours after transfection, cells were used for experiments. The efficiency of transfection assessed by expression of the GFP-containing plasmid was 70–80% in AR42J cells.
Experimental pancreatitis and preparation of tissue lysates.
Cerulein pancreatitis was induced in male (200–250 g) Sprague-Dawley rats by up to four hourly intraperitoneal injections of 20 μg/kg cerulein. Control animals received similar injections of physiological saline. The animals were euthanized at 30 min, 1.5, and 3.5 h after the first injection, and the pancreas tissue was harvested and saved at −80°C. Portions of frozen tissue were homogenized on ice in lysis buffer as described above. After sonication the lysates were rotated for 40 min at 4°C and centrifuged at 4°C for 15 min at 16,000 g. The supernatants were collected and stored at −80°C.
RESULTS
PKD1 is the predominant PKD isoform expressed in rat pancreatic acinar cells.
Initially, we examined which PKD isoform(s) is expressed in rat pancreatic acinar cells by Western blot analysis of the cell lysate with specific antibodies of PKD1, PKD2, and PKD3 (see Supplemental Fig. S1) (the online version of this article contains supplemental data). Our results indicate that PKD1 protein is the predominant PKD isoform endogenously expressed in rat pancreatic acinar cells. Thus the rat pancreatic acinar cells provide a model system to study the regulation and function of PKD1 without significant contribution of the other PKD isoforms.
CCK-8 and CCh induce both PKD1 phosphorylation/activation and NF-κB activation.
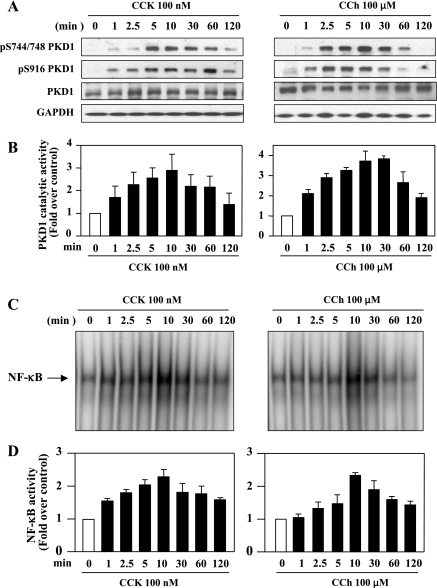
To characterize the regulation of PKD1 by CCK-8 and CCh in pancreatic acinar cells and determine a possible relation between PKD1 activation and NF-κB activation, we observed the kinetics of CCK-8- and CCh-induced PKD1 phosphorylation and activation as well as NF-κB activation. Isolated rat pancreatic acinar cells were stimulated with supramaximal concentrations of CCK-8 (100 nM) or CCh (100 μM) for various times (1–120 min) (Fig. 1A). The lysates were analyzed by Western blotting using antibodies that detected the phosphorylated state of the activation loop Ser744/748 and the COOH-terminal autophosphorylation site Ser916 of PKD1 (21). Stimulation of acinar cells with CCK-8 or CCh induced a rapid and prominent PKD1 phosphorylation at both Ser744/748 and Ser916 in a time-dependent manner (Figs. 1A). Furthermore, the data from in vitro kinase assays measuring synthetic substrate syntide-2 phosphorylation by PKD1 showed that treatment of the acinar cells with CCK-8 or CCh induced a rapid and dramatic (up to 3- to 4-fold in 5–10 min of stimulation) increase in PKD1 kinase activity (Figs. 1B). These results indicate that PKD1 phosphorylation and activation is regulated by CCK-8 and CCh in pancreatic acinar cells.
Fig. 1.
CCK-8 and carbachol (CCh) induce PKD1 phosphorylation and activation and NF-κB activation in rat pancreatic acinar cells. Rat pancreatic acinar cells were incubated with 100 nM CCK-8 (CCK) or 100 μM CCh for the indicated time periods. A: Western blotting analyses of the time course of CCK8- and CCh-induced PKD1 phosphorylation by use of PKD1 pS744/748 antibody or PKD1 pS916 antibody. Blots were reblotted for PKD1 expression with PKD C-20 antibody, and then for GAPDH to verify equal protein loading. All Western blots shown are representative of 3 independent experiments. B: time course of CCK8- and CCh-induced PKD-1 catalytic activation. The acinar lysates were immunoprecipitated using PKD C-20 antibody, and PKD1 activity in the immunocomplexes was determined by in vitro kinase assay. Results are expressed as a fold increase over unstimulated control in activity. Values are means ± SE (n = 3). C: time course of CCK-8 and CCh-induced NF-κB binding activity measured by EMSA. D: NF-κB band intensities were quantified in the PhosphorImager and normalized on the band intensity in unstimulated control. Values are means ± SE (n = 3).
Both CCK-8 and CCh dose dependently stimulated PKD1 phosphorylation and activation (see Supplemental Fig. S2). CCK-8 or CCh induced PKD1 phosphorylation and activation at the concentration as low as 0.1 nM or 1 μM, respectively, a physiological level of CCK-8 or CCh that causes maximal digestive enzyme secretion. The maximal effects of these agonists on PKD1 were achieved at 10–100 nM CCK-8 or at 100–1,000 μM CCh, i.e., at their supramaximal concentrations that cause inhibition of enzyme secretion and activation of the inflammatory response (2, 8, 9, 51). These results indicate that PKD1 is activated by CCK-8 and CCh at both physiological and pathological doses.
We next compared these PKD1 results with the kinetics of NF-κB activation induced by CCK-8 or CCh. The nuclear extracts from rat pancreatic acinar cells in the same experiments were submitted to EMSA to measure NF-κB DNA binding activity (Fig. 1, C and D). Both CCK-8 and CCh stimulated a time-dependent activation of NF-κB that was correlated with PKD1 phosphorylation/activation. Increases in both PKD1 activity and NF-κB activity were detected as early as 1 min after the addition of the agonists, reached a maximum in 10 min, and remained detectable in ∼60 min of stimulation. These parallel relationships suggest the possibility that PKD1 may be involved in NF-κB activation by these two agonists.
Inhibition of PKCɛ and δ prevents CCK-8- and CCh-induced PKD1 and NF-κB activation.
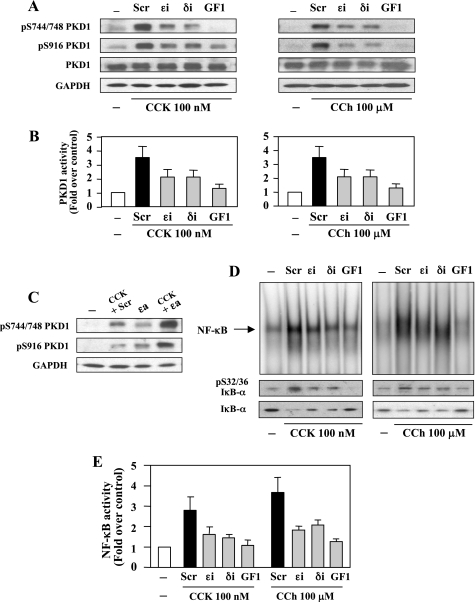
Our previous studies indicated that PKCδ and ɛ activity, but not PKCα and ζ, are necessary for mediation of NF-κB activation caused by CCK-8 in pancreatic acinar cells (38). Here we hypothesized that PKD1 mediates PKCδ- and/or ɛ-dependent NF-κB activation in response to CCK-8 and cholinergic signaling. We applied isoform-specific and cell-permeable PKC inhibitory peptides to examine whether the novel isoforms PKCδ and ɛ mediate both PKD1 activation and NF-κB activation in acinar cells treated with CCK-8 or CCh.
Rat pancreatic acinar cells were treated for 3 h with a broad-spectrum PKC inhibitor GF1 (3.5 μM), a specific PKCδ translocation inhibitor (δi, 10 μM), PKCɛ translocation inhibitor (ɛi, 10 μM), or a scrambled peptide (Scr, 10 μM), as described in previous studies (36, 38). Then the acinar cells were stimulated for 10 min with CCK-8 (100 nM) or CCh (100 μM). All three inhibitors prevented PKD1 phosphorylation (Fig. 2A) and catalytic activation (Fig. 2B) induced by CCK-8 or CCh. The broad-spectrum PKC inhibitor GF1 that has been demonstrated to not directly inhibit PKDs in multiple cell types (6, 33, 35, 58) almost completely prevented PKD1 activation. On the other hand, inhibitors δi and ɛi partially (50–70%) suppressed PKD1 activation, indicating that these two novel PKCs both contribute to the CCK-8- and CCh-induced PKD1 activation.
Fig. 2.
PKC inhibitors prevent CCK-8- and CCh-stimulated PKD1 and NF-κB activation in pancreatic acinar cells. Rat pancreatic acinar cells were incubated for 3 h with PKCɛ translocation inhibitor (ɛi), PKC-δ translocation inhibitor (δi), or scrambled peptide (Scr) (10 μM each), or with the broad-spectrum PKC inhibitor GF1 (3.5 μM) before stimulation with 100 nM CCK-8 or 100 μM CCh for 10 min. A: cell lysates were analyzed by Western blot using PKD1 pS744/748 antibody or PKD1 pS916 antibody. The blots were reprobed for PKD1 then for GAPDH to verify equal protein loading. B: the acinar cell lysates were immunoprecipitated with PKD C-20 antibody, and PKD1 catalytic activity in the immunocomplexes was determined by in vitro kinase assay. The results are expressed as a fold increase over unstimulated control in activity. Values are means ± SE (n = 3). C: rat pancreatic acinar cells were incubated for 3 h with PKCɛ translocation activator (ɛa) or scrambled peptide (10 μM each), and 0.1 nM CCK was added during the last 10 min of the incubation. Cell lysates were analyzed by Western blot using PKD1 pS744/748 antibody or PKD1 pS916 antibody. The blots were reprobed for GAPDH to verify equal protein loading. D: NF-κB binding activity was measured in nuclear extracts by EMSA. IκΒ-α phosphorylation and degradation were measured in cytosolic extracts from the same samples by Western blot analysis. E: NF-κB band intensities were quantified in the PhosphorImager and normalized on the band intensity in unstimulated control acini. Values are means ± SE (n = 3).
Multiple in vivo and in vitro studies demonstrated that PKCɛ play an important role in GPCR agonist-induced PKD1 activation (32, 35, 48). In particular, the plasma membrane translocation of PKCɛ was necessary for the activation loop phosphorylation of PKD1 (32, 35). Here, our results show that, in addition to PKCδ, PKCɛ is also involved in PKD1 activation in pancreatic acinar cells. However, a recent report (4) indicated that CCK-8-induced PKD1 activation in pancreatic acinar cells was predominantly PKCδ dependent. To further confirm the role of PKCɛ in CCK-8-induced PKD1 activation in acinar cells, we treated the cells with the combination of a PKCɛ translocation activator (ɛa) and a low-dose CCK-8 (0.1 nM). Our previous studies demonstrated that 0.1 nM CCK-8 activated PKCδ only (36). As illustrated in Fig. 2C, either 0.1 nM CCK-8 alone or PKCɛa alone caused partial activation of PKD1 demonstrated by phosphorylation at Ser744/748 and Ser916, suggesting that the activation of either PKCɛ or PKCδ alone is not sufficient to produce full PKD1 activation. When acinar cells were incubated with 0.1 nM CCK-8 together with the PKCɛa, a marked increase in PKD1 phosphorylation of Ser744/748 and Ser916 were observed (Fig. 2C). These results demonstrate that full PKD1 activation requires the activation of both PKCδ and PKCɛ.
To determine whether conventional or atypical PKCs are involved in PKD1 activation, we pretreated the acinar cells with a specific inhibitor of conventional PKC isoforms, Gö6976, or a PKCζ inhibitor, PKCζ pseudosubstrate, before stimulation with CCK-8 or CCh. Neither of these inhibitors suppressed PKD1 activation (data not shown).
We then determined NF-κB activation by measuring NF-κB DNA binding activity and IκB-α phosphorylation and degradation. Similar to PKD1 inhibition, both the CCK-8- and CCh-induced NF-κB activation were partially prevented by the specific inhibitor δi or ɛi (50–70%) and almost completely abolished by the broad-spectrum PKC inhibitor GF1 (Fig. 2, D and E). IκB-α phosphorylation and degradation, the prelude to NF-κB activation, correlated with the increased NF-κB binding activity in CCK-8- or CCh-treated cells. Either δi or ɛi blocked IκB-α phosphorylation and degradation consistently with their inhibitory effects on NF-κB binding activity (Fig. 2D). These data are in accord with those reported in our previous studies (38).
Thus our results indicate that the novel isoforms PKCδ and ɛ which are essential mediators of NF-κB activation in pancreatic acinar cells (36, 38) are also responsible for CCK-8- and CCh-stimulated PKD1 activation, suggesting that PKCδ and ɛ are upstream kinases in PKD1 and NF-κB activation induced by these two secretagogues. These results support our hypothesis that PKD1 is transducing the upstream PKC signal to activate NF-κB, and both CCK-8 and CCh induce NF-κB activation through a PKCs/PKD1 phosphorylation cascade.
Genetic deletions of PKCδ and PKCɛ prevent PKD1 activation stimulated by CCK-8 or CCh in pancreatic acinar cells.
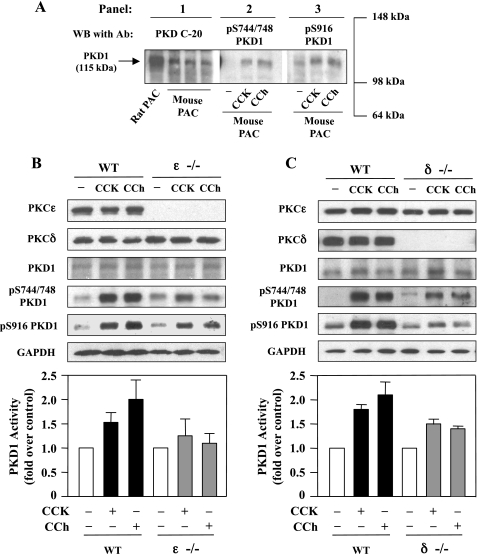
We further probed the roles of PKCδ and ɛ in PKD1 activation by using PKCδ or ɛ knockout mice (Fig. 3). We first examined whether PKD1 was expressed in mouse pancreatic acinar cells and activated by CCK-8 and CCh. Western blot analysis with PKD1 C-20 antibody (Fig. 3A, panel 1) showed that PKD1 was present in mouse acinar cells although its expression level was relatively lower than that in rat pancreatic acinar cells. Similar to the rat, both CCK-8 and CCh induced PKD1 phosphorylation at Ser744/748 and the COOH-terminal Ser916 in mouse acinar cells (Fig. 3A, panels 2 and 3). Although the anti-pS744/748 PKD antibody recognizes the phosphorylated serines in the activation loop of all three PKD isoforms and the anti-pS916 PKD antibody recognizes the phosphorylated COOH-terminal serine of PKD1 and PKD2, here we detected only a single phosphorylated PKD band that comigrates with PKD1.
Fig. 3.
PKD1 phosphorylation and activation induced by either CCK-8 or CCh is reduced in mice deficient in PKCɛ or PKCδ. A: mouse pancreatic acinar cells (PAC) were incubated for 10 min with 100 nM CCK-8 or with 100 μM CCh and the cell lysates were analyzed by Western blotting using antibodies against PKD C-20 (panel 1), pS744/748 PKD1 (panel 2), or pS916 PKD1 (panel 3). B and C: pancreatic acinar cells isolated from wild-type (WT) mice and mice deficient in PKCɛ (ɛ−/−) and PKCδ (δ−/−) were incubated with 100 nM CCK-8 or 100 μM CCh for 10 min at 37°C and the cell lysates were analyzed by Western blot with antibodies against PKCɛ, PKCδ, PKD1 C-20, pS744/748 PKD1, or pS916 PKD1. The blots were then reprobed for GAPDH to verify equal protein loading. Bar graphs: mouse acinar cell lysates were immunoprecipitated with PKD C-20 antibody and PKD1 catalytic activity in the immunocomplexes was determined by in vitro kinase assay. The results are expressed as a fold increase over unstimulated control in activity. Values are means ± SE (n = 2). Representative of 2 independent experiments.
Next, we examined the CCK-8- or CCh-induced PKD1 activation in pancreatic acinar cells isolated from mice deficient in PKCɛ or δ. With Western blotting, we confirmed that PKCɛ and δ expression were absent in PKCɛ knockout homozygote and PKCδ knockout homozygote, respectively (Fig. 3, B and C). Deficiency in either PKCɛ or δ did not affect the expression of PKD1 in acinar cells. Stimulation of wild-type mouse acinar cells with CCK-8 or CCh caused a dramatic increase in PKD1 phosphorylation in Ser744/748 and Ser916 and in PKD1 catalytic activity. Both the CCK-8- and CCh-stimulated PKD1 phosphorylation and catalytic activation were markedly reduced in either PKCɛ−/− or δ−/− acinar cells. These data further support the roles of PKCɛ and δ in PKD1 activation in pancreatic acinar cells.
PKD1 is the predominant PKD isoform in rat pancreatic acinar cell line AR42J and is phosphorylated by CCK-8 and CCh through PKC-dependent pathways.
The well-differentiated rat pancreatic acinar cell line AR42J is an established model for studying intracellular mechanisms involved in pancreatitis responses in vitro (20, 39). Different from primary pancreatic acinar cells that are difficult to keep responsive and viable in long-term culture for transfection, AR42J cells allow transfections and other molecular approaches (20, 30). Our previous studies (39) have shown that PKCδ and ɛ mediate NF-κB activation induced by TNF-α in AR42J cells. Here, we utilized this cell line to investigate the role of PKD1 in NF-κB activation induced by CCK-8 and CCh.
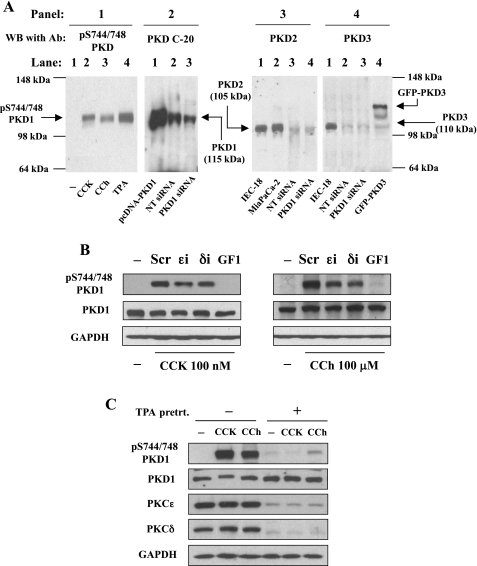
We first examined whether PKD1 as well as the other PKD isoforms are expressed in AR42J cells and whether PKD1 expression can be suppressed by transfection with siRNA specifically targeting PKD1 sequence. As illustrated in Fig. 4A, panel 2, AR42J cells transfected with a nontargeting siRNA (NT siRNA) yielded a very prominent single 115-kDa band that comigrated with the overexpressed PKD1 (as a positive control) in pcDNA3-PKD1-transfected AR42J cells. Transfection with specific PKD1 siRNAs (a pool of 4 siRNAs) resulted in a diminished intensity of the endogenous PKD1 band. However, in the PKD2 antibody blot (Fig. 4A, panel 3), we identified only a very faint band in AR42J cell lysate (NT siRNA, lane 3), which migrated with an apparent molecular mass of 105 kDa, corresponding to the PKD2 band in rat IEC-18 cells and Miapaca-2 cells known to highly express PKD2. Expression of PKD2 was not affected by specific PKD1 siRNA transfection. Similarly, with a specific PKD3 antibody that showed good immunoreactivity for GFP-PKD3 in transfected AR42J cells and endogenous PKD3 in IEC-18 cells (Fig. 4A, panel 4), we detected a very weak immunoreactive signal corresponding to endogenous PKD3 protein (110 kDa) in AR42J cells. Transfection with PKD1 siRNAs did not change the expression level of the endogenous PKD3 (Fig. 4A, panel 4). PKD1 was activated by CCK-8 and CCh as well as the phorbol ester TPA in AR42J cells. Similar to primary pancreatic acinar cells (see Supplemental Fig. S1), only a single phosphorylated PKD band was detected in AR42J, which comigrates with PKD1, but not with PKD2 or PKD3 (Fig. 4A, panel 1). Collectively, the results shown in Fig. 4A indicate that, similar to rat pancreatic acinar cells, PKD1 is also the predominant PKD isoform endogenously expressed in AR42J cells.
Fig. 4.
PKD1 is the predominant PKD isoform in AR42J cells and is activated by CCK-8 and CCh through a PKC-dependent pathway. A, panel 1: Western blot analysis using anti-pS744/748 PKD antibody for the lysate samples from AR42J cells incubated for 10 min at 37° with 10 nM CCK-8 or 100 μM CCh. Panel 2: lysates from AR42J cells transfected with pcDNA3-PKD1, nontargeting siRNAs (NT), or PKD1 siRNAs were analyzed by Western blot with PKD C-20 antibody that recognizes the COOH-terminal region of both PKD1 and PKD2. Panel 3: Western blot with a specific PKD2 antibody for the lysate samples from rat IEC-18, MiaPaCa-2, AR42J transfected with nontargeting siRNAs, or PKD1 siRNAs. Panel 4: Western blot with a specific PKD3 antibody for lysates from AR42J cells transfected with nontargeting siRNAs or PKD1 siRNAs using the lysates from IEC-18 cells and AR42J cells transfected with GFP-PKD3 as positive controls of PKD3. All Western blot results are representatives of 3 experiments. B: AR42J cells were incubated for 3 h with PKCɛ translocation inhibitor, PKCδ translocation inhibitor (δi), or scrambled peptide, 10 μM each, or with 3.5 μM broad-spectrum PKC inhibitor GF1. Then 100 nM CCK-8 or 100 μM CCh was added during the last 10 min of the incubation. Cell lysates were analyzed by Western blot using PKD1 pS744/748 antibody. The blots were reprobed for PKD1 expression by using PKD C-20 antibody and then for GAPDH to verify equal protein loading. C: serum-starved cultures of AR42J cells were incubated for 40 h either without (−) or with (+) 1 μM 12-O-tetradecanoylphorbol-13-acetate (TPA). The cells were washed and incubated for 4 h at 37°C in serum-free medium. Cells were then incubated for a further 10 min without or with CCK-8 (100 nM) or CCh (100 μM). Cell lysates were analyzed by Western blot with antibodies against pS744/748 PKD1, PKD1 C-20, PKCɛ, and PKCδ. To verify equal protein loading, the blots were reblotted for GAPDH.
Next, using a broad-spectrum PKC inhibitor GF1 and specific PKC inhibitor δi and ɛi, we showed that both CCK-8- and CCh-induced PKD1 phosphorylation at Ser744/748 were prevented partially by δi and ɛi and abolished by the broad-spectrum PKC inhibitor GF1 (Fig. 4B). We further explored the involvement of PKCδ and ɛ in PKD1 activation by experiments in which AR42J cells were chronically exposed to a biologically active phorbol ester that directly activates classical and novel PKCs and subsequently, with long-term exposure, promote their downregulation (34). As shown in Fig. 4C, prolonged treatment with 1 μM TPA caused a striking decrease in protein expression of PKCɛ and δ but had no effect on PKD1 expression level. The downregulation in PKCɛ and δ resulted in an almost complete inhibition in PKD1 phosphorylation at Ser744/748 elicited by subsequent CCK-8 or CCh stimulation (Fig. 4C). These findings indicate that similar to primary acinar cells, CCK-8- and CCh-stimulated PKD1 activation is also PKCδ and ɛ dependent in AR42J cell. Therefore, AR42J cells can be used as a model system to study the regulation and function of PKD1 in pancreatic acinar cells.
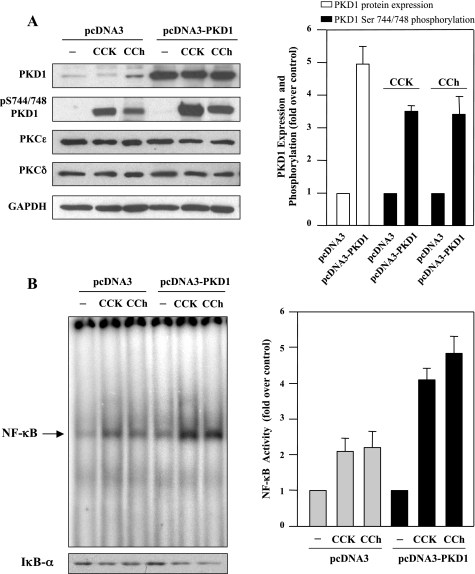
Overexpression of PKD1 potentiates NF-κB activation induced by CCK-8 or CCh.
Because our data indicated that CCK-8 and CCh caused a dramatic increase in PKCδ- and ɛ-dependent PKD1 activation and NF-κB activity, we hypothesized that these two events may lie in a linear signal transduction pathway, namely, PKC (δ and ɛ) → PKD1 → NF-κB. To directly determine whether PKD1 mediates NF-κB activation in response to CCK-8 or CCh, we increased the expression level of PKD1 in AR42J cells by transfection with an expression plasmid encoding wild-type PKD1, pCDNA3-PKD1. After 2–3 days, the transfected cells were stimulated with CCK-8 or CCh for 10 min. Whole cell lysates were prepared and analyzed by Western blotting, and nuclear extracts were prepared for NF-κB DNA binding activity determination. As illustrated in Fig. 5A, PKD1 protein expression levels were increased approximately four- to fivefold in AR42J cells transfected with pCDNA3-PKD1, compared with cells transfected with empty pCDNA3. Accordingly, CCK-8- and CCh-induced PKD1 activation loop phosphorylation were also markedly enhanced in cells overexpressing PKD1. Of note, PKD1 overexpression did not affect either PKCδ or ɛ protein levels. Furthermore, the increases in PKD1 expression and activity resulted in a marked enhancement in IκBα degradation and NF-κB activity stimulated by CCK-8 or CCh (Fig. 5B). These results suggest that PKD1 expression facilitates NF-κB activation and PKD1 may act as an upstream kinase that mediates NF-κB activation induced by CCK-8 and CCh.
Fig. 5.
Overexpression of PKD1 enhances NF-κB activation induced by CCK-8 or CCh. AR42J cells were transfected with the empty vector pcDNA3 or pcDNA3-PKD1. Two days after transfection, the cells were washed and incubated in serum-free F-12K medium for 3 h. Then the cells were incubated for 10 min in the absence (−) or presence (+) of 100 nM CCK-8 or 100 μM CCh. A, left: Western blot with antibodies against PKD C-20 for PKD1 expression or against pS744/748 for PKD1 phosphorylation. Western blots were also probed for the expression of PKCɛ and δ and then further probed for GAPDH to verify equal protein loading. Shown are representative blots from at least 3 independent experiments. Right: intensity of the Western blot bands was quantified by densitometry for the level of PKD1 expression (open bars) and PKD1 Ser744/748 phosphorylation (solid bars), expressed as fold increase relative to the controls (pcDNA3-transfected cells). Values are means ± SE (n = 6). B, left: NF-κB DNA binding activity in nuclear extracts was measured by EMSA, and IκΒ-α degradation in cytosolic extracts were measured by Western blot analysis. Right: NF-κB band intensities were quantified in the PhosphorImager and normalized on the band intensity in unstimulated control acinar cells. Values are means ± SE (n = 3).
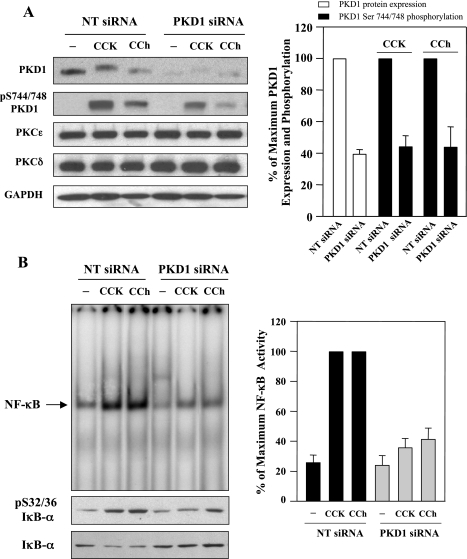
Knockdown of PKD1 reduces NF-κB activation induced by CCK-8 or CCh.
Next, we determined whether endogenous PKD1 mediates CCK-8- or CCh-induced NF-κB activation in AR42J cells. The cells were transfected with either NT siRNAs or with siRNAs targeting distinct PKD1 sequences. At 48 h after transfection, the cells were stimulated with 100 nM CCK-8 or 100 μM CCh for 10 min. Western blot analysis of the cell lysate (Fig. 6A) showed that both PKD1 protein expression and CCK-8- or CCh-stimulated phosphorylation at Ser744/748 were markedly decreased (∼60%) by transfection of pooled PKD1 siRNAs (4 duplexes), compared with the cells transfected with NT siRNAs (pool of 4 duplexes). Neither PKCδ or ɛ protein levels were affected (Fig. 6A). We have also shown in Fig. 4A (panels 3 and 4) that PKD1 siRNAs did not interfere with the expression of PKD2 and PKD3. Taken together, these data demonstrated the selectivity of PKD1 gene silencing. The salient feature of the results shown in Fig. 6B is that transfection of siRNAs targeting PKD1 dramatically diminished CCK-8- and CCh-induced IκB-α phosphorylation and degradation as well as NF-κB activation. These findings indicate that PKD1 plays a major role in mediating CCK-8- or CCh-induced NF-κB activation.
Fig. 6.
PKD1 knockdown decreases NF-κB activation induced by CCK-8 and CCh. AR42J cells were transiently transfected with PKD1 siRNAs or nontargeting negative control siRNAs. Two days after transfection, the cells were washed and incubated in serum-free F-12K medium for 3 h. Then the cells were incubated for 10 min in the absence or presence of 100 nM CCK-8 or 100 μM CCh. A, left: Western blot with antibodies against PKD1 C-20 for PKD1 expression or against pS744/748 for PKD1 phosphorylation. Western blots were also probed for the expression of PKCɛ and δ and then further probed for GAPDH to verify equal protein loading. Shown are representative blots from at least 3 independent experiments. Right: the intensity of the Western blot bands was quantified by densitometry for the level of PKD1 expression (open bars) and PKD1 Ser744/748 phosphorylation (solid bars), expressed as % of maximum expression level of PKD1 (open bars) or % of maximum in PKD1 phosphorylation obtained with 100 nM CCK-8 or 100 μM CCh. The PKD1 expression level or Ser744/748 phosphorylation in NT siRNAs control were considered as 100%. Values are means ± SE (n = 6). B, left: NF-κB DNA binding activity in nuclear extracts was measured by EMSA; IκΒ-α phosphorylation and degradation in cytosolic extracts were measured by Western blot analysis. Right: NF-κB band intensities were quantified in the PhosphorImager and normalized on the band intensity in unstimulated control acinar cells. Values are means ± SE (n = 3).
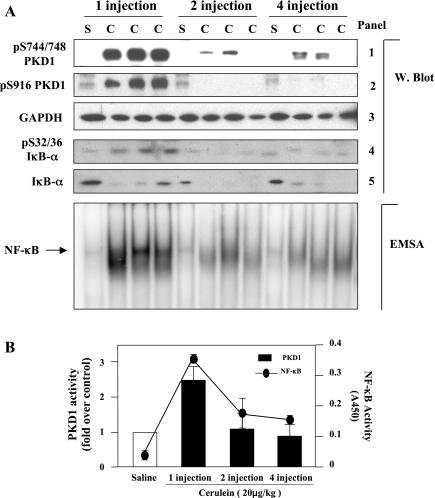
Kinetics of PKD1 phosphorylation and activation in cerulein-induced pancreatitis: associated with NF-κB activation.
Finally, we examined whether PKD1 is activated in vivo in experimental pancreatitis and whether there is a relationship between PKD1 activation and NF-κB activation. Acute pancreatitis was induced in rats by four hourly injections of the CCK-8 analog cerulein. The pancreas was collected in 30 min after the first, second, and fourth injection, and the tissue lysate was analyzed by Western blotting (Fig. 7A) and in vitro kinase assay (Fig. 7B). The data show that both PKD1 phosphorylation (at the activation loop or at Ser916) and its kinase activity increased dramatically at 30 min after first injection of cerulein and then decreased to basal level after second and fourth injection.
Fig. 7.
PKD1 and NF-κB are time dependently activated by cerulein in rat cerulein pancreatitis. Pancreatitis was induced in rats by 4 hourly intraperitoneal injections of cerulein (C, 20 μg/kg); control animals received similar injections of saline (S). Animals were then euthanized in 30 min after the 1st, 2nd, and 4th injection. Induction of pancreatitis was confirmed by elevated levels of serum amylase and lipase and by changes in pancreatic histology on tissue sections. A: time course of PKD1 phosphorylation and NF-κB activation in rat cerulein pancreatitis. Western blot: tissue lysates were analyzed by Western blot using PKD1 pS744/748 antibody (panel 1) or PKD1 pS916 antibody (panel 2). The blots were then reblotted with GAPDH antibody to verify equal protein loading (panel 3). IκΒ-α phosphorylation and degradation in cytosolic extracts were measured by Western blot analysis (panels 4 and 5). EMSA: time course of NF-κB activation in rat cerulein pancreatitis. EMSA was performed on nuclear extracts from the rat pancreas tissue. B: time course of PKD1 kinase activity and NF-κB activation in rat cerulein pancreatitis. The tissue lysates were immunoprecipitated with PKD1 C-20 antibody and PKD1 activity in the immunocomplexes was determined by in vitro kinase assay. The results (bar graphs) are expressed as a fold increase over saline control in activity. Values are means ± SE (n = 3). NF-κB binding activity of the nuclear extracts was determined by ELISA using ActiveMotif kit. Values presented in the curve figure are means ± SE (n = 3).
Furthermore, we measured cerulein-induced NF-κB activation with both EMSA (Fig. 7A) and ELISA methods (Fig. 7B). Interestingly, the time course of NF-κB activation was coincident with that of PKD1 activation (Fig. 7B). In particular, similar to PKD1, the extent of NF-κB activation after first cerulein injection was much greater than that after subsequent injections, indicating that both PKD1 activation and NF-κB activation are early events during acute pancreatitis. This close association of PKD1 activation with NF-κB activation strongly suggested a potential role of PKD1 in NF-κB activation in pancreatitis.
DISCUSSION
It is increasingly recognized that the inflammatory response occupies a central position in the mechanisms of pancreatitis and that NF-κB is a key regulator of the expression of the inflammatory molecules in pancreatitis (10, 28, 44, 47). Thus the elucidation of the intracellular signaling pathways leading to NF-κB activation in pancreas will contribute to understanding of the pathophysiological mechanisms of pancreatitis. Although our previous studies indicated that the activation and translocation of novel PKC isoforms PKCδ and ɛ are necessary for CCK-8-induced NF-κB activation (36, 38), the downstream signaling targets of PKCs within the NF-κB pathway in exocrine pancreas remain unidentified.
PKD1, the founding member of PKD family (PKCμ/PKD1, PKD2, and PKCν/PKD3) of CAMK-related protein kinases with distinct structural, enzymological, and dynamic properties (review in Ref. 35), occupies a crucial position in the signal transduction pathways initiated by DAG and PKC. Substantial evidences demonstrated that the novel isoforms of PKC including δ, ɛ, η, and θ are upstream kinases of PKD1. These novel PKC isoenzymes activate PKD1 by transphosphorylation at PKD1 activation loop Ser744 and Ser748 (41, 48, 50, 54). PKD family members have been extensively implicated in the regulation of multiple fundamental biological processes (7, 17, 35, 45). PKDs were also reported to play a critical role in gene transcription by interaction with transcription factors (14, 49). Particularly, PKD1 has been shown to mediate PKCδ-dependent NF-κB activation in response to oxidative stress (41–43) in Hela cells. PKD2 was demonstrated to be a critical mediator in lysophosphatidic acid-induced, PKC-dependent, NF-κB activation and interleukin-8 production in colonic epithelial cells (6). More recently, PKD1 was identified in rat pancreatic acinar cells and shown to be regulated by CCK-8 through predominantly a PKCδ-dependent pathway (4). But the functions of PKD1 in pancreatic acinar cells, specifically in NF-κB activation leading to pancreatitis, have not been elucidated. The present study was designed to determine whether PKD1 is a downstream signaling target of PKCδ and PKCɛ in the pathway mediating NF-κB activation induced by CCK-8 and the cholinergic agonist CCh in pancreatic acinar cells.
Initially, we demonstrated that PKD1 is the major PKD family protein detected in both primary rat pancreatic acinar cells and rat AR42J cell line. Either CCK-8 or CCh dose dependently induced a rapid and striking increase in PKD1 catalytic activity and multisite phosphorylation, which closely correlated with NF-κB activation induced by these agonists.
Several lines of evidence in the present study indicate that PKD1 is a downstream convergent point of PKCδ and ɛ in the signal transduction pathways triggered by CCK-8 and cholinergic stimulation. Full activation of PKD1 requires both PKCδ and PKCɛ activation in pancreatic acinar cells. Treatment with either specific translocation inhibitors for PKCδ and PKCɛ or genetic deletion of PKCδ or PKCɛ resulted in a partial reduction in PKD1 activity induced by CCK-8 and CCh in pancreatic acinar cells. However, treatment with GF1, a broad-spectrum PKC inhibitor, or downregulation of both PKCδ and PKCɛ by long-term exposure to phorbol ester nearly abolished CCK-8- or CCh-induced PKD1 activation. In contrast, neither the inhibitor of conventional PKC isoforms nor the PKCζ inhibitor prevented the PKD1 activation.
Our previous studies demonstrated that the PKCɛ translocation activator (PKCɛa) could specifically stimulate PKCɛ translocation and activation whereas low-dose (0.1 nM) CCK-8 activated only PKCδ and that the combination of these two agonists was necessary to mediate NF-κB activation (36). To further clarify the role of PKCδ and PKCɛ in PKD1 activation, we used a low-dose CCK-8 and PKCɛa to test whether PKD1 can be activated by signaling through PKCɛ or PKCδ alone. Our results showed that PKD1 was only partially activated by cell stimulation with either PKCɛa or 0.1 nM CCK-8 alone. However, treatment with the combination of both PKCɛa and low-dose CCK-8 potentiated PKD1 activity. These data indicate that full activation of PKD1 requires stimulation by both PKCδ and ɛ in acinar cells.
Taken together, these data suggest that PKCδ and ɛ mediate both PKD1 activation and NF-κB activation in pancreatic acinar cells treated with CCK-8 or CCh. Therefore, we hypothesized that PKD1 is a novel signal transduction element mediating PKCδ- and ɛ-dependent NF-κB activation in response to CCK-8 or CCh in pancreatic acinar cells. We used two approaches to determine the role of PKD1 in NF-κB activation. We found that overexpression of PKD1 in pancreatic acinar AR42J cells augmented both PKD1 activity and NF-κB activation in response to CCK-8 or CCh. Conversely, PKD1 gene silencing with siRNAs markedly reduced CCK-8 or CCh-stimulated NF-κB activation in AR42J cells. These results demonstrate that both CCK-8 and CCh induce NF-κB activation via PKCδ and ɛ/PKD1 pathway and that PKD1 plays a critical role in NF-κB activation.
Finally, we observed the in vivo kinetics of PKD1 and NF-κB activation during experimental pancreatitis. Both PKD1 and NF-κB activation were early events during acute pancreatitis and the time courses of the two responses were closely correlated. This close association of PKD1 activation and NF-κB activation in pancreatitis further supports the role of PKD1 in NF-κB activation in acute pancreatitis.
In conclusion, our results identified PKD1 as a novel early convergent point of PKCδ and ɛ signaling pathways triggered through CCK-8 or cholinergic receptors in exocrine pancreas and demonstrated that PKD1 mediates NF-κB activation in pancreatitis.
GRANTS
This study was supported by the Department of Veterans Affairs Merit Grant and National Institute on Alcohol Abuse and Alcoholism Grant P50-A11999 (to USC-UCLA Research Center for Alcoholic Liver and Pancreatic Diseases). E. Rozengurt is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-55003.
Supplementary Material
Acknowledgments
We thank Dr. Anna S. Gukovskaya for advice and discussion in this research and a critical review of the manuscript. We acknowledge Drs. Joseph Reeve and David Kiere for PKC inhibitory peptides and activator and Dr. Richard Waldron for discussion.
We also thank Dr. Keiichi I. Nakayama (Kyushu University) and Dr. Robert O. Messing (University of California, San Francisco) for providing the initial breeding pairs of PKCδ knockout mice (from Dr. Nakayama) and PKCɛ knockout mice (from Dr. Messing).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adler G, Beglinger C, Braun U, Reinshagen M, Koop I, Schafmayer A, Rovati L, Arnold R. Interaction of the cholinergic system and cholecystokinin in the regulation of endogenous and exogenous stimulation of pancreatic secretion in humans. Gastroenterology 100: 537–543, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Hirohata Y, Okabayashi Y, Imoto I, Otsuki M. Supramaximal CCK and CCh concentrations abolish VIP potentiation by inhibiting adenylyl cyclase activity. Am J Physiol Gastrointest Liver Physiol 275: G1202–G1208, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bastani B, Yang L, Baldassare JJ, Pollo DA, Gardner JD. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochim Biophys Acta 1269: 307–315, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta 1773: 483–501, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 122: 448–457, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Chiu TT, Leung WY, Moyer MP, Strieter RM, Rozengurt E. Protein kinase D2 mediates lysophosphatidic acid-induced interleukin 8 production in nontransformed human colonic epithelial cells through NF-κB. Am J Physiol Cell Physiol 292: C767–C777, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Fugmann T, Hausser A, Schoffler P, Schmid S, Pfizenmaier K, Olayioye MA. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J Cell Biol 178: 15–22, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautam D, Han SJ, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J Pharmacol Exp Ther 313: 995–1002, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J Biol Chem 277: 22595–22604, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-κB activation is associated with hormone-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 275: G1402–G1414, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Han B, Logsdon CD. CCK stimulates mob-1 expression and NF-κB activation via protein kinase C and intracellular Ca2+. Am J Physiol Cell Physiol 278: C344–C351, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Hurd C, Waldron RT, Rozengurt E. Protein kinase D complexes with C-Jun N-terminal kinase via activation loop phosphorylation and phosphorylates the C-Jun N-terminus. Oncogene 21: 2154–2160, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop SER(744) and SER(748) phosphorylation. J Biol Chem 283: 12877–12887, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johannessen M, Delghandi MP, Rykx A, Dragset M, Vandenheede JR, Van Lint J, Moens U. Protein kinase D induces transcription through direct phosphorylation of the cAMP-response element-binding protein. J Biol Chem 282: 14777–14787, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron 24: 253–260, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koon HW, Zhao D, Zhan Y, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves protein kinase Cdelta activation. J Pharmacol Exp Ther 314: 1393–1400, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Chen LA, Townsend CM Jr, Evers BM. PKD1, PKD2, and their substrate Kidins220 regulate neurotensin secretion in the BON human endocrine cell line. J Biol Chem 283: 2614–2621, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Song L, Jope RS. Cholinergic stimulation of AP-1 and NF kappa B transcription factors is differentially sensitive to oxidative stress in SH-SY5Y neuroblastoma: relationship to phosphoinositide hydrolysis. J Neurosci 16: 5914–5922, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugea A, Nan L, French SW, Bezerra JA, Gukovskaya AS, Pandol SJ. Role of the cholinergic system in alcohol-induced pancreatitis (Abstract). Gastroenterology 130: A206, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malka D, Vasseur S, Bodeker H, Ortiz EM, Dusetti NJ, Verrando P, Dagorn JC, Iovanna JL. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology 119: 816–828, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase Cmu. J Biol Chem 274: 26543–26549, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, Scharenberg AM. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol 26: 1569–1577, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihailovic T, Marx M, Auer A, Van Lint J, Schmid M, Weber C, Seufferlein T. Protein kinase D2 mediates activation of nuclear factor kappaB by Bcr-Abl in Bcr-Abl+ human myeloid leukemia cells. Cancer Res 64: 8939–8944, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Newton AC Protein kinase C: structure function, regulation. J Biol Chem 270: 28495–28498, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Nishizuka Y Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Owyang C Physiological mechanisms of cholecystokinin action on pancreatic secretion. Am J Physiol Gastrointest Liver Physiol 271: G1–G7, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Page K, Li J, Zhou L, Iasvovskaia S, Corbit KC, Soh JW, Weinstein IB, Brasier AR, Lin A, Hershenson MB. Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J Immunol 170: 5681–5689, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Pandol SJ Acute pancreatitis. Curr Opin Gastroenterol 22: 481–486, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y, Zong Y, Solomon TE, Gukovskaya AS, Tsukamoto H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 117: 706–716, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Piiper A, Leser J, Lutz MP, Beil M, Zeuzem S. Subcellular distribution and function of Rab3A-D in pancreatic acinar AR42J cells. Biochem Biophys Res Commun 287: 746–751, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Profita M, Bonanno A, Siena L, Ferraro M, Montalbano AM, Pompeo F, Riccobono L, Pieper MP, Gjomarkaj M. Acetylcholine mediates the release of IL-8 in human bronchial epithelial cells by a NFkB/ERK-dependent mechanism. Eur J Pharmacol 582: 145–153, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Rey O, Reeve JR Jr, Zhukova E, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor-mediated phosphorylation of the activation loop of protein kinase D: dependence on plasma membrane translocation and protein kinase Cepsilon. J Biol Chem 279: 34361–34372, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Rey O, Yuan J, Young SH, Rozengurt E. Protein kinase C nu/protein kinase D3 nuclear localization, catalytic activation, and intracellular redistribution in response to G protein-coupled receptor agonists. J Biol Chem 278: 23773–23785, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Pena A, Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun 120: 1053–1059, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem 280: 13205–13208, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Satoh A, Gukovskaya AS, Reeve JR Jr, Shimosegawa T, Pandol SJ. Ethanol sensitizes NF-κB activation in pancreatic acinar cells through effects on protein kinase C-ɛ. Am J Physiol Gastrointest Liver Physiol 291: G432–G438, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut 44: 253–258, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR Jr, Shimosegawa T, Pandol SJ. PKC-δ and -ɛ regulate NF-κB activation induced by cholecystokinin and TNF-α in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G582–G591, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Satoh A, Gukovskaya AS, Edderkaoui M, Daghighian MS, Reeve JR Jr, Shimosegawa T, Pandol SJ. Tumor necrosis factor-alpha mediates pancreatitis responses in acinar cells via protein kinase C and proline-rich tyrosine kinase 2. Gastroenterology 129: 639–651, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Shukla A, Lounsbury KM, Barrett TF, Gell J, Rincon M, Butnor KJ, Taatjes DJ, Davis GS, Vacek P, Nakayama KI, Nakayama K, Steele C, Mossman BT. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-delta knockout mice. Am J Pathol 170: 140–151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storz P, Doppler H, Toker A. Activation loop phosphorylation controls protein kinase D-dependent activation of nuclear factor kappaB. Mol Pharmacol 66: 870–879, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol 24: 2614–2626, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J 22: 109–120, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107: 7–11, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trauzold A, Schmiedel S, Sipos B, Wermann H, Westphal S, Roder C, Klapper W, Arlt A, Lehnert L, Ungefroren H, Johannes FJ, Kalthoff H. PKCmu prevents CD95-mediated apoptosis and enhances proliferation in pancreatic tumour cells. Oncogene 22: 8939–8947, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA 91: 8572–8576, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-κB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 280: G1197–G1208, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J Biol Chem 276: 32606–32615, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Waldron RT, Whitelegge JP, Faull KF, Rozengurt E. Identification of a novel phosphorylation site in c-jun directly targeted in vitro by protein kinase D. Biochem Biophys Res Commun 356: 361–367, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldron RT, Iglesias T, Rozengurt E. The pleckstrin homology domain of protein kinase D interacts preferentially with the eta isoform of protein kinase C. J Biol Chem 274: 9224–9230, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Williams JA Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol 63: 77–97, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Yuan J, Slice LW, Gu J, Rozengurt E. Cooperation of Gq, Gi, and G12/13 in protein kinase D activation and phosphorylation induced by lysophosphatidic acid. J Biol Chem 278: 4882–4891, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Yuan J, Rozengurt E. PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem 103: 648–662, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Yuan J, Bae D, Cantrell D, Nel AE, Rozengurt E. Protein kinase D is a downstream target of protein kinase Ctheta. Biochem Biophys Res Commun 291: 444–452, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates interleukin-8 expression through modulation of I kappa B alpha phosphorylation and p65 transcriptional activity: involvement of protein kinase C alpha. Mol Pharmacol 67: 2025–2031, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Zheng H, Chen D, Zhang J, Tian Y. Involvement of M3 cholinergic receptor signal transduction pathway in regulation of the expression of chemokine MOB-1, MCP-1 genes in pancreatic acinar cells. J Huazhong Univ Sci Technolog Med Sci 24: 140–143, 157, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Zugaza JL, Waldron RT, Sinnett-Smith J, Rozengurt E. Bombesin, vasopressin, endothelin, bradykinin, and platelet-derived growth factor rapidly activate protein kinase D through a protein kinase C-dependent signal transduction pathway. J Biol Chem 272: 23952–23960, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J 15: 6220–6230, 1996. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.