Abstract
Calponin contributes to the regulation of smooth muscle contraction through its interaction with F-actin and inhibition of the actin-activated Mg-ATPase activity of phosphorylated myosin. Previous studies have shown that the contractile agonist acetylcholine induced a direct association of translocated calponin and PKC-α in the membrane. In the present study, we have determined the domain of PKC-α involved in direct association with calponin. In vitro binding assay was carried out by incubating glutathione S-transferase-calponin aa 92-229 with His-tagged proteins of individual domains and different combinations of domains of PKC-α. Calponin was found to bind directly to the full-length PKC-α. Calponin bound to C2 and C4 domains but not to C1 and C3 domains of PKC-α. When incubated with proteins of different combination of domains, calponin bound to C2-C3, C3-C4, and C2-C3-C4 but not to C1-C2 or C1-C2-C3. To determine whether these in vitro bindings mimic the in vivo associations, and in vivo binding assay was performed by transfecting colonic smooth muscle cells with His-tagged proteins of individual domains and different combinations of domains of PKC-α. Coimmunoprecipitation of calponin with His-tagged truncated forms of PKC-α showed that C1-C2, C1-C2-C3, C2-C3, and C3-C4 did not associate with calponin. Calponin associated only with full-length PKC-α and with C2-C3-C4 in cells in the resting state, and this association increased upon stimulation with acetylcholine. These data suggest that calponin bound to fragments that may mimic the active form of PKC-α and that the functional association of PKC-α with calponin requires both C2 and C4 domains during contraction of colonic smooth muscle cells.
Keywords: tropomyosin, smooth muscle, contraction, cytoskeleton, signaling
phosphorylation of myosin light chain by calcium-calmodulin-dependent myosin light chain kinase is the primary mechanism of smooth muscle contraction. Nevertheless, during sustained contractions of tonic smooth muscle, force is maintained whereas calcium and myosin light chain phosphorylation is reduced. Additional regulatory mechanisms at the level of the thin filaments have been postulated. Thin-filament regulation involves the actin-binding contractile regulatory proteins tropomyosin (5, 14), caldesmon (8, 18), and calponin (2, 40).
Calponin is an actin-associated protein found in smooth muscle and nonmuscle cells. Calponin was originally discovered in chicken gizzard smooth muscle (45). Three isoforms of calponin, viz., acidic, neutral, and basic calponin, have been classified on the basis of their isoelectric point (29). Basic calponin is distributed specifically in smooth muscle tissues (6) and has been well characterized in vitro. Calponin inhibits the actin-activated Mg-ATPase activity of phosphorylated smooth muscle myosin. In vitro motility assays demonstrated that calponin inhibits relative movement of actin and myosin filaments (3). It has also been reported that exogenously added calponin reduces the active tension and maximal shortening velocity in skinned smooth muscle (41). Calponin has thus been suggested as an important modulator of smooth muscle contraction. Calponin has also been shown to be associated with other contractile proteins, tropomyosin (46), myosin (31), and calmodulin (20).
Contraction of smooth muscle has been correlated with phosphorylation of calponin (43, 44). PKC catalyzes the phosphorylation of calponin at Ser175 and Thr184, which have been postulated to result in loss of its actomyosin ATPase inhibitory activity (23, 31). Phosphorylation of calponin by PKC has been shown to modulate porcine arterial smooth muscle contraction. Thus PKC regulation of calponin phosphorylation is of physiological importance (29). Phosphorylation of calponin results in its dissociation from actin (26) and from tropomyosin (28). In its unphosphorylated state, calponin binds to actin and inhibits Mg-ATPase of myosin. Upon phosphorylation by PKC, inhibiting activity of calponin is lost (22).
Upon stimulation with contractile agonists, calponin has also been shown to relocate to the membrane in coronary smooth muscle cells (28, 29). Histochemical and digital imaging microscopy studies have shown that calponin is distributed more toward the center rather than the periphery in a resting cell and distributed with the cytoskeleton upon agonist stimulation in chicken gizzard smooth muscle cells (15). In resting vascular smooth muscle cells of the ferret, calponin is distributed throughout the cytosol associated with filamentous structures, and upon stimulation with a contractile agonist the cellular distribution of calponin changed from primarily cytosolic to surface (12).
Tonic contraction is activated by activation of protein kinase C (16). We have previously shown that PKC-α translocates to the membrane during agonist-induced contraction of smooth muscle cells from the rabbit colon (30). Reports indicate that PKC-ξ/PKC-α interacts with calponin (15, 24). Calponin is also shown to form a substrate for Rho kinase in vitro (34). In addition, it was reported that calponin may facilitate ERK-dependent signaling, thus playing a significant role in regulation of vascular smooth muscle contraction (7).
Previous studies from this laboratory showed that acetylcholine induced a sustained increase in the association of translocated calponin and PKC-α in the particulate fraction (27). Present investigation reports different domains of PKC-α involved in direct interaction with calponin.
PKC is characterized by 1) an NH2-terminal regulatory domain, which mediates membrane association and activation; 2) a small central hinge region; and 3) a COOH-terminal catalytic domain containing the active site. There are four conserved domains of PKC: 1) C1 domain containing a Cys-rich motif, which forms the diacylglycerol-phorbol ester binding site. 2) C2 domain containing the Ca2+-binding site. The C2 domain mediates Ca2+-dependent binding to the membrane lipid phosphatidylserine, which induces a conformational change that activates the enzyme. 3) C3 domain possessing the binding site for ATP, the phosphate donor for phosphotransferase activity. 4) C4 domain, which is the catalytic domain that possesses the binding site for substrates (27).
To determine the specific domains in direct interaction with calponin, His-tagged fragments of individual PKC-α domains and different combinations of PKC-α domains were used. Studies were also conducted using the colonic smooth muscle cells (CSMC) transfected with His-tagged fragments of individual PKC-α domains and different combinations of PKC-α domains. Data showed a direct binding of calponin to full-length PKC-α both in vitro and in transfected cells. In vitro binding studies showed that, among the different domains of PKC-α, C2 and C4 have significantly higher binding compared with C1 and C3. Among the truncated forms of PKC-α, calponin bound to C2-C3-C4, C2-C3, and C3-C4 in vitro but only to C2-C3-C4 in transfected cells. Calponin did not bind to C1-C2-C3 and C1-C2 either in vitro or in transfected cells. In transfected CSMC, the association increased upon stimulation with acetylcholine. The studies using cells transfected with truncated forms of PKC-α thus indicate that PKC-α (C1-C2), PKC-α (C1-C2-C3), PKC-α (C2-C3), and PKC-α (C3-C4) did not associate with calponin. Only full-length PKC-α and PKC-α (C2-C3-C4) associated directly with calponin. The data therefore suggest that calponin bound to fragment that mimics the active form of PKC-α and that functional association of PKC-α with calponin may require both C2 and C4 domains.
EXPERIMENTAL PROCEDURES
Materials
Collagenase type II was purchased from Worthington Biochemical (Freehold, NJ). Protein G-Sepharose was from Pharmacia Biotech (Uppsala, Sweden). Polyvinylidene fluoride (PVDF) membranes were from Bio-Rad (Hercules, CA). Full-length PKC-α protein was purchased from Cytoskeleton, Denver, CO. Lipofectamine reagent and Plus reagent were purchased from Invitrogen, Carlsbad, CA. Mouse monoclonal anti-PKC-α and anti-calponin antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Isopropyl-β-d-thiogalactopyranoside (IPTG) was purchased from Invitrogen. Anti-His antibodies and Enhanced Chemiluminescence (ECL) detection reagents were from Amersham Biosciences, Amersham, UK. Anti-glutathione S-transferase (GST) antibody, acetylcholine, and other reagents were purchased from Sigma, St. Louis, MO.
Methods
Construction and purification of recombinant GST-calponin aa 92-229 protein.
A 1.3-kbp cDNA (gift from Dr. J. M. Miano, University of Rochester Medical Center, Rochester, NY) was used for amplifying the coding regions of the human basic calponin gene with forward primer 5′ ATC AAG GCC ATC ACC AAG TAT (nucleotide 352–372) and reverse primer 3′ CTA GGC GGA ATT GTA GTA GTT (nucleotide 952–972). The restriction site of the forward primer was BamHI whereas the restriction site of the reverse primer was EcoRI. The PCR products (622 bp) were double digested with the restriction enzymes BamHI and EcoRI. The digested PCR products were fused in frame with the NH2-terminal GST-tag vector, pGEX-KT (gift from Dr. J. E. Dixon, University of California-San Diego, San Diego, CA). The vector consisted of GST DNA sequences immediately following an IPTG-inducible promoter region and a transcription start codon ATG. The newly inserted DNA would fuse with the GST at its 3′ end such that, when a protein is expressed, it would form as an NH2-terminal GST fusion protein. The GST fusion calponin protein was expressed and purified with glutathione-agarose beads as described by Hakes and Dixon (7) with slight modification. Five milliliters of overnight culture were inoculated in 500 ml fresh LB media with 100 μg/ml ampicillin. The culture was grown with vigorous shaking at 37°C until it reached to 0.4–0.5 OD at 600 nm (∼3 h). IPTG was then added to the culture at 1 mM final concentration and the cultures were grown for another 3–4 h with vigorous shaking at 37°C. The cells were then collected by centrifugation at 4,000 rpm for 10 min. The cell pellet was resuspended in 1/10th volume of ice-cold mouse tonicity phosphate buffered saline (MTPBS; 150 mM NaCl, 16 mM Na2HPO4, 4 mM NaH2PO4, pH 7.3, 1% Triton X-100) + 0.1% β-mercaptoethanol and lysozyme at a final concentration of 100 μg/ml (freshly prepared from 10 mg/ml stock), followed by an incubation on ice for 30 min. The cells were lysed by mild sonication and centrifuged at 10,000 rpm for 15 min. The supernatant was mixed with glutathione agarose beads (50 μl of each 500-μl supernatant) and rocked at 4°C for 2 h. The beads were washed thrice with five bead volumes of MTPBS. The fusion protein was eluted with one bead volume of elution buffer (50 mM Tris·HCl, pH 8.0 containing 10 mM reduced glutathione). The fusion proteins were analyzed by immunoblotting using anti-GST and anti-calponin antibodies.
Construction of recombinant His-tagged full-length PKC-α and His-tagged different fragments of PKC-α.
To define the domains of PKC-α involved in direct association with calponin, in vitro binding assay was performed using purified fragments of different combinations of PKC-α domains: His-PKC-α (C1-C2-C3), His-PKC-α (C2-C3-C4), His-PKC-α (C1-C2), His-PKC-α (C2-C3), His-PKC-α (C3-C4), His-PKC-α (C1), His-PKC-α (C2) and His-PKC-α (C3), and His-PKC-α (C4). Different combination of domains used for the studies were constructed as described previously (27).
In vitro binding assay of fusion protein GST-calponin aa 92-229 with full-length PKC-α and His-tagged different constructs of PKC-α proteins.
Either 25 μg (0.48 μmol) of GST-calponin aa 92-229 or 13 μg (0.48 μmol) of GST was mixed with 200 μl of glutathione agarose beads and rocked for 2 h at 4°C. Unbound GST-calponin aa 92-229 fusion proteins or GST were removed by washing five times with MTPBS. All the washes were retained for further analysis, and 10 μg of full-length PKC-α protein or 25 μg of His-tagged different constructs of PKC-α proteins were added to the GST-calponin aa 92-229-bound beads or GST-bound beads and rocked for 1 h at 4°C. Unbound PKC-α protein was removed by washing five times with MTPBS. All the washes were retained for further analysis. The bound proteins were eluted twice with one bead volume of elution buffer (50 mM Tris·HCl, pH 8.0 containing 10 mM reduced glutathione). Ten microliters of the first, third, and fifth washes and the elution were spotted on three PVDF membranes. The membranes were blocked with 5% nonfat dry milk and immunoblotted with anti-GST antibody, anti-calponin antibody, anti-PKC-α antibodies, or anti-His antibody, respectively. The spots were detected by ECL.
Dot blot.
Dry blotting paper and the PVDF membrane were cut according to the dot-blot apparatus size. The cut PVDF membrane was soaked in 100% methanol for 2–3 min followed by equilibration in 1× PBS for 5 min. Dot blot was performed adding 10 μl of the sample into each slot following manufacturer's instructions. The membrane was incubated in 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST) and rocked for 1 h at room temperature. The membrane was washed three times for 10 min with TBST. The membrane was then incubated in the appropriate dilution of primary antibody for 1 h with rocking at room temperature. Again, the membrane was washed three times for 15 min with TBST. The membrane was then incubated in the appropriate dilution of secondary antibody for 1 h with rocking at room temperature, followed by washing three more times for 15 min with TBST. The membrane was then incubated with ECL reagent for 1 min. The proteins were detected on the membrane by immediately exposing the membrane to the film.
Isolation of smooth muscle cells from rabbit colon.
New Zealand White rabbits were euthanized according to University of Michigan University Committee on Use and Care of Animals animal care guidelines. The internal anal sphincter, consisting of the distalmost 3 mm of the circular muscle layer, ending at the junction of skin, and mucosa was removed by sharp dissection. A 10-cm length of the colon to the junction of jejunum was dissected and used for further isolation of smooth muscle cells. Cells were isolated as previously described. Circular muscle tissue was incubated for two successive 1-h periods at 31°C in 15 ml of HEPES buffer (pH 7.4) (in mM): 115 NaCl, 5.7 KCl, 2.0 KH2PO4, 24.6 HEPES, 1.9 CaCl2, 0.6 MgCl2, 5.6 glucose containing 0.1% (wt/vol) collagenase type II, 0.01% (wt/vol) soybean trypsin inhibitor, and 2 mg/ml Dulbecco's modified Eagle's medium (DMEM). At the end of the second enzymatic incubation period, the medium was filtered through a 500-μm Nitex filter. The partially digested tissue left on the filter was washed four times with 10 ml of collagenase-free buffer solution. Tissue was then transferred into 15 ml of fresh collagenase-free buffer solution, and cells were gently dispersed. After a cell count, the harvested cells were diluted in collagenase-free HEPES buffer (pH 7.4). Each colon yielded 10–20 × 106 cells.
Transfection of smooth muscle cells with full-length PKC-α and different combinations of PKC-α domains.
Fresh CSMC from rabbit were cultured in DMEM with 10% FBS and 3% penicillin-streptomycin. Cells were passaged on the day before transfection and allowed to reach 70% confluence on the day of transfection. CSMC used were from passage 1 to rule out the risk of change in the phenotype. pcDNA3.1 with full-length PKC-α, PKC-α (C1-C2-C3), PKC-α (C1-C2), PKC-α (C2-C3), PKC-α (C2-C3-C4), and PKC-α (C3-C4) cDNAs (27) were transfected into the cells by using Invitrogen Lipofectamine reagent as described previously (27). Briefly, the cDNA was diluted with serum-free DMEM and mixed with Plus reagent, followed by incubation at room temperature for 15 min. The Lipofectamine reagent was diluted with serum-free DMEM. Then the precomplexed DNA and diluted Lipofectamine reagent were mixed and incubated for 15 min. The transfection complex was then overlaid on the cells. After 4 h of incubation, the medium was changed to culture medium. After the cells reached confluence, they were used for the experiments. The overexpression of full-length PKC-α and truncated forms of PKC-α was confirmed by Western blot (27).
Immunoprecipitation and immunoblotting.
Confluent transfected rabbit colon smooth muscle cells were treated with acetylcholine for 4 min, followed by wash with cold PBS three times. Lysates from the transfected cells were analyzed for protein content by using Bio-Rad protein assay reagent. Anti-calponin antibody (4 μl) was added to 500 μg of protein sample in 500 μl of lysis buffer and rocked overnight at 4°C, and 50 μl of 50% protein G-Sepharose bead slurry was added to the overnight mixture and rocked at 4°C for 2 h. The beads bound with proteins were then collected by centrifuging at 14,000 g for 3 min at 4°C. Supernatant was discarded and the bead pellet was washed three times at room temperature with TBS bead wash buffer (20 mM Tris·HCl, 150 mM NaCl, pH 7.6) (17). The beads were then resuspended in 25 μl of 2× Laemmli sample buffer and boiled for 5 min. Proteins from the immunoprecipitates were separated on SDS-PAGE and transferred to a PVDF membrane. The membrane was immunoblotted with anti-PKC-α antibody.
Data Analysis
Bands from the Western blot spots were quantitated using a densitometer (Bio-Rad model GS-700, Bio-Rad Laboratories) and the band density (absorbance units × mm2) was calculated and expressed as percent of total density. The control bands intensities were standardized to 100%, and the band intensities of samples from treated cells were compared with the control and expressed as percent change from the control. Each experiment had its own control. All the means were compared and analyzed by ANOVA. Band data are within the linear range of detection for each antibody used.
Dot-blot spots were quantified by use of a densitometer (model GS-700, Bio-Rad Laboratories), and spot volumes (absorbance units × mm2) were calculated and expressed as a percentage of the total volume. Spot data are within the linear range of detection for each antibody used. The control spot intensity was standardized to 100%. The spot intensities of eluted fractions of the PKC-α were compared with the control and expressed as a percent change from the control. All the means were compared and analyzed by Student's t-test.
Spots were quantitated using a densitometer (model GS-700, Bio-Rad Laboratories), and spot densities (absorbance units × mm2) were calculated and expressed as a percentage of the total density. Spot data are within the linear range of detection for each antibody used. In addition, spots for standard proteins (of GST and PKC-α) in serial dilutions of 500 to 1 ng were analyzed. The combined intensity of the eluted fractions of the fusion protein (GST-calponin aa 92-229) and the combined intensity of the eluted fractions of the binding protein (PKC-α) were converted to molar quantities by the plot drawn against the standard protein intensities. Hence the molar ratios of the proteins interacting were calculated.
RESULTS
Expression and Purification of GST-Fused Calponin
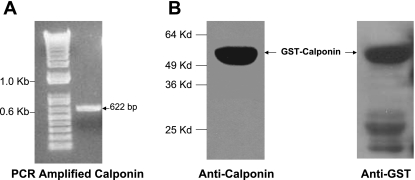
GST-tagged calponin was constructed to carry out in vitro binding assay to investigate the possibility of direct protein-protein interaction between PKC-α and calponin. PCR amplified 622 bp calponin cDNA (nucleotide 351-972 corresponding to amino acid 92-229) (Fig. 1A) was cloned into GST tag containing vector, pGEX-KT at BamHI and EcoRI sites to express calponin as NH2-terminal GST-fused proteins. The clone was then confirmed to be in the correct open reading frame by sequencing.
Fig. 1.
Construction and purification of glutathione S-transferase (GST)-tagged calponin. A: PCR amplification of calponin cDNA: ∼622 bp of PCR fragment of calponin was amplified from pBS KS+/calponin clone. B: purified GST-calponin aa 92-229 was subjected to SDS-PAGE followed by Western blot with anti-calponin (left) and anti-GST (right) antibodies. Molecular mass marker is marked on the left side.
The GST-calponin aa 92-229 fusion protein was expressed by induction with 1 mM IPTG. The expressed fusion protein was purified by using the glutathione agarose beads as described in Methods and analyzed by immunoblotting using anti-calponin and anti-GST antibodies (Fig. 1B). A band of ∼56-kDa molecular mass was detected on the blot both by anti-calponin antibody and anti-GST antibody.
Calponin Directly Binds Full-Length PKC-α In Vitro
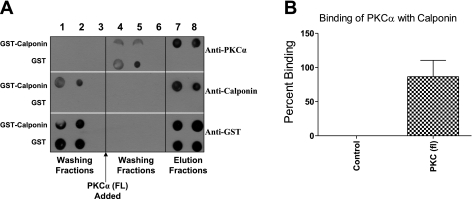
To study the direct association of calponin with PKC-α, in vitro binding assay was carried out using GST-calponin aa 92-229 fusion protein and purified full-length PKC-α. GST-calponin aa 92-229 protein was conjugated to glutathione agarose beads and incubated with full-length PKC-α protein. The incubation with PKC-α allowed its binding to the immobilized GST-calponin aa 92-229. The GST-calponin aa 92-229 fusion proteins bound with PKC-α were eluted from the glutathione agarose beads with reduced glutathione buffer (pH 8.0) as described in Methods. The washing fractions (Fig. 2A, lanes 3–5) and eluted fractions (Fig. 2A, lanes 7–8) were analyzed on dot blot using anti-calponin, anti-PKC-α, and anti-GST antibodies. The PKC-α dots were observed in the elution (Fig. 2A, lanes 7–8) from glutathione agarose-bound GST-calponin aa 92-229 fraction. Glutathione agarose beads conjugated to GST alone were used as controls to confirm that the binding of PKC-α was due to its binding with calponin, but not to GST (Fig. 2A). The percentage increase of binding of full-length PKC-α to calponin was 86.57 ± 23.90% above control (n = 3, P ≤ 0.01) (Fig. 2B). The in vitro data thus suggest that full-length PKC-α binds to calponin directly.
Fig. 2.
Direct association of GST-calponin aa 92-229 with PKC-α. A: representative dot blot showing the association of PKC-α with GST-calponin aa 92-229. GST alone conjugated to glutathione agarose beads was used as control. Fractions 1–3 were washing of unbound GST-calponin aa 92-229 protein. Fractions 4–6 were washing of unbound PKC-α protein. Fractions 7–8 were elution of GST-calponin aa 92-229 protein bound to the glutathione-agarose. Coelution of PKC-α with GST-calponin aa 92-229 was observed in lanes 7–8 (probed with anti-PKC-α, anti-calponin, and anti-GST antibodies), an indication of direct association of full-length (FL) PKC-α with GST-calponin aa 92-229. B: densitometry graph representing the percentage increase of binding of full-length PKC-α protein to GST-calponin aa 92-229 fusion protein. Percentage increase of binding of full-length PKC-α to GST-calponin aa 92-229 was 86.57 ± 23.90% (n = 3, **P ≤ 0.01) above control. GST alone was used as control.
In Vitro Association of Calponin With Different Truncated Forms of PKC-α
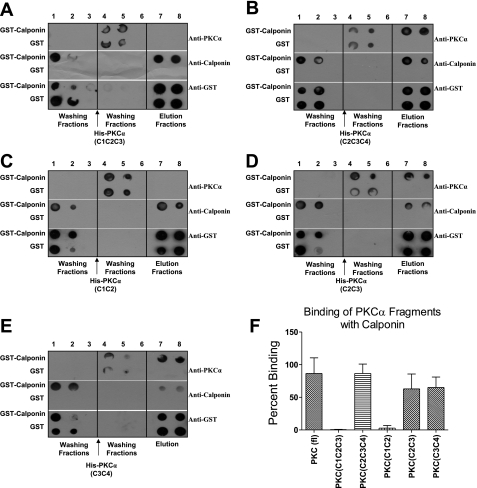
To define the domains of PKC-α involved in direct association with calponin, in vitro binding assay was performed using purified fragments of different combinations of PKC-α domains (38). Different combination of domains used were His-PKC-α (C1-C2-C3), His-PKC-α (C2-C3-C4), His-PKC-α (C1-C2), His-PKC-α (C2-C3), and His-PKC-α (C3-C4). Analysis of blots showed significant binding of GST-calponin aa 92-229 to His-PKC-α (C2-C3-C4) (86.54 ± 14.40%; n = 3, P ≤ 0.01) (Fig. 3B), His-PKC-α (C2-C3) (62.98 ± 22.71%) (Fig. 3D), and His-PKC-α (C3-C4) (65.10 ± 16.01%) (Fig. 3E) compared with the His-PKC-α (full-length) (n = 4, P ≤ 0.05) (Fig. 2). However, no significant binding of GST-calponin aa 92-229 to His-PKC-α (C1-C2-C3) (0.53 ± 0.36%) (Fig. 3A) and His-PKC-α (C1-C2) (Fig. 3C) (2.87 ± 3.99%) was observed compared with the His-PKC-α (full-length) (n = 4, P ≤ 0.05) (Fig. 2). Graphical representation of the binding data suggest that the C1 domain of PKC-α may not be essential for the binding of PKC-α to calponin. It also suggests that calponin binding site(s) may exist on the C2, C3, or C4 domains of PKC-α.
Fig. 3.
In vitro binding assay of GST-calponin aa 92-229 with various truncated forms of PKC-α. A representative dot blot showing the nonassociation of PKC-α (C1-C2-C3) with GST-calponin aa 92-229 and the association of PKC-α (C2-C3-C4) with GST-calponin aa 92-229. GST alone conjugated to glutathione agarose beads was used as control. Lanes 1–3 were washing fractions of unbound GST-calponin aa 92-229 protein, lanes 4–6 were washing fractions of unbound PKC-α domain fragments, and lanes 7–8 represent elution fractions. A: representative blot showing in vitro analysis of direct interaction of PKC-α (C1-C2-C3) protein with calponin. B: representative blot showing in vitro analysis of direct interaction of PKC-α (C2-C3-C4) protein with calponin. C: representative blot showing in vitro analysis of direct interaction of PKC-α (C1-C2) protein with calponin. D: representative blot showing in vitro analysis of direct interaction of PKC-α (C2-C3) protein with calponin. E: representative blot showing in vitro analysis of direct interaction of PKC-α (C3-C4) protein with calponin. F: densitometry graph representing the percentage increase in binding of full-length PKC-α (Fig. 2), PKC-α (C1-C2-C3) (0.53 ± 0.36%), PKC-α (C2-C3-C4) (86.54 ± 14.40%), PKC-α (C1-C2) (2.87 ± 3.99%), PKC-α (C2-C3) (62.98 ± 22.71%), and PKC-α (C3-C4) (65.1 ± 16.01%) to GST-calponin aa 92-229 (n = 4, P ≤ 0.05). There is a significant difference among full-length PKC-α, PKC-α (C1-C2-C3), and PKC-α (C1-C2) binding to GST-calponin aa 92-229. There is no difference among full-length PKC-α, PKC-α (C2-C3-C4), PKC-α (C2-C3), and PKC-α (C3-C4) binding to GST-calponin aa 92-229.
Specific PKC-α Domain Involved in Direct Association of PKC-α With Calponin
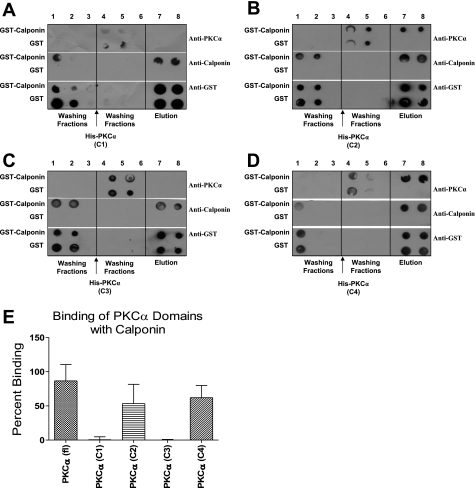
To map the precise domain that is responsible for the interaction of the two proteins, in vitro binding assay was performed by using His-tagged fragments of individual domain of PKC-α: His-PKC-α (C1), His-PKC-α (C2), His-PKC-α (C3), and His-PKC-α (C4). The binding data demonstrate direct binding of GST-calponin aa 92-229 to His-PKC-α (C2) (53.29 ± 21.89%) (Fig. 4B) and His-PKC-α (C4) (62.10 ± 17.74%) (Fig. 4D) but not to His-PKC-α (C1) (0.53 ± 4.31%) (Fig. 4A) and His-PKC-α (C3) (0.02 ± 0.73%) (Fig. 4C) (n = 3, P ≤ 0.05). Graphical representation of the in vitro binding data suggest that direct binding sites for calponin may exist on C2 and C4 domains of PKC-α, but not on the C1 or C3 domain. Interestingly, the fragments with C1 domain, His-PKC-α (C1-C2) and His-PKC-α (C1-C2-C3), did not bind to calponin. The presence of C1 may interfere with the in vitro interaction of PKC-α with calponin when C4 domain is not present. The data also indicated the importance of the C4 domain of PKC-α in the binding of PKC-α to calponin.
Fig. 4.
In vitro binding assay of GST-calponin aa 92-229 with individual domains of PKC-α. A representative dot blot showing in vitro assay of direct binding of GST-calponin aa 92-229 with PKC-α (C1) (A), PKC-α (C2) (B), PKC-α (C3) (C), and PKC-α (C4) (D). GST alone conjugated to glutathione agarose beads was used as control. Lanes 1–3 were loaded with washing fractions of unbound GST-calponin aa 92-229 protein, lanes 4–6 were loaded with washing fractions of unbound recombinant individual domains of PKC-α, and lanes 7–8 were loaded with elution fractions. Coelution of GST-calponin aa 92-229 with PKC-α (C2) (B) and PKC-α (C4) (D) were observed in lanes 7–8, an indication of direct association of recombinant PKC-α (C2) with GST-calponin aa 92-229. Coelution of PKC-α (C1) (A) and PKC-α (C3) (C) with GST-calponin aa 92-229 were not observed in lanes 7–8, an indication of nonassociation of PKC-α (C1) and PKC-α (C3) with GST-calponin aa 92-229. E: densitometry graph representing the percentage increase of binding of full-length PKC-α (from Fig. 2), PKC-α (C1) (0.53 ± 4.31%), PKC-α (C2) (53.29 ± 21.89%), PKC-α (C3) (0.02 ± 0.73%), and PKC-α (C4) (62.10 ± 17.74%) to GST-calponin aa 92-229 (n = 3, P ≤ 0.05). There is a significant difference in binding of full-length PKC-α, PKC-α (C1), and PKC-α (C3) with GST-calponin aa 92-229. There is no difference among full-length PKC-α, PKC-α (C2), and PKC-α (C4) binding to GST-calponin aa 92-229.
Association of Calponin With Different Truncated Forms PKC-α In Vivo
To confirm the binding of truncated forms of PKC-α with calponin in CSMC, full-length PKC-α and different His-truncated forms of PKC-α were subcloned into pcDNA3.1 and were transiently transfected into cultured rabbit CSMC. The transfection efficiency was over 80%. The overexpression of full-length PKC-α or the truncated forms of PKC-α was confirmed by Western blot (30).
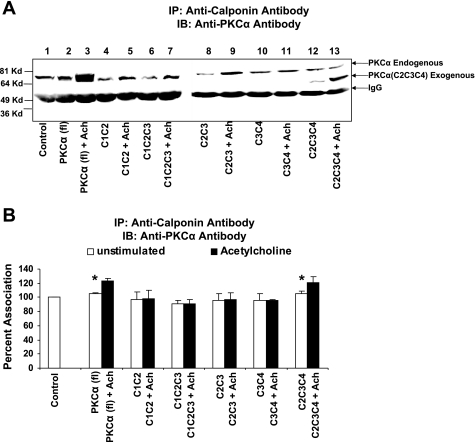
Confluent transfected CSMC were stimulated with 0.1 μM acetylcholine for 4 min. The stimulated transfected cells, unstimulated transfected cells and untransfected rabbit colon smooth muscle cells were immunoprecipitated with anti-calponin antibody. The immunoprecipitates were then subjected to SDS-PAGE. In Fig. 5A, lanes 1, 2, 4, 6, 8, 10, and 12 were loaded with untreated sample whereas lanes 3, 5, 7, 9, 11, and 13 were loaded with samples treated with acetylcholine. The SDS-PAGE separated proteins were transferred to PVDF membrane. The membrane was immunoblotted with anti-PKC-α antibody. The band intensity was observed in CSMC transfected with full-length PKC-α, and in CSMC transfected with PKC-α (C2-C3-C4). The association of full-length PKC-α and fragments PKC-α (C2-C3-C4) to calponin increased upon stimulation with acetylcholine (Fig. 5A, lanes 3 and 13). Acetylcholine-induced significant increases in the association of PKC-α with calponin in CSMC transfected with full-length PKC-α and PKC-α (C2-C3-C4) are 123.76 ± 3.24 and 120.78 ± 8.99%, respectively, compared with untransfected smooth muscle cells (control) (n = 3, P ≤ 0.05) (Fig. 5B). The amounts of calponin immunoprecipitated remained constant in all the experiments (data not shown). The association of the PKC-α (C1-C2-C3), PKC-α (C1-C2), PKC-α (C2-C3), and PKC-α (C3-C4) with calponin was not observed in vivo (Fig. 5A).
Fig. 5.
Coimmunoprecipitation of PKC-α with calponin in rabbit colon smooth muscle cells transfected with full-length PKC-α, various truncated forms of PKC-α in response to acetylcholine. Cells transfected with full-length PKC-α or various truncated forms of PKC-α were treated with 0.1 μM acetylcholine for 4 min. Untreated cultured cells served as controls. Cell lysate were immunoprecipitated with anti-calponin antibody and were further subjected to SDS-PAGE followed by Western blot with anti-PKC-α and anti-calponin antibodies. A: Western blot showing an increase in the association of PKC-α with calponin in cells transfected with full-length PKC-α (78 kDa) and PKC-α (C2-C3-C4) (53 kDa) in the presence of acetylcholine. Lane 1, Control (untransfected cells); lane 2, PKC-α (full-length) transfected cells; lane 3, PKC-α (full-length) transfected cells stimulated with acetylcholine; lane 4, PKC-α (C1-C2) transfected cells; lane 5, PKC-α (C1-C2) transfected cells stimulated with acetylcholine; lane 6, PKC-α (C1-C2-C3) transfected cells; lane 7, PKC-α (C1-C2-C3) transfected cells stimulated with acetylcholine; lane 8, PKC-α (C2-C3) transfected cells; lane 9, PKC-α (C2-C3) transfected cells stimulated with acetylcholine; lane 10, PKC-α (C3-C4) transfected cells; lane 11, PKC-α (C3-C4) transfected cells stimulated with acetylcholine; lane 12, PKC-α (C2-C3-C4) transfected cells; lane 13, PKC-α (C2-C3-C4) transfected cells stimulated with acetylcholine. B: stimulation of cells transfected with either full-length PKC-α or PKC-α (C2-C3-C4) with acetylcholine (0.1 μM) resulted in a significant increase in the association with calponin (123.76 ± 3.24, 120.78 ± 8.99%, respectively, for full-length and C2-C3-C4; P < 0.05, n = 3). Calponin failed to associate with truncated forms of PKC-α in cells transfected with either PKC-α (C1-C2), PKC-α (C1-C2-C3), PKC-α (C2-C3), and PKC-α (C3-C4) or upon stimulation with acetylcholine (98.28 ± 11.22, 91.16 ± 5.82, 96.63 ± 9.64, 96.19 ± 1.09%, respectively, for C1-C2, C1-C2-C3, C2-C3, and C3-C4). IB, immunoblot; IP, immunoprecipitation.
Graphical representation of immunoprecipitation data also suggests that in smooth muscle cells, full-length PKC-α, and the fragments PKC-α (C2-C3-C4) associated with calponin in cells, and the association increased upon stimulation with acetylcholine (Fig. 5B), whereas the fragments PKC-α (C1-C2-C3), PKC-α (C1-C2), PKC-α (C2-C3), and PKC-α (C3-C4) did not bind calponin either before or after stimulation with acetylcholine (Fig. 5B). These data suggest that the functional association of PKC-α with calponin may require both the C2 and C4 domain.
DISCUSSION
Calponin is hypothesized to be a cytoskeleton regulatory protein functioning as a bridge between actin and the intermediate filament network in smooth muscles (9). Potential binding partners for calponin include calmodulin, s100 proteins, tropomyosin, myosin, and caldesmon (10). A direct interaction of calponin with phospholipids and with HSP90 has also been suggested (19).
Immunoprecipitation with anti-calponin antibody followed by immunoblot against anti-PKC-α antibody indicated an increase in the amount of associated proteins in the particulate fraction in response to acetylcholine in CSMC. The increase was evident at 30 s after stimulation with acetylcholine and was sustained at 4 min. There is concomitant decrease in the acetylcholine-induced association of calponin with PKC-α in the cytosolic fractions. This is indicative of increased association of translocated calponin with translocated PKC-α in response to acetylcholine in the CSMC (19). Agonist-induced smooth muscle contractions are not entirely dependent on myosin-light chain phosphorylation (42). Myosin light chain phosphorylation did not change in ferret aorta smooth muscles loaded with antisense calponin RNA during phenylephrine-induced contraction. Ferret aorta smooth muscle strips loaded with antisense calponin RNA also showed decreases in the amplitude of phenylephrine-induced contraction (28, 29). Matthew et al. (19) reported an increase in shortening velocity of smooth muscle from the bladder and vas deferens of calponin-knockout mouse. Agonist-induced contraction was not addressed in mice lacking calponin (15). In the present studies, we have used isolated permeabilized smooth muscle cells to examine the involvement of calponin during agonist-induced contraction. Preincubation of cells with anti-calponin antibody inhibit agonist-induced smooth muscle contraction. Walsh (42) has suggested that calponin might play an important role in regulation of agonist-induced contraction of tonic smooth muscles.
Association and redistribution of calponin along with PKC upon agonist stimulation has been previously reported in vascular smooth muscle. In the relaxed state of the cell, calponin is distributed throughout the cell cytoplasm of vascular smooth muscle cells (21). In the present studies, reduction of the associated proteins in the cytosolic fraction indicated a translocation of the protein. Leinweber et al. (15) have shown that calponin interacts with PKC-α at the regulatory domain in vitro and that aa 160–182 of calponin seems to be necessary for its interaction with PKC-α. Calponin has been shown to inhibit actin-activated myosin ATPase activity in reconstituted contractile protein systems, and this inhibition is reversed by phosphorylation catalyzed in vitro by PKC or Ca+2/calmodulin-dependent protein kinase II (CaM kinase II) (39). Calponin is also a well-established in vitro substrate for PKC as well as a possible in vivo substrate for PKC (11). Rokolya et al. (32) have demonstrated that the physiological kinase for calponin phosphorylation is protein kinase C. Studies from other laboratories have indicated that PKC activity is related to its subcellular localization (33, 35). Many investigators have described association of PKC to the plasma membrane upon stimulation of smooth muscles (4). Membrane association is reflected in a shift in subcellular localization and translocation from cytosolic PKC to membrane compartments. This process is controlled by protein-protein interactions that play an important role in localization and function of PKC isozymes. The interaction between PKCs and cytoskeletal proteins is isozyme selective.
In smooth muscle cells, PKC can interact with several proteins. Recent data from our laboratory indicate that acetylcholine induces a significant and sustained increase in the association of thin-filament tropomyosin with PKC-α in the particulate fraction of CSMC (36). In response to acetylcholine stimulation, calponin translocates to particulate fraction with an increase in the association of calponin with HSP27, tropomyosin and PKC-α in CSMC (30). The interactions of PKC with other proteins play an important role in the functions of PKC itself and the other proteins with which it interacts. In the present study, we have investigated the domains of PKC-α essential for its binding to calponin. In vitro and in vivo studies were carried out to investigate the specific domains of PKC-α involved in direct interaction calponin with PKC-α. In vitro studies were carried out using GST-tagged-calponin fusion protein. Although the fusion protein contained calponin aa 92-298, it included the putative phosphorylation sites (Ser175 and Thr184) (34). The fusion protein could also be identified by anti-calponin monoclonal antibody, indicating that the expressed fusion protein was functional. The fusion protein was immobilized on glutathione agarose and was tested for its interaction with recombinant PKC-α. Examination of the dot blots of the fractions collected from the in vitro binding studies indicated a direct association of recombinant PKC-α with GST-calponin aa 92-229 fusion protein (Fig. 2A). Furthermore, to test whether the association was specific, we used GST alone as control and the elution did not reveal any binding of PKC-α with GST alone (Fig. 2A). Direct association of calponin with PKC-α explains the direct association of calponin with PKC-α in CSMC.
In vitro binding data indicate that calponin binding sites exist on C2, the regulatory domain, and C4, the catalytic domain of PKC-α. Most of the protein binding sequences are localized to the regulatory domain of PKC (15). The C2 domain has dual roles in the regulation of PKC-α activity. In addition to its proposed lipid or Ca2+-lipid binding sites, this domain regulates protein-protein interactions. The C2 region contains the RACK binding site (37) and the GAP43 binding site in PKC-δ (25). The C2 region in PKC-β also possesses the pseudo-RACK binding site (25). The RACKs bind PKC in its active conformation and increase PKC phosphorylation of substrates (25). In this study, we found the calponin binding sites exist within the regulatory domain, which provide further evidence of the direct interaction between PKC and the cytoskeletal protein calponin. The COOH terminus is largely conserved among the different PKC isoforms, suggesting the possibility of common binding sites, which bestows a possible role of the catalytic domain in targeting PKC isozymes to specific cellular sites and possible interactions with other proteins. Our data indicate that the presence of possible calponin binding sites on C2 domain of PKC-α. However, PKC-α (C1-C2) and PKC-α (C1-C2-C3) did not bind to GST-calponin aa 92-229. It is possible that the C1 domain interferes with the binding of C1-C2 and C1-C2-C3 domain(s) with calponin. This result is consistent with other studies (24). Leinweber and coworkers (15) found that the C2 and C1B domains of PKC-α interacted in the intact regulatory domain, since both C2 and C1B bound to the cytoskeletal protein calponin better than the whole regulatory domain. In the study of regulation of PKC-α activity, Slater et al. (37) found that the existence of C1-C2 domain interactions retained the PKC-α molecule in an inactive conformation. In this study we found without C1 domain, C2-C3, C3-C4 and C2-C3-C4 bound to calponin in vitro. However, in transfected cells the two fragments, C2-C3 and C3-C4 did not bind to calponin. Only fragment C2-C3-C4 bound to calponin. These results indicated that individual domains, C2 or C4 may not play a role in structural stabilization in the binding of PKC-α to calponin. Both C2 and C4 domains may be required for PKC to function well, which is different from binding of PKC-α to RhoA (27). In cells, the membrane association of PKC occurs through C1 or C2 domains (25). In vivo situations, recruitment of PKC to membranes by both the C1 and C2 domains results in a remarkably high-affinity interaction that depends on the presence of both diacylglycerol (or phorbol esters) and phosphatidylserine. This tight binding results in a release of the pseudosubstrate from the active site, thus allowing substrate binding and catalysis.
All PKCs, with the possible exception of PKC-μ, contain an autoinhibitory pseudosubstrate domain that maintains PKC in an inactive conformation by sterically blocking the active site. Activation of PKC is always coupled to removal of this autoinhibitory domain from the active site. Newton (24) proposed the model for PKC's regulation. PKC adopts a conformation such that pseudosubstrate occupies the active site of the catalytic domain of PKC. The high-affinity binding of the pseudosubstrate sequence to the catalytic cleft blocks substrate access and, hence, catalytic activity, thus maintaining it in the inactive form. Our results indicated that PKC-α (C2-C3-C4) bound to calponin in vitro. Furthermore, in cells transfected with this fragment, their association with calponin increased upon stimulation with acetylcholine. The constructs of PKC-α (C2-C3-C4) may mimic the active form of PKC-α. Our results are consistent with the model in which activation of PKC-α by phorbol esters or Ca2+ exposes regions in the catalytic domains that interact with PKC-α binding proteins and may explain the ability of wild-type PKC-α to be translocated to the membranes under certain conditions.
In summary, C2, the regulatory domain, and C4, the catalytic domain of PKC-α, are essential for the direct interaction of calponin with PKC-α in CSMC. Furthermore, calponin being actin-binding proteins, the direct interaction between calponin and PKC-α would suggest the possibility of interplay between signaling and contractile proteins. We thus hypothesize that direct interaction of calponin with PKC-α results in phosphorylation of calponin, which leads to agonist smooth muscle contraction of colon. Thus our results are in agreement with other reports indicating an important role for calponin in agonist-induced smooth muscle contraction.
GRANTS
This study was supported by National Institutes of Health Grant 5 RO1 DK-057020.
Acknowledgments
We thank Haiyan Pang for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andrea JE, Walsh MP. Protein kinase C of smooth muscle. Hypertension 20: 585–595, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Carmichael JD, Winder SJ, Walsh MP, Kargacin GJ. Calponin and smooth muscle regulation. Can J Physiol Pharmacol 72: 1415–1419, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Childs TJ, Watson MH, Novy RE, Lin JJ, Mak AS. Calponin and tropomyosin interactions. Biochim Biophys Acta 1121: 41–46, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Dekker LV, Parker PJ. Regulated binding of the protein kinase C substrate GAP-43 to the VO/C2 region of protein kinase C-delta. J Biol Chem 272: 12747–12753, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Eaton BL Tropomyosin binding to F-actin induced by myosin heads. Science 192: 1337–1339, 1976. [DOI] [PubMed] [Google Scholar]
- 6.Haeberle JR Calponin decreases the rate of cross-bridge cycling and increases maximum force production by smooth muscle myosin in an in vitro motility assay. J Biol Chem 269: 12424–12431, 1994. [PubMed] [Google Scholar]
- 7.Hakes DJ, Dixon JE. New vectors for high level expression of recombinant proteins in bacteria. Anal Biochem 202: 293–298, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Huang R, Li L, Guo H, Wang CL. Caldesmon binding to actin is regulated by calmodulin and phosphorylation via different mechanisms. Biochemistry 42: 2513–2523, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Ibitayo AI, Tsunoda Y, Nozu F, Owyang C, Bitar KN. Src kinase and PI 3-kinase as a transduction pathway in ceramide-induced contraction of colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 275: G705–G711, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Je HD, Gangopadhyay SS, Ashworth TD, Morgan KG. Calponin is required for agonist-induced signal transduction—evidence from an antisense approach in ferret smooth muscle. J Physiol 537: 567–577, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin JP, Walsh MP, Sutherland C, Chen W. A role for serine-175 in modulating the molecular conformation of calponin. Biochem J 350: 579–588, 2000. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko T, Amano M, Maeda A, Goto H, Takahashi K, Ito M, Kaibuchi K. Identification of calponin as a novel substrate of Rho-kinase. Biochem Biophys Res Commun 273: 110–116, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Khalil RA, Morgan KG. Phenylephrine-induced translocation of protein kinase C and shortening of two types of vascular cells of the ferret. J Physiol 455: 585–599, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol 302: 593–606, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Leinweber B, Parissenti AM, Gallant C, Gangopadhyay SS, Kirwan-Rhude A, Leavis PC, Morgan KG. Regulation of protein kinase C by the cytoskeletal protein calponin. J Biol Chem 275: 40329–40336, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Leinweber BD, Leavis PC, Grabarek Z, Wang CL, Morgan KG. Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. Biochem J 344: 117–123, 1999. [PMC free article] [PubMed] [Google Scholar]
- 17.Mabuchi K, Li B, Ip W, Tao T. Association of calponin with desmin intermediate filaments. J Biol Chem 272: 22662–22666, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Marston SB, Huber PAJ. Caldesmon. In: Biochemistry of Smooth Muscle Contraction, edited by Barany M and Barany K. San Diego, CA: Academic, 1996, p. 77–90.
- 19.Matthew JD, Khromov AS, McDuffie MJ, Somlyo AV, Somlyo AP, Taniguchi S, Takahashi K. Contractile properties and proteins of smooth muscles of a calponin knockout mouse. J Physiol 529: 811–824, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mino T, Yuasa U, Naka M, Tanaka T. Phosphorylation of calponin mediated by protein kinase C in association with contraction in porcine coronary artery. Biochem Biophys Res Commun 208: 397–404, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Mochly-Rosen D Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science 268: 247–251, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Morano I Tuning smooth muscle contraction by molecular motors. J Mol Med 81: 481–487, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura F, Mino T, Yamamoto J, Naka M, Tanaka T. Identification of the regulatory site in smooth muscle calponin that is phosphorylated by protein kinase C. J Biol Chem 268: 6194–6201, 1993. [PubMed] [Google Scholar]
- 24.Newton AC Protein kinase C: structure, function, regulation. J Biol Chem 270: 28495–28498, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Newton AC, Johnson JE. Protein kinase C: a paradigm for regulation of protein function by two membrane-targeting modules. Biochim Biophys Acta 1376: 155–172, 1998. [DOI] [PubMed] [Google Scholar]
- 26.North AJ, Gimona M, Cross RA, Small JV. Calponin is localised in both the contractile apparatus and the cytoskeleton of smooth muscle cells. J Cell Sci 107: 437–444, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Pang H, Bitar KN. Direct association of RhoA with specific domains of PKC-α. Am J Physiol Cell Physiol 289: C982–C993, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Parker CA, Takahashi K, Tang JX, Tao T, Morgan KG. Cytoskeletal targeting of calponin in differentiated, contractile smooth muscle cells of the ferret. J Physiol 508: 187–198, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker CA, Takahashi K, Tao T, Morgan KG. Agonist-induced redistribution of calponin in contractile vascular smooth muscle cells. Am J Physiol Cell Physiol 267: C1262–C1270, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Patil SB, Pawar MD, Bitar KN. Direct association and translocation of PKC-α with calponin. Am J Physiol Gastrointest Liver Physiol 286: G954–G963, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Pohl J, Winder SJ, Allen BG, Walsh MP, Sellers JR, Gerthoffer WT. Phosphorylation of calponin in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 272: L115–L123, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Rokolya A, Walsh MP, Singer HA, Moreland RS. Protein kinase C-catalyzed calponin phosphorylation in swine carotid arterial homogenate. J Cell Physiol 176: 545–552, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA 91: 839–843, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J 13: 1658–1676, 1999. [PubMed] [Google Scholar]
- 35.Ron D, Luo J, Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of B protein kinase C in vivo. J Biol Chem 270: 24180–24187, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Ron D, Mochly-Rosen D. An autoregulatory region in protein kinase C: the pseudo anchoring site. Proc Natl Acad Sci USA 92: 492–496, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slater SJ, Seiz JL, Stagliano A, Stubbs CD. Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry 40: 4437–4445, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Small JV, Gimona M. The cytoskeleton of the vertebrate smooth muscle cell. Acta Physiol Scand 164: 341–348, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Somara S, Pang H, Bitar KN. Agonist-induced association of tropomyosin with protein kinase Cα in colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 288: G268–G276, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Szymanski PT Calponin (CaP) as a latch-bridge protein—a new concept in regulation of contractility in smooth muscles. J Muscle Res Cell Motil 25: 7–19, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Szymanski PT, Goyal RK. Calponin binds to the 20-kilodalton regulatory light chain of myosin. Biochemistry 38: 3778–3784, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Walsh MP Calponin—knocked out but not down! J Physiol 529: 517, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winder SJ, Allen BG, Clement-Chomienne O, Walsh MP. Regulation of smooth muscle actin-myosin interaction and force by calponin. Acta Physiol Scand 164: 415–426, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Winder SJ, Sutherland C, Walsh MP. Biochemical and functional characterization of smooth muscle calponin. Adv Exp Med Biol 304: 37–51, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Winder SJ, Walsh MP. Calponin. Curr Top Cell Regul 34: 33–61, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Winder SJ, Walsh MP, Vasulka C, Johnson JD. Calponin-calmodulin interaction: properties and effects on smooth and skeletal muscle actin binding and actomyosin ATPases. Biochemistry 32: 13327–13333, 1993. [DOI] [PubMed] [Google Scholar]