Abstract
The complex interaction of genetic, microbial, and environmental factors may result in continuous activation of the mucosal immune system leading to inflammatory bowel disease (IBD). Most present treatments for IBD involve altering or suppressing the aberrant immune response; however, the role of the intestinal microbiota in the pathophysiology of IBD is becoming more evident. The epithelial layer is essential for the proper functioning of the gastrointestinal tract, and its increased permeability to the luminal antigens may lead to the inflammatory processes and mucosal damage observed in IBD. Factors affecting the efficacy of the epithelial barrier include presence of pathogenic bacteria (e.g., Helicobacter spp.), presence of probiotic bacteria, availability of selected nutrients, and others. Defective function of the mucosal barrier might facilitate the contact of bacterial antigens and adjuvants with innate and adaptive immune cells to generate prolonged inflammatory responses. This review will briefly describe the complex structure of the epithelial barrier in the context of bacterial-mucosal interactions observed in human IBD and mouse models of colitis.
Keywords: enteric bacteria, permeability, inflammation
the wall of the intestine consists of four layers: 1) the mucosa, separated by a thin layer of smooth muscle from 2) the submucosa, 3) layers of smooth muscle called the muscularis propria, and 4) an outer layer of connective tissue called the serosa. The mucosa consists of a single layer of epithelial cells that borders the lumen, with underlying loose connective tissue, termed the lamina propria, that contains numerous immune cells. The presence of the epithelial layer is essential for proper functioning of the gastrointestinal (GI) tract. Besides secretion and absorption, the epithelium prevents unwanted solutes, microorganisms, and luminal antigens from entering the body (19, 118). Thus, although the epithelium has to provide an effective barrier against harmful macromolecules and microorganisms, it must be permeable to nutrients and macromolecules while allowing a controlled exposure of the mucosal immune system to microbial factors (21). Impairment of this barrier function leading to the increased permeability to luminal antigens has been proposed as an initiating factor in the pathogenesis of chronic human inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn's disease (CD) that are characterized by aberrant immune responses against microorganisms that are present in the intestine. Thus increased permeability may play a central role in the inflammatory process and may be linked to the chronic mucosal damage observed in IBD.
Most present treatments for IBD involve altering or suppressing the aberrant immune responses; however, the role of the intestinal microbiota in the pathophysiology of IBD is becoming more evident (102). Recent discoveries in animal models have unraveled several additional factors that can affect the efficacy of the epithelial barrier. These factors include presence of certain pathogenic bacteria (41), role of probiotic bacteria in competitive exclusion (61), availability of selected nutrients (57), and others. For example many investigators have reported previously that IBD can be accelerated in IBD-susceptible IL-10−/− mice by infection with a variety of murine Helicobacter species (13, 18, 35, 125). Overall, the complex interaction of genetic, microbial, and environmental factors results in continuous activation of the mucosal immune system in IBD. Modification of types or numbers of colitis-inducing (colitogenic) bacteria present within the luminal microenvironment or prevention of their interaction with the immune system may offer alternative approaches that can synergize with existing therapies (50).
Potentially colitogenic bacterial antigens and adjuvants are present within the intestine of all mice not kept in highly artificial germ-free (gnotobiotic) facilities. However, the absence of such bacteria can prevent the development of IBD in susceptible mouse strains (88). IBD can also occur in a variety of mouse strains that are genetically deficient in immune regulatory factors. For example, nongerm-free mice deficient in the immunoregulatory cytokine IL-10 develop a colitis that closely resembles CD in humans. Mouse strains with defects that affect intestinal permeability also have been described to develop IBD. An example here is the mdr (multidrug resistance) 1a−/− mouse (described further in Disruption of the Epithelial Barrier), (68). Characteristics of animal models of IBD have been reviewed extensively (28, 96).
On the basis of our present knowledge, we propose that the development of IBD requires three factors: 1) the presence of bacterial antigens and adjuvants within the intestine, 2) a host defect in immune regulation that allows induction of immune responses against these antigens, and 3) defective function of the mucosal barrier so that the bacterial antigens and adjuvants present within the intestine can come in contact with the innate and adaptive immune cells to generate responses. The three-factor model can potentially explain how the known susceptibility alleles and IBD-related triggers in existing murine models result in the development of chronic colitis. This review will briefly describe the complex structure of the mucosal barrier and specifically focus on factors that affect bacterial-mucosal interactions.
Epithelial Barrier
Epithelial cells.
Both human studies and various animal models have demonstrated the importance of maintaining the epithelial barrier (43). Colonic epithelial cells are the first line of defense against enteric antigens and bacteria (23). The luminal surface of the intestine consists of a single layer of epithelial cells joined together by tight junctions. This forms a selective barrier that facilitates nutrient absorption while, at the same time, limiting uptake of antigens or potentially toxic material from the lumen (83). In normal host, the epithelial barrier restricts both transcellular (through the cells of the epithelium) and paracellular (between the cells) permeation of antigens, allowing only small quantities of molecules to cross into the mucosa and interact with the mucosal immune system. Epithelial cells respond to several neuronal and immune mediators by modifying their function, including permeability (100). The sustained enhancement of paracellular permeability facilitates the constant passage of luminal antigens through the mucosa and can lead to chronic inflammation in susceptible individuals (121). In contrast, transcellular permeability typically only increases during active inflammation (83). Specialized cholesterol-rich membrane domains called lipid rafts are believed to play an important physiological role in macromolecular transport and permeability in the intestinal epithelium (79).
Lipid rafts.
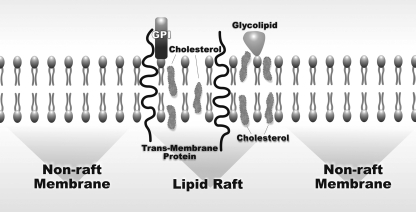
Lipid rafts are plasma membrane domains that are composed of cholesterol, glycosphingolipids, and glycosylphosphatidylinositol (GPI)-anchored proteins (123). Clustered lipid rafts that are made up of an assembly of smaller units have been implicated in a variety of cellular functions including signaling and growth regulation, as well as cellular transport (Fig. 1) (79). Numerous bacterial pathogens have evolved mechanisms of coopting host endocytic pathway via clustered lipid rafts, including Escherichia coli (E. coli) (1) and Campylobacter jejuni (58). An important virulence factor of E. coli, FimH adhesin, acts via a mechanism dependent on membrane cholesterol as well as caveolin-1 presence in lipid raft domains. FimH is a mannose-binding lectin on the tips of bacterial fimbrial appendages that was first identified in uropathogenic E. coli (49). Subsequently, the receptor for FimH-expressing E. coli was identified as the GPI-anchored protein, CD 48 (7) located within lipid rafts. Thus FimH allows the bacteria to bind to cells and colonize mucosal surfaces. In addition, the mechanism of transcellular passage of Campylobacter jejuni involves tyrosine phosphorylation of certain host cell substrates (58). Several other microorganisms use different mechanisms during the host infection. For example, Helicobacter pylori bacteria cause gastric pathology, in part, via affecting the cholesterol gradient and extracting the lipid from plasma membranes of epithelial cells for subsequent glucosylation, eventually leading to the induction of inflammation (120).
Fig. 1.
Structure of the epithelial membrane including nonraft membrane and a lipid raft. Lipid rafts are enriched with cholesterol and studded with proteins. GPI, glycosylphosphatidylinositol-anchored cell surface glycoproteins.
A study by Flanagan et al. (32) focused on discerning the gene expression pattern of intestinal epithelial cells during the development and progression of IBD. Those authors identified the LY6A gene superfamily as strongly upregulated in inflamed intestinal epithelial cells. The majority of LY6 family members are GPI-anchored cell surface glycoproteins with broad distribution on cells of hematopoietic origin (32). Surface expression of LY6A was induced by IL-22 and interferon (IFN)-γ and resulted in production of a number of chemokines known to be involved in the immunopathogenesis of IBD. These authors suggest that LY6 upregulation of chemokine secretion is dependent on cholesterol biosynthesis, suggesting a role for lipid raft reorganization in LY6-mediated chemokine production. Furthermore, that data suggests that LY6 family members may be involved in the pathogenesis of IBD by establishing and maintaining an unresolved chemokine gradient in the colon, which eventually leads to the alteration in mucosal permeability.
Tight junctions.
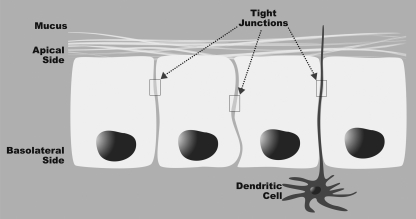
Tight junctions (zonulae occludentes, ZO) are typically found at the boundary between the apical and the basolateral membrane domains of adjacent epithelial cells (34). A tight junction consists of a cluster of proteins that forms a physiologically active barrier at the level of the intestinal epithelial cell that can change its permeability on the basis of the cellular environment (Fig. 2). The transmembrane proteins constituting the tight junctions are attached to the cytoskeleton, thereby linking cell-to-cell adhesion sites sealing the paracellular space. Occludin has been identified as a tight junction-specific integral membrane protein that is regulated by immune mediators, whose expression restricts paracellular transport of macromolecules (37). Occludin is ∼65 kDa and is attached to the cytoskeleton by another group of proteins including cingulin and 7H6 (48). Proteins ZO-1, -2, and -3 bind the cytoplasmic tail of occludin and link the tight junction to the actin cytoskeleton (34). Furthermore, proteins of the ZO family can shuttle to the nucleus to influence transcriptional processes in cellular proliferation and differentiation (36). A major mechanism of altering tight junctions is the phosphorylation of these proteins. Recently it was reported that atypical protein kinase C (a subgroup of the protein kinase C family with distinct protein structure and regulatory mechanisms) regulates tight junction formation through the phosphorylation of claudin-4, an integral membrane protein (3). In another study, it was suggested that increases in intestinal epithelial tight junction permeability are also associated with increases in phosphorylation of myosin II regulatory light chain kinase-dependent myosin light chain (31).
Fig. 2.
Structure of the mucosal barrier. Tight junctions link adjacent colon epithelial cells. The processes of dendritic cells pass between epithelial cells. The dendritic cells sample and process antigen material for presentation to other immune cells. Mucus produced by epithelial cells serves an additional physical barrier to prevent penetration of luminal antigens.
The expression of tight junction proteins has been shown to be decreased in IBD (38). This is accompanied by an increase in intestinal permeability, decrease in transepithelial resistance, and an increased possibility that luminal antigens will come in contact with immune cells located in the lamina propria. At least in colitis attributable to oral administration of the chemical irritant dextran sulfate sodium, it was suggested that alterations in the tight junction complex occur before and not as a consequence of the onset of intestinal inflammation and that these changes may facilitate development of the inflammatory infiltrate seen in colitis (81). Also, it is possible that alterations in the structure and complexity of tight junctions act in conjunction with mucus production (see section below) to modulate epithelial permeability.
Goblet cells and intestinal mucus.
Specialized intestinal epithelial cells called goblet cells produce a viscous mucus layer that covers the intestinal epithelium (42). Recent studies have revealed the role of goblet cells in the pathogenesis of colitis. Two of the characteristic histological features of IBD (especially UC) are goblet cell depletion and thinning of the mucus layer (42). Anti-goblet cell autoantibodies have been described in up to 40% of patients with IBD (44) and also in 20% of first-degree relatives of patients with IBD (33). Those results suggest that anti-goblet cell autoantibodies may represent a marker characterizing susceptibility to IBD.
The intestinal mucus layer is a dense, carbohydrate-rich matrix that consists primarily of O-glycosylated mucins (2) that form the protein building blocks of the mucus layer. Regulatory roles of mucin proteins Muc2 and Muc3 have been previously demonstrated to be important in IBD (46, 113). Muc2 is the major mucin present in the intestinal mucus barrier and it has been shown that its deficiency leads to inflammation in the colon (42). Mice with mutation in the Muc2 gene showed aberrant mucin biosynthesis, less stored mucin in goblet cells, a diminished mucus barrier, and increased susceptibility to colitis induced by a luminal toxin (42). Muc2-deficient mice also demonstrate enhanced local production of IL-1β, tumor necrosis factor (TNF), and IFN-α in the distal colon, with a twofold increase in intestinal permeability. Spontaneous colitis in Muc2-deficient mice, which lack the recognizable goblet cells throughout the entire intestine, illustrates the importance of the mucus barrier as the protection against antigens present in the luminal environment (113). Muc3 was shown to play an active role in epithelial restitution and maintenance of the epithelial barrier (46). Although some studies propose that some Muc proteins participate in the suppression of colitis (46, 113), the definitive and detailed roles of the mucus as a protective agent against colitis remains to be fully determined. It should be noted that several studies have shown a remarkable degree of heterogeneity in human intestinal mucus that was associated with extensive differences in the glycosylation of the various mucins present in different regions of the intestine (6, 25, 77). For example, in situ hybridization has demonstrated that mucins from each section of the gastrointestinal tract have a typical Muc gene composition: Muc2 is localized to goblet cell vesicles in both small and large intestine, whereas Muc3, more abundant in the small intestine, is found in most surface epithelial cells and in the upper parts of the crypt cells. Moreover, Muc5B and Muc6 are weakly expressed in the colon and are absent in other parts of the digestive tract, whereas Muc4 is widely expressed in the GI tract.
Several additional studies have demonstrated the important barrier functions of mucus in IBD. Altered intestinal O-glycan expression in intestinal mucus has been observed in patients with UC and colorectal cancer. The enzyme β1,3-N-acetylglucosaminyltransferase (C3GnT) is responsible for the biosynthesis of core 3-derived O-glycans (2). C3GnT-deficient mice exhibit impaired colonic mucus layer formation, colon-specific reduction in Muc2, and increased intestinal permeability (2). Moreover, these mice were highly susceptible to experimental triggers of colitis and colorectal adenocarcinoma. Thus mucin depletion has been suggested to be a fundamental component of the pathogenesis of colitis.
Bacteria and the mucus.
A variety of bacteria are present within the intestine (89), and these bacteria may have different relationships with the mucus. The mucus layer is nearly bacteria-free in the mid to distal murine colon (107). However, bacteria may be present in the mucosa-adjacent and luminal regions of the cecum and proximal colon. Thus each segment of the mouse colon has a characteristic distribution of microbiota with respect to the mucus (107). There might be several explanations for the spatial organization of the microbiota. Oxygen concentrations, products of innate and acquired immunity (e.g., defensins, immunoglobulins, phosphatidylcholine, etc.), various nutrients, intraluminal pH, and mucus viscosity all vary depending on the intestinal region and the proximity to the colonic mucosa (17, 91). Each of these factors may interfere with bacterial growth. Microorganisms can coaggregate and form biofilms that display synergistic and antagonistic properties compared with their free-swimming (planktonic) counterparts (24, 92).
Mucosal biofilms are defined as organized arrays of bacteria that are adherent to the epithelial surface (106). Studies using ribosomal RNA-targeted fluorescence in situ hybridization have shown that biofilms are not generally present in normal intestine in humans (105, 110) or mice (107). In contrast, complex multispecies biofilms were identified in about two-thirds of the patients with IBD studied (105, 110). These biofilms penetrate intestinal mucus leading to direct contact between fecal bacteria and the mucosal epithelium. On the other hand, it was shown that, in normal mice, mucus prevents luminal (fecal) bacteria from contacting the epithelial surface in the mid to distal colon (107, 110). Once a biofilm is established, other pathogens can potentially settle within it and invade epithelial cells or stimulate immune cells. The presence of a biofilm may thus allow luminal antigens and toxins to reach the unshielded epithelial surface where they can trigger cascades of host inflammatory responses. Individual immunity and genetic disposition may determine whether the increased exposure to bacterial antigens that occurs as a result of biofilm formation leads to development and perpetuation of the intestinal inflammation that is characteristic of IBD.
Bacteria and IBD
Bacterial populations.
Involvement of bacterial communities in the pathogenesis of IBD has been demonstrated by multiple studies (41, 73, 105, 107). For example, it was reported that larger numbers of bacteria are associated with bowel biopsies collected from patients with CD compared with controls (22). This increased concentration relates to both anaerobes as well as facultative bacteria and may be partially due to a disruption in epithelial barrier function in susceptible individuals (119). It was suggested that microbiota-derived lipopolysaccharide is necessary for the development of inflammatory Th1-type T cells in colitis (54). These findings were further investigated in the studies using IL-10−/− mice that lacked any kind of toll-like receptors (TLRs) response as the result of the knockout of myeloid differentiation factor 88 (MyD88), an essential component of most TLR signaling pathways (85). Those mice failed to develop colitis, suggesting an important role of microbial antigens in the development of inflammation. In another study, the same authors also showed that activation of TLRs by commensal microflora is critical for the protection against gut injury and associated mortality (86). Their results suggest that this TLR-mediated protection may work through two possible, not mutually exclusive, mechanisms: the detection of luminally derived TLR ligands on commensal bacteria by TLRs expressed on colonic epithelium or inducing the production of protective factors upon epithelial damage by commensal-derived TLR ligands.
Recently, a number of scientific studies have focused on microbial composition in patients with IBD and in animal models (108, 110). Interestingly, a reduced biodiversity in the fecal and biopsy-associated samples was shown in patients with CD (102) compared with controls. For example, clostridial cluster IV (Clostridium leptum subgroup) formed a smaller proportion of the fecal community in patients with CD than in controls. This phylogenetic group contains several butyrate-producing bacteria. Butyrate and other short-chained volatile fatty acids are believed to be important sources of energy for colonic epithelial cells and may have immunomodulatory and anti-inflammatory properties. Thus the decrease in these bacteria in the colon might have a detrimental effect on the colonic epithelium (53, 99).
Colon biopsies from healthy humans without IBD are nearly free of bacteria once they are washed of fecal contents (105). In contrast, the concentrations of bacteria in biopsies from patients with IBD were high even after being washed. The bacteria that remained after being washed were tightly adherent to the mucosa and thus have been termed “mucosal bacteria.” Numbers of mucosal bacteria increased progressively from self-limited colitis to UC to CD (107). Additionally, mucosal bacterial concentrations are low in patients with IBD in remission and high in patients with active IBD. Also, they are inversely correlated to the daily doses of 5-aminosalicylate therapy and to the use of antibiotics (110). The types of mucosal bacteria seen in patients with IBD were also seen in normal subjects without IBD but are not associated with the mucosa in normal subjects (105). Other studies have shown the difference in bacterial profiles in patients with UC and CD (109) and suggested that the suppressing effects of antibiotics on the mucosal microbiota in IBD are accompanied by massive rebound of both the microbiota and inflammation when antibiotics are discontinued (106).
In another study, fluorescence in situ hybridization with probes specific for bacterial 16S rRNA combined with conventional histological techniques was used to study the relationships between various species of intestinal bacteria and the mucosa in mice with and without colitis (107). Those studies have demonstrated that, in normal mice, most bacterial groups are separated from the mucosal surface by either a mucus layer that excludes bacteria that is present from the mid-colon distally or by an “interlaced” layer in the cecum and proximal colon that is composed of tightly packed bacteria. The interlaced and/or mucus layers thus limit the contact of the bulk of the intestinal bacteria with the mucosal epithelium. However, situations that cause defects in the epithelial surface or degrade the protective qualities of mucus and/or the interlaced layer may allow contact of bacterial antigens and adjuvants with immune cells located in the lamina propria and lead to generation of immune responses that result in IBD. Whether the increased exposure to bacterial antigens that occurs as a result of biofilm formation leads to the development and perpetuation of the intestinal inflammation that is characteristic of IBD may depend on genetic factors that cause variations in the innate and/or adaptive immune responses of individual host.
Probiotics and IBD.
Pathogens can affect the integrity of the epithelial barrier, allowing them easy access to the gut lamina propria and systemic spreading, eventually leading to bacteremia (55, 98). However, certain types of bacteria (usually called “probiotic” or beneficial bacteria) are known to enhance and contribute to the epithelial barrier (17, 67). Probiotic bacteria have both direct and indirect beneficial effects on the epithelia, including enhancement of epithelial barrier function via altering intestinal microarchitecture (17), modulation of the mucosal immune system (15), and alteration of the intestinal environment (70). Probiotics inhibit pathogenic enteric bacteria via competitive exclusion, i.e., decreasing luminal pH, secretion of bacteriocidal proteins, and blocking bacterial binding to epithelium (20). Probiotic bacteria can also alter immunoregulation (39), including the induction of the expansion of T regulatory cells (26). Probiotics have also been shown to induce intestinal production of anti-inflammatory cytokines (e.g., IL-10 and transforming growth factor-β) while reducing production of proinflammatory cytokines (e.g., TNF) (30). Oral administration of the VSL#3 consortium of probiotics (16) was shown to normalize barrier function in IL-10−/− mouse model of IBD (67). VSL#3 is a probiotic cocktail consisting of eight different gram-positive organisms: Bifidobacterium (B.) longum, B. infantis, B. breve, Lactobacillus (L.) acidophilus, L. casei, L. delbrueckii spp. bulgaricus, L. plantarum, and Streptococcus salivarius spp. Thermophilus (67). The characteristics of probiotic bacteria suggest that some bacterial species may be efficacious in the treatment of IBD by strengthening the epithelial barrier. For example, VSL#3 treatment of IL-10−/− mice with IBD resulted in a normalization of colonic physiological function and barrier integrity along with a reduction in mucosal levels of proinflammatory cytokines and a significant improvement in histological disease by secretion of soluble factors enhancing the barrier integrity (66). Other studies have confirmed that probiotic bacteria may enhance the integrity of the tight junctions between the intestinal epithelial cells during infections or inflammatory conditions (17). Thus colonization with probiotic bacteria may lead to decreased exposure of immune cells to the bacterial antigens that are believed to drive IBD.
Rachmiliwitz et al. (84) showed that protective effects of probiotic microorganisms (VSL#3) in a dextran sulfate sodium model of experimental colitis are mediated by DNA that was recognized by the mucosal TLR9 receptor. This interaction subsequently led to an increased endogenous production of β-defensins, antibacterial peptides that regulate bacterial survival. Additionally, it was reported that the treatment of cultured intestinal epithelial cells with VSL#3 led to an increase of the transepithelial electrical resistance, a change that correlates with decreased permeability (78). In that study, incubation of intestinal epithelial cells with this probiotic consortium also induced the expression of several mucins, leading to decreased adhesion of microorganisms and their components to the epithelial surface.
Interestingly, considering the properties of the biofilms (described in Epithelial Barrier), the ability of a probiotic strain to adhere to mucus and epithelial cell surfaces is one of the main selection criteria for candidate probiotics (51). The binding of bacteria to the mucus layer is a prerequisite for adhesion and prevents the bacteria from being removed by intestinal peristalsis (29). It has been suggested that Lactobacilli can colonize the nonsecretory gastric epithelium by attaching to epithelial cells and can continuously inoculate gastric contents and the lower regions of the intestinal tract (10, 47, 93). It is likely that the beneficial effects of probiotics are the sum of a complex, multivariate series of alterations in gut microbial and whole body metabolism (16). These alterations might include whole body and immune function (15), absorption of nutrients, and beneficial changes in the intestinal architecture (17). It is also possible that some probiotic species can communicate with the epithelial cells and/or the immune system, as well as modulating tissue physiology. For clinical applications, probiotic strains must be resistant to acid and bile and have the ability to be metabolically active within the luminal microenvironment where they should ideally survive but not persist in the long term (39).
Antigen sampling and antigen-presenting cells.
Generally, presentation of antigens to T cells by antigen-presenting cells such as dendritic cells (DC) is a critical event for the activation of adaptive immune responses. Overall, there are three mechanisms describing antigen sampling in the gut. In the first one, the antigen-transport mechanism from the lumen to the lamina propria is explained by a pathway mediated by the microfold (M) cells found in the epithelium overlying Peyers patches (115). Briefly, in a germ-free host, introduction of a commensal organism into the GI tract is followed by its entry into the Peyers patches and mesenteric lymph nodes (65); IgA responses selectively induced by DC may limit its further entry. In a second proposed pathway, lamina propria DC directly capture the luminal antigens by extending their dendritic processes between epithelial cells into the intestinal lumen (90). Finally, Yoshida et al. (122) described recently a third pathway in which neonatal Fc receptor serves as the vehicle that transports lamina propria-associated IgG across the colonic epithelial layer into the lumen where it can bind enteric bacterial antigens. The receptor then recycles the antigen/IgG complex back across the epithelial cells into the lamina propria for processing by DC, which further present the antigens to the T cells. A similar CD23/IgE-dependent mechanism has been also documented (63).
In the case of colitis, it was shown that enteric bacterial antigens are required for the activation of pathogenic T cells (103). It is evident that the biological functions of colonic epithelial cells under inflammatory conditions vary significantly depending on several factors, such as the phase of colitis (acute vs. chronic) and the specific diagnosis of UC vs. CD. For example, antibacterial peptides are commonly upregulated in colonic epithelial cells in both acute and chronic colitis (71), whereas enhanced expression of antigen-presenting molecules on colonic epithelial cells is specifically observed in chronic, but not in acute, colitis (72). RegIII-γ is a C type lectin that allows bacteria to bind without recruiting complement components (14). Production of RegIII-γ by colonic epithelial cells is markedly increased under chronic and acute intestinal inflammatory conditions but not in the recovery phase of colitis (72). Studies of CD have shown an increased expression of IL-12/IL-23, IFN-γ, and TNF cytokines by intestinal lamina propria lymphocytes (5, 45). Additionally, the ability of colonic epithelial cells to serve as antigen-presenting cells has been well documented in patients with IBD (23).
Disruption of the Epithelial Barrier
In a genetically predisposed animal model (e.g., IL-10−/−), microbiota that is not harmful in the normal host is capable of causing intestinal inflammatory response after disruption of the intestinal barrier has occurred (9), eventually leading to severe, chronic IBD. Such results were described in recent studies, where authors used nonsteroidal anti-inflammatory drugs (NSAIDs) piroxicam or sulindac to trigger the onset of IBD in IL-10−/− mice (9, 40). Results showed that exposure to piroxicam disrupted the epithelial barrier by enhancing intestinal epithelial apoptosis, thus facilitating adhesion and invasion of intestinal bacteria into mucosal tissues (40). It was suggested that other NSAIDs such as indomethacin (111) and celecoxib (64) may have similar effects.
Helicobacter effects on the mucosal barrier.
Many investigators have reported previously that IBD can be triggered in IL-10−/− mice by infection with Helicobacter (H.) hepaticus, H. bilis, and other murine enteric Helicobacter species (13, 35, 125). For example, our laboratory has demonstrated that IL-10−/− mice neonatally coinfected with Helicobacter rodentium and H. typhlonius rapidly developed severe colitis (41). In addition to developing more severe IBD at an earlier age than noninfected IL-10−/− mice, Helicobacter-infected mice had also a high rate of rectal prolapse and colonic neoplasia. However, Dieleman and colleagues (27) reported that germ-free IL-10−/− mice monoassociated with H. hepaticus did not develop IBD. Furthermore, simultaneous infection with H. hepaticus did not increase the severity of IBD in germ-free mice associated with specific pathogen-free flora. These seemingly conflicting findings can be reconciled by the “three-factor” model of IBD pathogenesis presented earlier. According to that model, development of IBD requires 1) the presence of bacterial antigens and adjuvants within the intestine, 2) a host defect in immune regulation that allows induction of immune responses against these antigens, and 3) defective function of the mucosal barrier so that the bacterial antigens and adjuvants present within the intestine can come in contact with the innate and adaptive immune cells to generate responses.
In this view, intestinal Helicobacter spp. function as “barrier breakers” that generate a breach in the mucosal barrier to allow the bacterial antigens and adjuvants already within the intestine to contact immune cells rather than sources of antigen per se. The germ-free mice studied by Dieleman et al. (27) already had a defective barrier attributable to decreased mucus production and absence of the protective interlaced layer (107) that contains probiotic bacteria (factor 2). The IL-10-deficient mice had an inherent genetic defect in immune regulation attributable to absence of the immunoregulatory cytokine IL-10 (factor 3). The lack of IBD in response to monoassociation with H. hepaticus indicates that these bacteria do not by themselves possess the appropriate antigens and adjuvants to induce an immune response that can initiate and perpetuate IBD (factor 1). However, in other studies involving Helicobacter infection and IL-10−/− mice that led to colitis (41, 60, 101), mice were not kept in a germ-free environment and were thus exposed to the wide variety of potentially colitigenic microbial antigens. Enhanced permeability of the barrier induced by Helicobacter infection that allows contact of colitogenic luminal bacteria with immune cells present in the lamina propria can initiate an aberrant immune response in mice with defective immune regulation that eventually results in colitis.
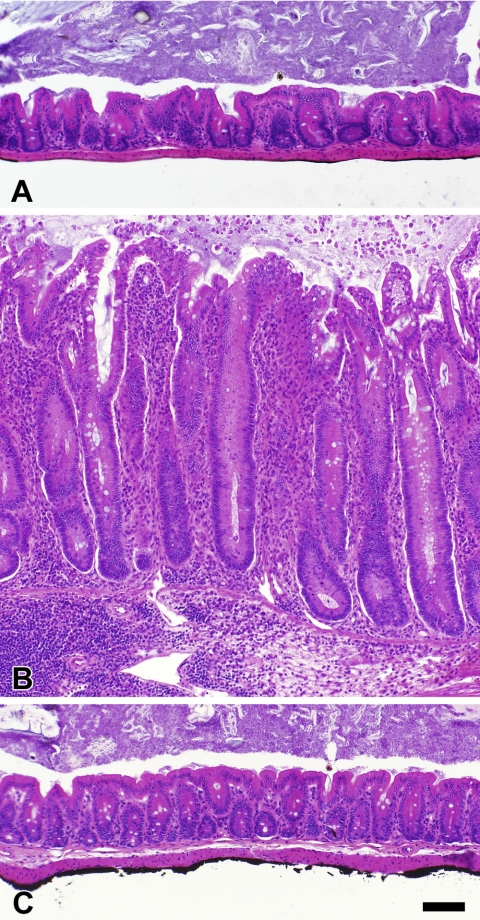
The concept of enteric Helicobacter species as barrier breakers in the intestine is attractive since a gastric-infecting member of this genus has previously been shown to degrade barrier functions in the stomach. H. pylori, the causative organism of peptic ulcers, attaches to the stomach wall and burrows through the mucus to grow adjacent to the gastric epithelium. This degrades the protective properties of the gastric mucus, allowing acid to contact and damage the epithelial surface. In case of the intestinal Helicobacter spp., the injurative leakage is hypothesized to consist of colitigenic bacterial antigens and adjuvants rather than acid, and this can lead to the development of IBD in susceptible hosts. Figure 3 shows the mucosal layer in control uninfected and helicobacter-infected IL-10−/− mice with or without anti-helicobacter antibiotic treatment. Infected mice develop IBD characterized by mucosal hyperplasia and inflammatory infiltrates that can be transmural. Focal ulcerations and architectural distortion may also be present. These inflammatory changes are abrogated by anti-helicobacter antibiotic treatment (Fig. 3C). Since the antibiotics used have broad activity against a range of microbial groups, it is not clear whether this improvement is due to direct effects on helicobacter organisms that prevent leakage of colitigenic antigens from the lumen, effects on other non-helicobacter microbiota, or other mechanisms. Clearly, additional studies will be required to specifically address the mechanisms by which intestinal Helicobacter spp. are involved in pathogenesis of IBD.
Fig. 3.
Colon histology in Helicobacter-infected and -noninfected IL-10−/− adult mice. The histological appearance of the cecum is shown for noninfected control mice (A) and mice coinfected with H. rodentium and H. typhlonius in the absence of treatment (B) or following treatment with a commercially available 4-drug anti-Helicobacter regimen (C). Untreated IL-10−/− mice infected with H. rodentium plus H. typhlonius had marked mucosal hyperplasia with prominent inflammatory infiltrates. The mucosal hyperplasia and infiltrates were totally abrogated by anti-Helicobacter treatment. All photomicrographs were taken at the same magnification; bar represents 100 μm.
Gut permeability in IBD.
One of the constant features in IBD seems to be an increase in intestinal permeability that can precede inflammatory lesions and trigger mucosal inflammation (116). Furthermore, increased mucosal permeability can continuously stimulate mucosal immune system. Increased paracellular permeability was reported in both acute and chronic colitis in humans (69, 95), as well as in IBD mouse models, including mdr1a−/− and IL-10−/− mice (21, 104). Also a number of studies have shown a potential role for inflammatory cytokines like TNF and IFN-γ in directly increasing intestinal epithelial permeability. Several mouse models have demonstrated that such inflammatory responses influence barrier function by inducing disassembly of tight junctions in epithelial cells (11, 12, 82, 112, 117). Bacteria were hypothesized to play a critical role in regulating this process. However, the specific bacteria species that may play this role in the development of IBD have not yet been determined.
Adherent invasive E. coli directly and indirectly induce host intestinal epithelial cells to upregulate CEACAM6, a receptor that facilitates its adherence and invasion (8). Similarly, the host has the ability to alter molecular interactions between commensals and the intestine. Ryu et al. (94) have shown that host transcription factors that regulate production of antimicrobial peptides can markedly affect the gut microbiota in Drosophila. In that study, inhibiting production of the Caudal transcription factor led to dominance of a previously minor strain of bacteria that led to gut cell apoptosis and increased host mortality. Reintroduction of the missing transcription factor restored the normal microbial balance and normalized host survival (94). As suggested by these data, the distinction between pathogen and commensal may be quite fuzzy, depending on the balance between various bacterial species and the overall physiological state of the organism.
The common intestinal bacterium Pseudomonas aeruginosa is induced to express PA-I lectin, a virulence-related attachment factor that causes increased paracellular permeability, under conditions of host stress that result in intestinal epithelial hypoxia (56). Increased intestinal permeability may benefit local bacteria by increasing nutrients that are available but has also been strongly implicated in susceptibility to IBD. Pseudomonas aeruginosa also has the ability to transform apical membrane into basolateral membrane, a change that allows it to bind and be internalized into polarized intestinal epithelial cells (52).
Collet et al. (21) reported increase in chemokine secretion and a distinctive pattern of mucosal gene expression in the mdr1a−/− mice that are deficient in the epithelial transport protein P-glycoprotein. Additionally, it was demonstrated that the corresponding MDR-1 molecule in humans acts as an upstream component in a cascade of events that regulates traffic of leukocytes, particularly DC, out of tissues via lymphatic conduits (87). The mdr1a−/− mice spontaneously develop IBD when colonized with normal enteric microbiota. In this IBD model, P-glycoprotein has not been linked to a primary defect in the mucosal immune system, which makes it a very valuable tool for studying permeability factors. That study demonstrated a distinctive pattern of mucosal gene expression in mdr1a−/− mice before the development of active colitis and in the absence of increased colonic permeability. That pattern includes upregulation of the inflammatory genes, e.g., IFN-γ, IL-6, and downregulation of the anti-inflammatory PAP/RegIII-γ genes along with increased sensitivity of epithelial cells to the bacterial LPS (21). The vast majority of the genes involved in the mentioned pattern are associated with increased recognition of enteric bacteria. That suggests a primary role for P-glycoprotein in controlling the host relationship with the enteric microbiota.
Studies by Lewis et al. (62) suggested that the metabolic stress could interfere with the normal interaction between enterocytes and the commensal flora, resulting in an increase in epithelial permeability. Those authors demonstrated that uncoupling oxidative phosphorylation (i.e., inducing metabolic stress) in epithelial monolayers by treatment with dinitrophenol in the presence of E. coli resulted in increased paracellular and transcellular permeability. These data support previous studies, where abnormal mitochondria have been observed in epithelial cells in tissues collected from patients with CD, indicating metabolic stress in these individuals (75).
Another potential factor influencing barrier function is altered balance in the expression of α- and β-defensins (80), small proteins produced by the immune cells that are active against bacteria and fungi. Such imbalance could allow intestinal microbes to invade the mucosa or permit colonization of intestinal crypts (107, 114). An abnormality in α-defensin expression could contribute to the onset of inflammation especially since NOD2 mutation that predisposes to the development of CD in humans (76) is associated with α-defensin abnormalities (45).
Present treatments of IBD have focused on drugs able to decrease inflammation. However, limiting antigen access to the submucosa by reinforcing the epithelial barrier is an alternative approach that shows promise for treatment of IBD. Zeissig et al. (124) reported that molecular mechanisms for increased permeability include upregulation of pore-forming claudin-2, downregulation and redistribution of tight junction components claudin-5 and claudin-6, and finally increased epithelial apoptosis. Several treatments for improving protection of the mucosal barrier have been proposed. Laharie et al. (59) reported that rebamipide, a mucosal protective agent, reinforces mucosal barrier integrity in basal and inflammatory conditions and inhibits the increase in macromolecular transepithelial transport. Mechanisms of rebamipide action include scavenging of cytokine-induced hydroxyl radicals (74) and induction of prostaglandin production (59). In that study, mice deficient in IL-10 that were treated with rebamipide demonstrated reinforcement of the distal colonic epithelial barrier via increased transepithelial resistance, increased proliferation of cells in mesenteric lymph nodes, and increase in IFN-γ and IL-12 secretion. Those results indicate that this protective agent, beside reinforcing the colonic barrier, also has a slight Th1 immunostimulatory effect of mesenteric lymph nodes. Other treatments aiming at the keeping of the integrity of the epithelial barrier include probiotics (97), antibiotics (106), and antibodies (4).
Summary
The data summarized in this review demonstrate the importance of the epithelial barrier in maintaining health of the organism. Beside its major role in the absorption and secretion, the intestinal epithelium must provide an effective barrier to harmful macromolecules and microorganisms while allowing the mucosal immune system to sample luminal antigens. Its unique structure and complexity are vulnerable to various aberrant interactions with the intestinal microbiota, which eventually may lead to chronic inflammation and IBD.
GRANTS
This work was supported by National Institutes of Health Grant R01-CA115480 to L. Hale.
REFERENCES
- 1.Abraham SN, Duncan MJ, Li G, Zaas D. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J Investig Med 53: 318–321, 2005. [DOI] [PubMed] [Google Scholar]
- 2.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med 204: 1417–1429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aono S, Hirai Y. Phosphorylation of claudin-4 is required for tight junction formation in a human keratinocyte cell line. Expl Cell Res. In press. [DOI] [PubMed]
- 4.Ardizzone S, Bianchi PG. Biologic therapy for inflammatory bowel disease. Drugs 65: 2253–2286, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Asakura H, Suzuki K, Honma T. Recent advances in basic and clinical aspects of inflammatory bowel disease: which steps in the mucosal inflammation should we block for the treatment of inflammatory bowel disease? World J Gastroenterol 13: 2145–2149, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem 41: 1479–1485, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, Abraham SN. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389: 636–639, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. CEACAM6 acts as a receptor for adherent-invasive E coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest 117: 1566–1574, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology 123: 1527–1542, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Bomba A, Nemcová R, Gancarcíková S, Herich R, Guba P, Mudronová D. Improvement of the probiotic effect of micro-organisms by their combination with maltodextrins, fructo-oligosaccharides and polyunsaturated fatty acids. Br J Nutr, 88 Suppl 1: S95–S99, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Brown SJ, Abreu MT. Antibodies to tumor necrosis factor-alpha in the treatment of Crohn's disease. Curr Opin Drug Discov Devel 8: 160–168, 2005. [PubMed] [Google Scholar]
- 12.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19: 923–933, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, Viney JL, Maggio-Price L. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G764–G778, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313: 1126–1130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chichlowski M, Croom J, McBride BW, Daniel L, Davis G, Koci MD. Direct-fed microbial primalac and salinomycin modulate whole-body and intestinal oxygen consumption and intestinal mucosal cytokine production in the broiler chick. Poult Sci 86: 1100–1106, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Chichlowski M, Croom J, McBride BW, Havenstein GB, Koci MD. Metabolic and physiological impact of probiotics or direct-fed-microbials on poultry: a brief review of current knowledge. Int J Poult Sci 6: 694–704, 2007. [Google Scholar]
- 17.Chichlowski M, Croom WJ, Edens FW, McBride BW, Qiu R, Chiang CC, Daniel LR, Havenstein GB, Koci MD. Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult Sci 86: 1121–1132, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Chichlowski M, Sharp JM, Vanderford DA, Myles MH, Hale LP. Helicobacter typhlonius and H. rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL-10-deficient mice. Comp Med. In press. [PMC free article] [PubMed]
- 19.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 84: 282–291, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Collado MC, Isolauri E, Salminen S. Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol Lett 285: 58–64, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Collett A, Higgs NB, Gironella M, Zeef LA, Hayes A, Salmo E, Haboubi N, Iovanna JL, Carlson GL, Warhurst G. Early molecular and functional changes in colonic epithelium that precede increased gut permeability during colitis development in mdr1a (−/−) mice. Inflamm Bowel Dis 14: 620–631, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 55: 1760–1767, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahan S, Roth-Walter F, Arnaboldi P, Agarwal S, Mayer L. Epithelia: lymphocyte interactions in the gut. Immunol Rev 215: 243–253, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davey ME, O'Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64: 847–867, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bolós C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology 109: 723–734, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 174: 3237–3246, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Dieleman LA, Arends A, Tonkonogy SL, Goerres MS, Craft DW, Grenther W, Sellon RK, Balish E, Sartor RB. Helicobacter hepaticus does not induce or potentiate colitis in interleukin-10-deficient mice. Infect Immun 68: 5107–5113, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elson CO, Cong Y, Lorenz R, Weaver CT. New developments in experimental models of inflammatory bowel disease. Curr Opin Gastroenterol 20: 360–367, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Erickson AK, Willgohs JA, McFarland SY, Benfield DA, Francis DH. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these glycoproteins with the adhesive phenotype. Infect Immun 60: 983–988, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis 10: 286–299, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Feighery L, Cochrane S, Quinn T, Baird A, O'Toole D, Owens SE, O'Donoghue D, Mrsny R, Brayden D. Myosin light chain kinase inhibition: correction of increased intestinal epithelial permeability in vitro. Pharm Res 25: 1377–1386, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan K, Modrusan Z, Cornelius J, Chavali A, Kasman I, Komuves L, Mo L, Diehl L. Intestinal epithelial cell up-regulation of LY6 molecules during colitis results in enhanced chemokine secretion. J Immunol 180: 3874–3881, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Folwaczny C, Noehl N, Tschöp K, Endres SP, Heldwein W, Loeschke K, Fricke H. Goblet cell autoantibodies in patients with inflammatory bowel disease and their first-degree relatives. Gastroenterology 113: 101–106, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Forster C Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol 130: 55–70, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox JG, Gorelick PL, Kullberg MC, Ge Z, Dewhirst FE, Ward JM. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun 67: 1757–1762, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci 109: 429–435, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Furuse M, Fujita K, Hiiragi T, Fujimoto K, ST. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 281: G216–G228, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Gionchetti P, Rizzello F, Campieri M. Probiotics and antibiotics in inflammatory bowel disease. Curr Opin Gastroenterol 17: 331–335, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Hale LP, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis 11: 1060–1069, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Hale LP, Perera D, Gottfried MR, Maggio-Price L, Srinivasan S, Marchuk D. Neonatal co-infection with Helicobacter species markedly accelerates the development of inflammation-associated colonic neoplasia in IL-10 −/− mice. Helicobacter 12: 598–604, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 5: e54, 2008. [DOI] [PMC free article] [PubMed]
- 43.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 270: 1203–1207, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Hibi T, Ohara M, Kobayashi K, Brown WR, Toda K, Takaishi H, Hosoda Y, Hayashi A, Iwao Y, Watanabe M. Enzyme linked immunosorbent assay (ELISA) and immunoprecipitation studies on anti-goblet cell antibody using a mucin producing cell line in patients with inflammatory bowel disease. Gut 35: 224–230, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124: 993–1000, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Ho SB, Dvorak LA, Moor RE, Jacobson AC, Frey MR, Corredor J, Polk DB, Shekels LL. Cysteine-rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology 131: 1501–1517, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Adams MC. An in vitro model for investigating intestinal adhesion of potential dairy propionibacteria probiotic strains using cell line C2BBe1. Lett Appl Microbiol 36: 213–216, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Jiang WG, Bryce RP, Horrobin DF, Mansel RE. Regulation of tight junction permeability and occludin expression by polyunsaturated fatty acids. Biochem Biophys Res Commun 244: 414–420, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JR Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev 4: 80–128, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanauchi O, Mitsuyama K, Araki Y, Andoh A. Modification of intestinal flora in the treatment of inflammatory bowel disease. Curr Pharm Des 9: 333–346, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Kankaanpaa P, Yang B, Kallio H, Isolauri E, Salminen S. Effects of polyunsaturated fatty acids in growth medium on lipid composition and on physicochemical surface properties of lactobacilli. Appl Environ Microbiol 70: 129–136, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kierbel A, Gassama-Diagne A, Rocha C, Radoshevich L, Olson J, Mostov K, Engel J. Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J Cell Biol 177: 21–27, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res 1: 855–862, 2003. [PubMed] [Google Scholar]
- 54.Kobayashi M, Kweon MN, Kuwata H, Schreiber RD, Kiyono H, Takeda K, Akira S. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest 111: 1297–1308, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohler H, McCormick B, Walker W. Bacterial-enterocyte crosstalk: cellular mechanisms in health and disease. J Pediatr Gastroenterol Nutr 36: 175–185, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Kohler JE, Zaborina O, Wu L, Wang Y, Bethel C, Chen Y, Shapiro J, Turner JR, Alverdy JC. Components of intestinal epithelial hypoxia activate the virulence circuitry of Pseudomonas. Am J Physiol Gastrointest Liver Physiol 288: G1048–G1054, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Konkel ME, Hayes SF, Joens LA, Cieplak W. Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cell cultures. Microb Pathog 13: 357–370, 1992. [DOI] [PubMed] [Google Scholar]
- 59.Laharie D, Menard S, Asencio C, Vidal-Martinez T, Rullier A, Zerbib F, Candalh C, Megraud F, Heyman M, Matysiak-Budnik T. Effect of rebamipide on the colonic barrier in interleukin-10-deficient mice. Dig Dis Sci 52: 84–92, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Laharie D, Ménard S, Asencio C, Vidal-Martinez T, Rullier A, Zerbib F, Candalh C, Mégraud F, Heyman M, Matysiak-Budnik T. Effect of rebamipide on the colonic barrier in interleukin-10-deficient mice. Dig Dis Sci 52: 84–92, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Lee HS, Han SY, Bae EA, Huh CS, Ahn YT, Lee JH, Kim DH. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int Immunopharmacol 8: 574–580, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Lewis K, Caldwell J, Phan V, Prescott D, Nazli A, Wang A, Soderholm JD, Perdue MH, Sherman PM, McKay DM. Decreased epithelial barrier function evoked by exposure to metabolic stress and nonpathogenic E. coli is enhanced by TNF-α. Am J Physiol Gastrointest Liver Physiol 294: G669–G678, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Nowak-Wegrzyn A, Charlop-Powers Z, Shreffler W, Chehade M, Thomas S, Roda G, Dahan S, Sperber K, Berin MC. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology 131: 47–58, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Lönnroth C, Andersson M, Arvidsson A, Nordgren S, Brevinge H, Lagerstedt K, Lundholm K. Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumor tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immun 8: 5, 2008. [PMC free article] [PubMed] [Google Scholar]
- 65.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303: 1662–1665, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, Iritani BM, Zeng W, Nicks A, Tsang M, Shows D, Morrissey P, Viney JL. Dual infection with Helicobacter bilis and Helicobacter hepaticus in P-glycoprotein-deficient mdr1a−/− mice results in colitis that progresses to dysplasia. Am J Pathol 166: 1793–1806, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol 23: 379–383, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol 17: 204–210, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Mizoguchi A, Mizoguchi E. Inflammatory bowel disease, past, present and future: lessons from animal models. J Gastroenterol 43: 1–17, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Mizoguchi E, Xavier RJ, Reinecker HC, Uchino H, Bhan AK, Podolsky DK, Mizoguchi A. Colonic epithelial functional phenotype varies with type and phase of experimental colitis. Gastroenterology 125: 148–161, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Myles MH, Dieckgraefe BK, Criley JM, Franklin CL. Characterization of cecal gene expression in a differentially susceptible mouse model of bacterial-induced inflammatory bowel disease. Inflamm Bowel Dis 13: 822–836, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Naito Y, Yoshikawa T, Tanigawa T, Sakurai K, Yamasaki K, Uchida M, Kondo M. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med 18: 117–123, 1995. [DOI] [PubMed] [Google Scholar]
- 75.Nazli A, Yang PC, Jury J, Howe K, Watson JL, Soderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol 164: 947–957, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohkusa T, Nomura T, Sato N. The role of bacterial infection in the pathogenesis of inflammatory bowel disease. Intern Med 43: 534–539, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Ohmori H, Dohrman AF, Gallup M, Tsuda T, Kai H, Gum JR Jr, Kim YS, Basbaum CB. Molecular cloning of the amino-terminal region of a rat MUC 2 mucin gene homologue. Evidence for expression in both intestine and airway. J Biol Chem 269: 17833–17840, 1994. [PubMed] [Google Scholar]
- 78.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol 286: G613–G626, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4: 724–738, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Pingel LC, Kohlgraf KG, Hansen CJ, Eastman CG, Dietrich DE, Burnell KK, Srikantha RN, Xiao X, Belanger M, Progulske-Fox A, Cavanaugh JE, Guthmiller JM, Johnson GK, Joly S, Kurago ZB, Dawson DV, Brogden KA. Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol Cell Biol. In press. [DOI] [PubMed]
- 81.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 140: 12–19, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Poritz LS, Garver KI, Tilberg AF, Koltun WA. Tumor necrosis factor alpha disrupts tight junction assembly. J Surg Res 116: 14–18, 2004. [DOI] [PubMed] [Google Scholar]
- 83.Porras M, Martin MT, Yang PC, Jury J, Perdue MH, Vergara P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm Bowel Dis 12: 843–852, 2006. [DOI] [PubMed] [Google Scholar]
- 84.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126: 520–528, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity 25: 319–329, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Randolph GJ, Beaulieu S, Pope M, Sugawara I, Hoffman L, Steinman RM, Muller WA. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci USA 95: 6924–6929, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rath HC, Schultz M, Freitag R, Dieleman LA, Li F, Linde HJ, Scholmerich J, Sartor RB. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun 69: 2277–2285, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rescigno M The pathogenic role of intestinal flora in IBD and colon cancer. Curr Drug Targets: 395–403, 2008. [DOI] [PubMed]
- 90.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2: 361–367, 2001. [DOI] [PubMed] [Google Scholar]
- 91.Rhodes JM Colonic mucus and mucosal glycoproteins: the key to colitis and cancer? Gut 30: 1660–1666, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol 11: 94–100, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Rojas M, Conway PL. Colonization by lactobacilli of piglet small intestinal mucus. J Appl Bacteriol 81: 474–480, 1996. [DOI] [PubMed] [Google Scholar]
- 94.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319: 777–782, 2008. [DOI] [PubMed] [Google Scholar]
- 95.Sartor RB Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594, 2008. [DOI] [PubMed] [Google Scholar]
- 96.Sartor RB, Blumberg RS, Braun J, Elson CO, Mayer LF. CCFA microbial-host interactions workshop: highlights and key observations. Inflamm Bowel Dis 13: 600–619, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep 9: 497–507, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Scavizzi F, Raspa M. Helicobacter typhlonius was detected in the sex organs of three mouse strains but did not transmit vertically. Lab Anim 40: 70–79, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Segain JP, de la Bletiere DR, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappa B inhibition: implications for Crohn's disease. Gut 47: 397–403, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharkey KA, Mawe GM. Neuroimmune and epithelial interactions in intestinal inflammation. Curr Opin Pharmacol 2: 669–677, 2002. [DOI] [PubMed] [Google Scholar]
- 101.Sharp JM, Vanderford DA, Chichlowski M, M.H. M, Hale LP. Infection decreases reproductive success of IL-10-deficient mice. Comp Med 58: 447–453, 2008. [PMC free article] [PubMed] [Google Scholar]
- 102.Sokol H, Lay C, Seksik P, Tannock GW. Analysis of bacterial bowel communities of IBD patients: what has it revealed? Inflamm Bowel Dis 14: 858–867, 2008. [DOI] [PubMed] [Google Scholar]
- 103.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest 117: 514–521, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 294: G139–G147, 2008. [DOI] [PubMed] [Google Scholar]
- 105.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H. Mucosal flora in inflammatory bowel disease. Gastroenterology 122: 44–54, 2002. [DOI] [PubMed] [Google Scholar]
- 106.Swidsinski A, Loening-Baucke V, Bengmark S, Scholze J, Doerffel Y. Bacterial biofilm suppression with antibiotics for ulcerative and indeterminate colitis: consequences of aggressive treatment. Arch Med Res 39: 198–204, 2008. [DOI] [PubMed] [Google Scholar]
- 107.Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol 11: 1131–1140, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56: 343–350, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swidsinski A, Loening-Baucke V, Vaneechoutte M, Doerffel Y. Active Crohn's disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis 14: 147–161, 2008. [DOI] [PubMed] [Google Scholar]
- 110.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43: 3380–3389, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sydora BC, MacFarlane SM, Walker JW, Dmytrash AL, Churchill TA, Doyle J, Fedorak RN. Epithelial barrier disruption allows nondisease-causing bacteria to initiate and sustain IBD in the IL-10 gene-deficient mouse. Inflamm Bowel Dis 13: 947–954, 2007. [DOI] [PubMed] [Google Scholar]
- 112.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16: 5040–5052, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006. [DOI] [PubMed] [Google Scholar]
- 114.Vasquez N, Mangin I, Lepage P, Seksik P, Duong JP, Blum S, Schiffrin E, Suau A, Allez M, Vernier G, Tréton X, Doré J, Marteau P, Pochart P. Patchy distribution of mucosal lesions in ileal Crohn's disease is not linked to differences in the dominant mucosa-associated bacteria: a study using fluorescence in situ hybridization and temporal temperature gradient gel electrophoresis. Inflamm Bowel Dis 13: 684–692, 2007. [DOI] [PubMed] [Google Scholar]
- 115.Verstege MI, ten Kate FJ, Reinartz SM, van Drunen CM, Slors FJ, Bemelman WA, Vyth-Dreese FA, te Velde AA. Dendritic cell populations in colon and mesenteric lymph nodes of patients with Crohn's disease. J Histochem Cytochem 56: 233–241, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vetrano S, Rescigno M, Rosaria Cera M, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, Locati M, Malesci A, Dejana E, Danese S. Unique role of junctional adhesion molecule-A in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 135: 173–184, 2008. [DOI] [PubMed] [Google Scholar]
- 117.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166: 409–419, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Watson AJ, Chu S, Sieck L, Gerasimenko O, Bullen T, Campbell F, McKenna M, Rose T, Montrose MH. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology 129: 902–912, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Wehkamp J, Schmid M, Stange EF. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol 23: 370–378, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zahringer U, Mollenkopf HJ, Heinz E, Meyer TF. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med 12: 1030–1038, 2006. [DOI] [PubMed] [Google Scholar]
- 121.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434, 2007. [DOI] [PubMed] [Google Scholar]
- 122.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, Blumberg RS. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest 116: 2142–2151, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zaas DW, Duncan M, Rae Wright J, Abraham SN. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim Biophys Acta 1746: 305–313, 2005. [DOI] [PubMed] [Google Scholar]
- 124.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56: 61–72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang L, Danon SJ, Grehan M, Chan V, Lee A, Mitchell H. Natural colonization with helicobacter species and the development of inflammatory bowel disease in interleukin-10-deficient mice. Helicobacter 10: 223–230, 2005. [DOI] [PubMed] [Google Scholar]