Abstract
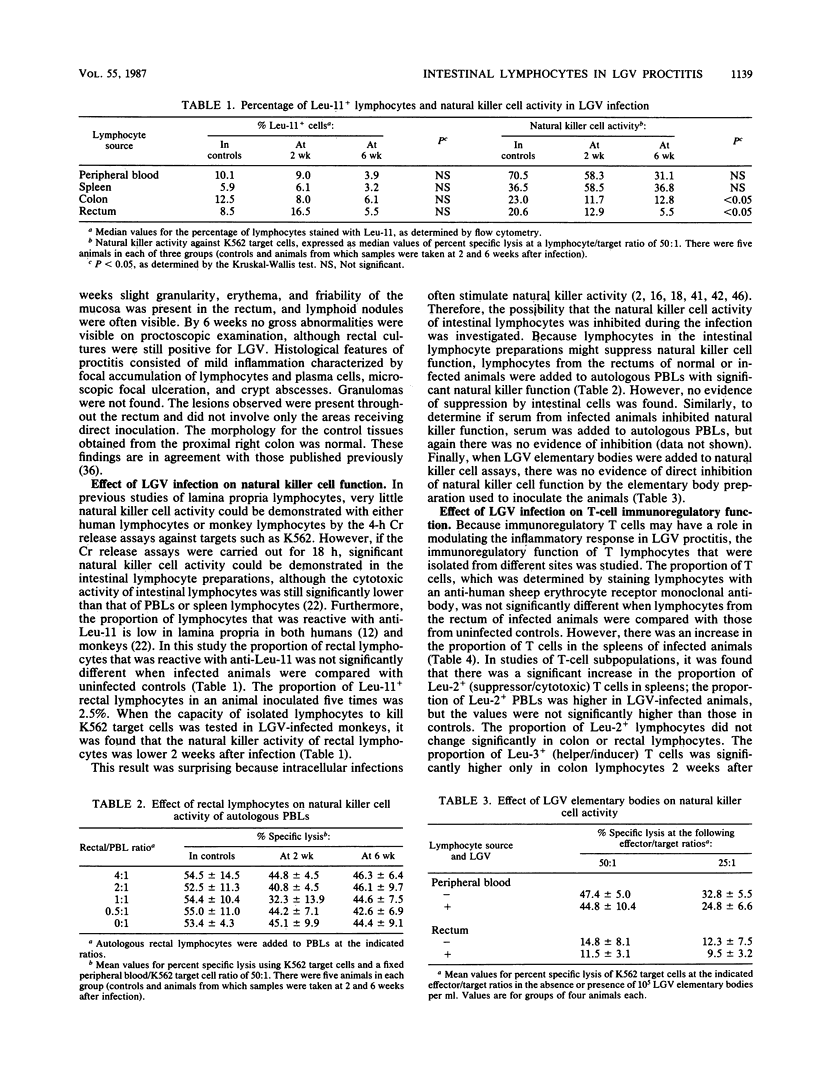
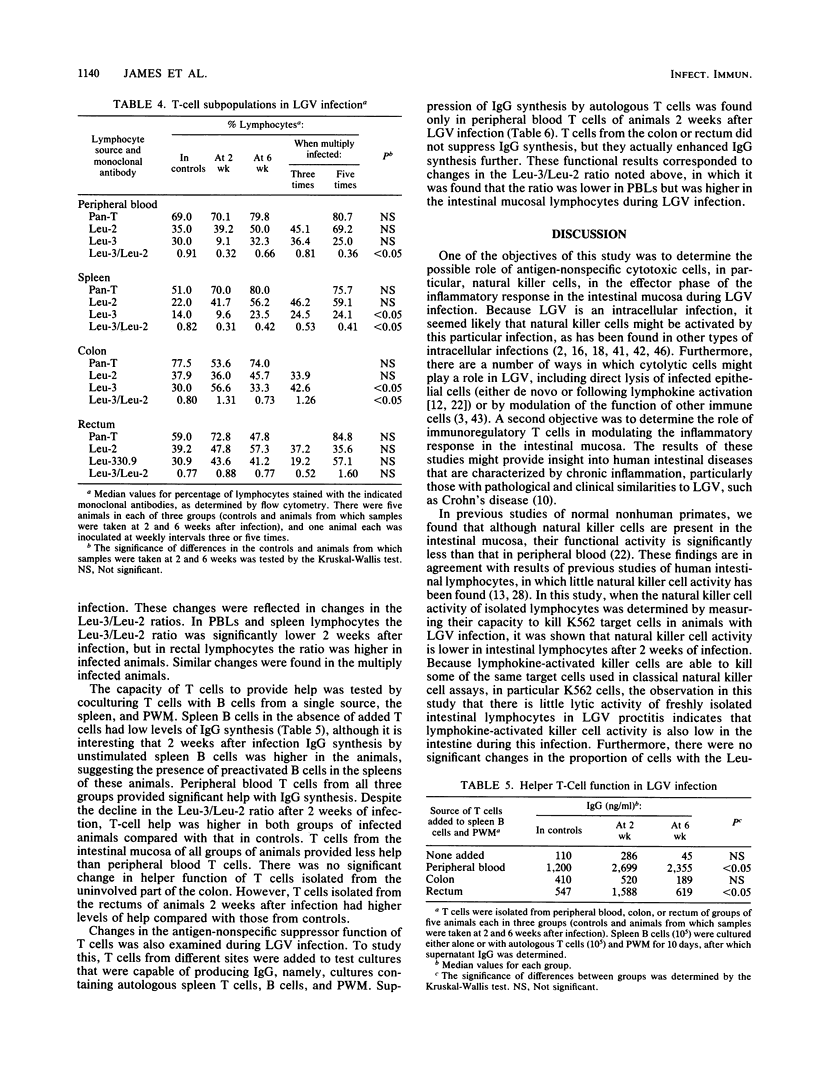
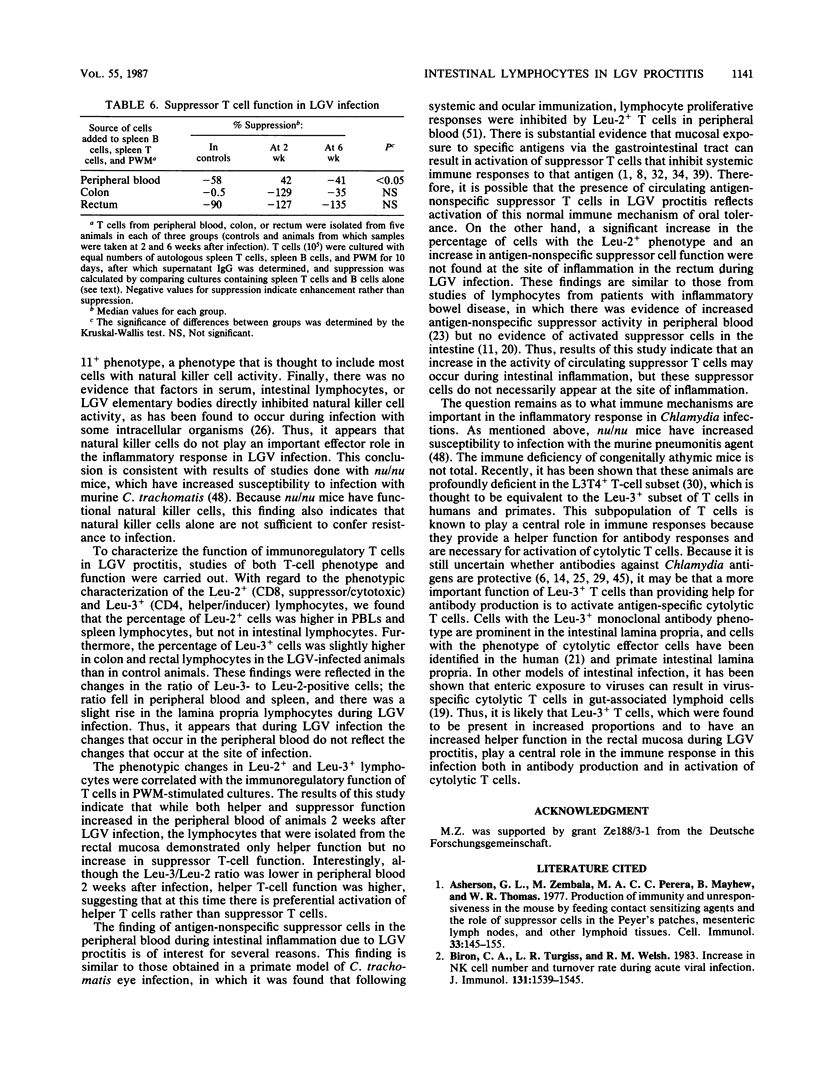
To study the role of natural killer cells and immunoregulatory T cells in the pathogenesis of proctitis due to Chlamydia trachomatis (L2 serovar), lymphocytes were obtained from the rectal mucosa and other sites of nonhuman primates and studied by using phenotypic and functional assays. In animals with lymphogranuloma venereum (LGV) proctitis, the percentage of lymphocytes with the natural killer cell phenotype (Leu-11+) was not significantly higher at any site in LGV infection, and natural killer cell function of lymphocytes isolated from the rectum was lower during LGV infection. This was not due to the suppressive effect of factors in serum, rectal lymphocytes, or LGV elementary bodies. In studies of regulatory T cells, the Leu-3+/Leu-2+ ratio was lower in the peripheral blood and the spleen during LGV infection, but the ratio did not decrease in lamina propria T cells. Both peripheral blood and rectal lymphocytes had higher helper T-cell function for polyclonal immunoglobulin G (IgG) synthesis in pokeweed mitogen-stimulated cultures 2 weeks following LGV infection. Increased suppressor T-cell function for pokeweed mitogen-stimulated IgG synthesis was found only in the peripheral blood of animals 2 weeks after infection, but not in isolated rectal lymphocytes. These results indicate that in LGV proctitis natural killer cells are not an important component of the inflammatory infiltrate at the site of infection, and helper T-cell function increases in peripheral blood and rectal lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Zembala M., Perera M. A., Mayhew B., Thomas W. R. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the peyer's patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1977 Sep;33(1):145–155. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- Biron C. A., Turgiss L. R., Welsh R. M. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983 Sep;131(3):1539–1545. [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J Immunol. 1984 Feb;132(2):611–615. [PubMed] [Google Scholar]
- Brunham R. C., Kuo C., Chen W. J. Systemic Chlamydia trachomatis infection in mice: a comparison of lymphogranuloma venereum and trachoma biovars. Infect Immun. 1985 Apr;48(1):78–82. doi: 10.1128/iai.48.1.78-82.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Martin D. H., Kuo C. C., Wang S. P., Stevens C. E., Hubbard T., Holmes K. K. Cellular immune response during uncomplicated genital infection with Chlamydia trachomatis in humans. Infect Immun. 1981 Oct;34(1):98–104. doi: 10.1128/iai.34.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux L. V., Schlossman S. F., Letvin N. L. Delineation of lymphocyte subsets in lymph nodes of nonhuman primates. Clin Immunol Immunopathol. 1984 Apr;31(1):96–101. doi: 10.1016/0090-1229(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Challacombe S. J., Tomasi T. B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980 Dec 1;152(6):1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Martin P. J., Hansen J. A., Ledbetter J. A. Evolution of epitopes on human and nonhuman primate lymphocyte cell surface antigens. Immunogenetics. 1983;18(6):599–615. doi: 10.1007/BF00345968. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Machelski E., Weiserbs D. B. T cell-B cell regulation in the intestinal lamina propria in Crohn's disease. Gastroenterology. 1985 Aug;89(2):321–327. doi: 10.1016/0016-5085(85)90332-4. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Tubbs R. R., Youngman K. R. Human intestinal mucosal mononuclear cells exhibit lymphokine-activated killer cell activity. Gastroenterology. 1985 Mar;88(3):625–637. doi: 10.1016/0016-5085(85)90130-1. [DOI] [PubMed] [Google Scholar]
- GRAYSTON J. T., WOOLRIDGE R. L., WANG S. Trachoma vaccine studies on Taiwan. Ann N Y Acad Sci. 1962 Mar 5;98:352–367. doi: 10.1111/j.1749-6632.1962.tb30558.x. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Jewell D. P. Local immune mechanisms in inflammatory bowel disease and colorectal carcinoma. Natural killer cells and their activity. Gastroenterology. 1986 Jan;90(1):12–19. doi: 10.1016/0016-5085(86)90068-5. [DOI] [PubMed] [Google Scholar]
- Hanna L., Schmidt L., Sharp M., Stites D. P., Jawetz E. Human cell-mediated immune responses to chlamydial antigens. Infect Immun. 1979 Feb;23(2):412–417. doi: 10.1128/iai.23.2.412-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Spontaneous lytic activity against allogeneic tumor cells and depression of specific cytotoxic responses in mice infected with Trypanosoma cruzi. J Immunol. 1981 Jun;126(6):2436–2442. [PubMed] [Google Scholar]
- Haynes B. F., Dowell D. L., Hensley L. L., Gore I., Metzgar R. S. Human T cell antigen expression by primate T cells. Science. 1982 Jan 15;215(4530):298–300. doi: 10.1126/science.6171885. [DOI] [PubMed] [Google Scholar]
- Holmberg L. A., Ault K. A. Characterization of natural killer cells induced in the peritoneal exudates of mice infected with Listeria monocytogenes: a study of their tumor target specificity and their expression of murine differentiation antigens and human NK-associated antigens. Cell Immunol. 1984 Nov;89(1):151–168. doi: 10.1016/0008-8749(84)90206-5. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B. The response of gut-associated T lymphocytes to intestinal viral immunization. J Immunol. 1984 Dec;133(6):2955–2960. [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Immunoregulatory function of lamina propria T cells in Crohn's disease. Gastroenterology. 1985 May;88(5 Pt 1):1143–1150. doi: 10.1016/s0016-5085(85)80073-1. [DOI] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology. 1986 Dec;91(6):1483–1489. [PubMed] [Google Scholar]
- James S. P., Graeff A. S. Spontaneous and lymphokine-induced cytotoxic activity of monkey intestinal mucosal lymphocytes. Cell Immunol. 1985 Jul;93(2):387–397. doi: 10.1016/0008-8749(85)90143-1. [DOI] [PubMed] [Google Scholar]
- James S. P., Neckers L. M., Graeff A. S., Cossman J., Balch C. M., Strober W. Suppression of immunoglobulin synthesis by lymphocyte subpopulations in patients with Crohn's disease. Gastroenterology. 1984 Jun;86(6):1510–1518. [PubMed] [Google Scholar]
- Johnson A. P., Osborn M. F., Rowntree S., Thomas B. J., Taylor-Robinson D. A study of inactivation of Chlamydia trachomatis by normal human serum. Br J Vener Dis. 1983 Dec;59(6):369–372. doi: 10.1136/sti.59.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P. Pathogenesis and immunology of chlamydial infections of the genital tract. Rev Infect Dis. 1985 Nov-Dec;7(6):741–745. doi: 10.1093/clinids/7.6.741. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Mechanisms of depression of splenic natural killer cell function in C57BL/6 mice infected with Leishmania donovani. Cell Immunol. 1984 Sep;87(2):601–612. doi: 10.1016/0008-8749(84)90028-5. [DOI] [PubMed] [Google Scholar]
- Kunimoto D., Brunham R. C. Human immune response and Chlamydia trachomatis infection. Rev Infect Dis. 1985 Sep-Oct;7(5):665–673. doi: 10.1093/clinids/7.5.665. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Bragdon M. J., Kodner I. J., Bertovich M. J. Deficient cell-mediated cytotoxicity and hyporesponsiveness to interferon and mitogenic lectin activation by inflammatory bowel disease peripheral blood and intestinal mononuclear cells. Gastroenterology. 1986 Jan;90(1):6–11. doi: 10.1016/0016-5085(86)90067-3. [DOI] [PubMed] [Google Scholar]
- MacDonald A. B., McComb D., Howard L. Immune response of owl monkeys to topical vaccination with irradiated Chlamydia trachomatis. J Infect Dis. 1984 Mar;149(3):439–442. doi: 10.1093/infdis/149.3.439. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Blanc C., Lees R. K., Sordat B. Abnormal distribution of T cell subsets in athymic mice. J Immunol. 1986 Jun 15;136(12):4337–4339. [PubMed] [Google Scholar]
- Martin L. N., Gormus B. J., Bozelka B. E. Functional analysis of monkey lymphocyte subsets defined by OKT4 and OKT8 Monoclonal Antibodies. Cell Immunol. 1983 Apr 15;77(2):338–347. doi: 10.1016/0008-8749(83)90034-5. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Hanson D. G. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979 Nov;123(5):2344–2350. [PubMed] [Google Scholar]
- Neubauer R. H., Marchalonis J. J., Strnad B. C., Rabin H. Surface markers of primate B and T lymphoid cell lines identified by antibodies to human and simian lymphocyte antigens. J Immunogenet. 1982 Aug;9(4):209–221. doi: 10.1111/j.1744-313x.1982.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Quinn T. C., Goodell S. E., Mkrtichian E., Schuffler M. D., Wang S. P., Stamm W. E., Holmes K. K. Chlamydia trachomatis proctitis. N Engl J Med. 1981 Jul 23;305(4):195–200. doi: 10.1056/NEJM198107233050404. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Taylor H. R., Schachter J. Experimental proctitis due to rectal infection with Chlamydia trachomatis in nonhuman primates. J Infect Dis. 1986 Nov;154(5):833–841. doi: 10.1093/infdis/154.5.833. [DOI] [PubMed] [Google Scholar]
- Qvigstad E., Digranes S., Thorsby E. Antigen-specific proliferative human T-lymphocyte clones with specificity for Chlamydia trachomatis. Scand J Immunol. 1983 Oct;18(4):291–297. doi: 10.1111/j.1365-3083.1983.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Effect of antithymocyte serum on the course of chlamydial genital infection in female guinea pigs. Infect Immun. 1983 Aug;41(2):876–879. doi: 10.1128/iai.41.2.876-879.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman L. K., Chiller J. M., Brown W. R., Hanson D. G., Vaz N. M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J Immunol. 1978 Dec;121(6):2429–2434. [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Verhoef J., Remington J. S. Enhancement of human natural killer cell activity by subcellular components of Toxoplasma gondii. Cell Immunol. 1984 Jul;86(2):317–326. doi: 10.1016/0008-8749(84)90386-1. [DOI] [PubMed] [Google Scholar]
- Stein-Streilein J., Bennett M., Mann D., Kumar V. Natural killer cells in mouse lung: surface phenotype, target preference, and response to local influenza virus infection. J Immunol. 1983 Dec;131(6):2699–2704. [PubMed] [Google Scholar]
- Storkus W. J., Dawson J. R. B cell sensitivity to natural killing: correlation with target cell stage of differentiation and state of activation. J Immunol. 1986 Mar 1;136(5):1542–1547. [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967 May;63(5 Suppl):1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr Mouse natural killer cells: induction specificity, and function. J Immunol. 1978 Nov;121(5):1631–1635. [PubMed] [Google Scholar]
- Whittum-Hudson J. A., Taylor H. R., Farazdaghi M., Prendergast R. A. Immunohistochemical study of the local inflammatory response to chlamydial ocular infection. Invest Ophthalmol Vis Sci. 1986 Jan;27(1):64–69. [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Coalson J. J., Grubbs B. Cellular immunity to the mouse pneumonitis agent. J Infect Dis. 1984 Apr;149(4):630–639. doi: 10.1093/infdis/149.4.630. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J. Role of cell-mediated immunity in chlamydial infection: implications for ocular immunity. Rev Infect Dis. 1985 Nov-Dec;7(6):754–759. doi: 10.1093/clinids/7.6.754. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Weiner M. H., Grubbs B. Antibody in host defense against mouse pneumonitis agent (murine Chlamydia trachomatis). Infect Immun. 1984 Sep;45(3):674–678. doi: 10.1128/iai.45.3.674-678.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Taylor H. R. Immune mechanisms in chlamydial eye infection: cellular immune responses in chronic and acute disease. J Infect Dis. 1984 Nov;150(5):745–751. doi: 10.1093/infdis/150.5.745. [DOI] [PubMed] [Google Scholar]
- de la Monte S. M., Hutchins G. M. Follicular proctocolitis and neuromatous hyperplasia with lymphogranuloma venereum. Hum Pathol. 1985 Oct;16(10):1025–1032. doi: 10.1016/s0046-8177(85)80280-x. [DOI] [PubMed] [Google Scholar]


