Abstract
Polycystic kidney diseases (PKD) are inherited as autosomal dominant (ADPKD) or autosomal recessive (ARPKD) traits and are characterized by progressive enlargement of renal cysts. Aberrant cell proliferation is a key feature in the progression of PKD. Cux1 is a homeobox gene that is related to Drosophila cut and is the murine homolog of human CDP (CCAAT Displacement Protein). Cux1 represses the cyclin kinase inhibitors p21 and p27, and transgenic mice ectopically expressing Cux1 develop renal hyperplasia. However, Cux1 transgenic mice do not develop PKD. Here, we show that a 246 amino acid deletion in Cux1 accelerates PKD progression in cpk mice. Cystic kidneys isolated from 10-day-old cpk/Cux1 double mutant mice were significantly larger than kidneys from 10-day-old cpk mice. Moreover, renal function was significantly reduced in the Cux1 mutant cpk mice, compared with cpk mice. The mutant Cux1 protein was ectopically expressed in cyst-lining cells, where expression corresponded to increased cell proliferation and apoptosis, and a decrease in expression of the cyclin kinase inhibitors p27 and p21. While the mutant Cux1 protein altered PKD progression, kidneys from mice carrying the mutant Cux1 protein alone were phenotypically normal, suggesting the Cux1 mutation modifies PKD progression in cpk mice. During cell cycle progression, Cux1 is proteolytically processed by a nuclear isoform of the cysteine protease cathepsin-L. Analysis of the deleted sequences reveals that a cathepsin-L processing site in Cux1 is deleted. Moreover, nuclear cathepsin-L is significantly reduced in both human ADPKD cells and in Pkd1 null kidneys, corresponding to increased levels of Cux1 protein in the cystic cells and kidneys. These results suggest a mechanism in which reduced Cux1 processing by cathepsin-L results in the accumulation of Cux1, downregulation of p21/p27, and increased cell proliferation in PKD.
Keywords: p27, cyst formation, apoptosis, cellular polarity, multiorgan hyperplasia, p21, cell cycle, cell proliferation
polycystic kidney disease (PKD) is a term applied to a group of inherited disorders characterized by the presence of renal cysts; however, multiple organs are usually affected. Human autosomal dominant PKD (ADPKD) results from mutations in one of two genes, PKD1 or PKD2, that encode polycystin-1 and polycystin-2 proteins, respectively (39, 58–60). Human autosomal recessive PKD (ARPKD) results from mutations within a single gene, polycystic kidney and hepatic disease 1 (PKHD1), encoding fibrocystin/polyductin (47, 66). Proteins that are mutated in human PKD (polycystin-1, polycystin-2, fibrocystin/polyductin), and in animal models of PKD (cystin, polaris), colocalize to the primary cilia (26, 46, 50, 69). This suggests that mutations in ciliary proteins affect common or overlapping signaling pathways resulting in PKD (43). The process of cyst formation in PKD is thought to involve various mechanisms including cell proliferation, fluid secretion, dedifferentiation, abnormal basement membrane formation, matrix remodeling, apoptosis, and alteration in cellular polarity (10, 19, 22, 27). Growing evidence suggests that PKD is a developmental disorder (4, 7, 23, 26, 33, 34, 48, 67).
The murine transcription factor Cux1 is structurally related to the Drosophila homeodomain protein cut and contains four putative DNA binding domains (3 cut repeats, 1 homeodomain) (1, 2, 44, 45, 51, 64, 70). Drosophila cut is required for the proper development of the Malpighian tubules, the insect excretory and osmoregulatory organs (5, 6). Cux1 represses the expression of the cyclin kinase inhibitor (CKI) p21 in S phase and is part of the network controlling G1-S transition (12). Cux1 also represses the CKI p27, and ectopic expression of Cux1 in transgenic mice results in multiorgan hyperplasia from the aberrant downregulation of p27 (30). In the kidney, Cux1 expression is spatially and temporally regulated, with highest expression in the nephrogenic zone, where it is associated with cell proliferation (30, 64). During normal kidney development, p27 is absent from the nephrogenic zone, but is expressed in maturing glomeruli and tubules following the downregulation of Cux1 (11). Thus, Cux1 is a cell cycle-dependent transcription factor that promotes cell proliferation during the early stages of nephrogenesis by repressing p27 gene expression.
In addition to the full-length isoform, called Cux1 (p200), there are several truncated isoforms. A nuclear isoform of the cysteine protease cathepsin-L has been identified that proteolytically processes Cux1 (p200) in S-phase (21). While the full-length Cux1 protein (p200) contains three cut repeats and the homeodomain, the proteolytically processed Cux1 (p110 or p90) contains only cut repeats 2 and 3, together with the homeodomain (21). Cux1 (p200) exhibits transient DNA binding activity and functions as a transcriptional repressor, whereas p110 stably binds DNA and can function as a transcriptional activator (40). Other Cux1 isoforms result from alternate splicing (CASP) (31), alternate promoter use (p75) (20), or proteolytic processing (p150) (37). Another Cux1 transcript, found exclusively in testis, encodes a 55-kDa protein; however, it is not known whether it originates by alternate splicing or an alternate promoter (65).
Cux1 (p200) is highly expressed in cystic kidneys isolated from both Pkd1 null and cpk mice, murine models of ADPKD and ARPKD, respectively (53). In cystic kidneys from Pkd1 null mice, Cux1 is highly expressed in both cyst-lining cells and in normal appearing tubule epithelium where expression of Cux1 is associated with increased cell proliferation. In cystic kidneys from cpk mice, Cux1 is not abnormally expressed until late stages of cystogenesis where expression of Cux1 is associated with increased apoptosis in cyst-lining cells. However, CMV/Cux1 transgenic mice do not develop cystic kidney disease. However, it was recently shown that transgenic mice ectopically expressing the p75 isoform of Cux1 develop renal abnormalities including renal tubule hyperplasia and cystic dilations with long latency (9).
In the present study, we generated Cys1cpk mice carrying a mutated allele of Cux1 to determine whether changes in Cux1 affect the progression of disease. The mutated Cux1 allele, called Cux1tm1Ejn, carries an in-frame deletion of Cux1, encompassing exons 15 and 16 (63). The Cux1tm1Ejn mice display wavy hair and curly vibrissae, and females are unable to support pups because of lactation defects. However, the kidneys of these mice are phenotypically normal. The Cux1tm1Ejn phenotype is similar to previously described mouse mutants in the EGFR pathway, including waved-1 (wa-1) (35, 38) and waved-2 (wa-2) (16, 36). The role of EGFR in PKD has been well-described (18, 29, 32, 49, 54). Moreover, blocking EGFR activity, either genetically using the wa-2 mutation, or pharmacologically, results in decreased cyst formation and improved kidney function (49, 55, 56). To determine whether the Cux1tm1Ejn would similarly alter cyst progression, we crossed the Cux1tm1Ejn mice with Cys1cpk mice to generate Cux1tm1Ejn/Cys1cpk mice.
MATERIALS AND METHODS
Animals.
Cys1cpk/+ and Cux1tm1Ejn/J/+ mice were purchased from Jackson Laboratory (Bar Harbor, ME), and stock colonies are maintained at the University of Kansas Medical Center. The Cux1tm1Ejn was generated by a targeted insertion of a construct to replace the first cut repeat introducing a nonsense mutation that would result in a truncated protein of ∼60 kDa. However, the resulting mice expressed a mutant form of the protein with an internal deletion of 246 amino acids encompassing the first cut repeat domain, while the CR2, CR3, HD, and C-tail of Cux1 remained intact. The mRNA produced from the mutant mice allele was missing two exons, the exon encoding the first cut repeat and the subsequent exon. Identification of +/+, Cys1cpk/+, and Cys1cpk genotypes was determined by PCR, as described (26). The presence of the Cux1tm1Ejn mutation was identified by Southern blot analysis, as described (63). Males homozygous for Cux1tm1Ejn and heterozygous for Cys1cpk were crossed with female mice heterozygous for both Cux1tm1Ejn and Cys1cpk to generate double homozygote Cux1tm1Ejn/Cys1cpk mice. All Cux1tm1Ejn, Cys1cpk, and Cux1tm1Ejn/Cys1cpk mice analyzed were on the same B6129SF1/J mixed genetic background. All protocols were approved by the University of Kansas Medical Center Animal Care and Use Committee. The University of Kansas Medical Center is fully accredited by the American Association of the Accreditation of Laboratory Animal Care.
Characterization of cystic phenotype.
Cystic kidneys were collected from 3- and 10-day-old cpk and Cux1tm1Ejn/Cys1cpk mice. The weight of both cystic kidneys (KW) was divided by body weight (BW) and calculated to determine KW as a percent BW. Kidney length measurements of bilateral kidneys were performed and divided by crown-rump measured in centimeters (cm). Cystic kidney midsagittal sections (5 μm) were utilized to stage cysts, as described previously (61). Cysts were considered early stage if there were less than 50 cyst-lining epithelial cells, intermediate if there were 51–200 cyst-lining epithelial cells, and advanced stage if greater than 200 cyst-lining cells.
Serum chemistry.
Blood was collected by intracardiac puncture and immediately centrifuged at 2,000 g for serum collection. Blood urea nitrogen (BUN) was determined using an autoanalyzer (Physicians Reference Laboratory, LLC, Overland Park, KS).
Immunohistochemistry.
Immunohistochemistry was performed as previously described (53). Kidney sections were immersion fixed in 4% paraformaldehyde and blocked in paraffin. Sections were washed in PBS containing 1% Tween 20 (PBST) and blocked in 10% normal goat serum (NGS) at room temperature for 1 h. Rabbit Cux1 (Santa Cruz Biotechnology), mouse PCNA (Sigma), or rabbit Cathepsin-L (Calbiochem) primary antibody was applied to sections incubated at room temperature for 1 h at a concentration of 1:100, 1:3,000, and 1:50, respectively. Biotinylated goat anti-rabbit (1:400) was used to detect Cux1 and Cathepsin-L antibody. A horse anti-mouse Texas red conjugated secondary antibody (Vector) was utilized to detect PCNA antibody (1:400). Sections were then washed in PBST and incubated with either FITC-conjugated avidin (Vector) or incubated with avidin-biotin-peroxidase complex (ABC-Elite: Vector) and DAB. All sections were washed with PBST and then mounted with either Vectashield medium with Dapi (Vector) or dehydrated with graded ethanols, mounted with Permount (fisher), and covered with glass coverslips. All images were captured with an Optronics Magnafire digital camera.
Western blot analysis.
Human ADPKD and normal human kidney (NHK) were harvested and nuclear extracts were prepared as previously described (8, 18). Nuclear extracts (45 μg) were loaded onto 4–15% SDS-PAGE gels and transferred to PVDF membranes where they were blocked in 5% milk PBST. Membranes were probed with Cux1 (1:50), Cathepsin-L (1:2,000), p27 (1:100), or p21 (1:100; Santa Cruz Biotechnology) primary antibody followed by PBST washes and horseradish peroxidase (1:10,000) secondary antibody application.
TUNEL assay.
Sections were processed for terminal deoxynucleotidal transferase (TdT)-mediated dUTP-nick-end labeling (TUNEL) with the ApopTag Red InSitu Apoptosis Detection kit (Intergen) according to the manufacturer's instructions. Sections were counterstained with DAPI, coverslipped, and visualized on a fluorescence microscope. Images were captured with an Optronics Magnafire digital camera.
Statistics.
In all studies, a one-way ANOVA was performed. If significance between the genotypes existed (P ≤ 0.05), post hoc analysis by least significant difference (LSD) was performed to determine statistical significance (P ≤ 0.05) between groups. All statistical analyses were performed using the Statview statistical program.
RESULTS
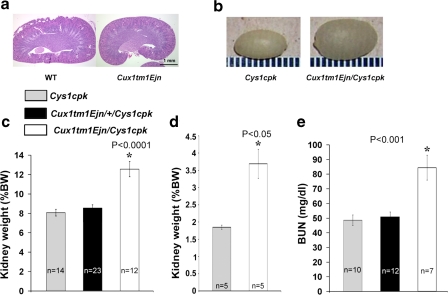
We crossed Cux1tm1Ejn mice, which carry a 246 amino acid deletion in Cux1 that includes the first cut repeat (Cux1ΔCR1) (63), with Cys1cpk mice to generate mice homozygous for both genes (Cux1tm1Ejn/Cys1cpk). Mice carrying the Cux1ΔCR1 mutation have curly whiskers, wavy hair, and lactation defects (63), but have normal kidneys (Fig. 1a). Surprisingly, when this mutation was combined with the Cys1cpk mutation, the double homozygous (Cux1tm1Ejn/Cys1cpk) mice developed cystic kidneys that were significantly larger than the cystic kidneys from mice carrying only the Cys1cpk mutation (Fig. 1). Bilateral kidneys were harvested at postnatal day 10 (P10) and total KW and BW measurements were collected to calculate KW standardized as a percentage of BW, standard measurements to quantify renal cystic disease severity (24, 41). By gross appearance, kidneys from Cux1tm1Ejn/Cys1cpk mice were significantly larger than kidneys from Cys1cpk mice (Fig. 1b). Cux1tm1Ejn/Cys1cpk kidneys were significantly larger compared with Cys1cpk in regard to KW/BW, both at postnatal day 3 and 10 (Fig. 1, c and d). While Cys1cpk mice heterozygote for the Cux1tm1Ejn mutation showed a slight increase in KW/BW, it was not significant. Large cystic kidneys are directly correlated with reduced renal function and high BUN levels (13, 62). Accordingly, Cux1tm1Ejn/Cys1cpk animals had higher BUN levels than Cys1cpk mice (Fig. 1e), indicating impaired renal function.
Fig. 1.
Increased kidney size and decreased kidney function in Cux1tm1Ejn/Cys1cpk mice. a: Cux1tm1Ejn mutation results in normal kidneys. Brightfield image of midsagittal sections of 10-day-old age-matched kidneys from wild-type (WT) or Cux1tm1Ejn mice. Hematoxylin and eosin staining shows no phenotypic differences in between kidneys from Cux1tm1Ejn and WT mice. Bar = 1 mm. b: Gross appearance of Cys1cpk and Cux1tm1Ejn/Cys1cpk cystic 10-day-old kidneys. c and d: Kidney weight (KW) standardized as a percentage of body weight (BW) represented as mean values ± SE. *Kidneys from Cux1tm1Ejn/Cys1cpk 10-day-old mice were significantly larger (P ≤ 0.0001) than kidneys from Cux1tm1Ejn/+/Cys1cpk or Cys1cpk 10-day-old mice in c and that kidneys from Cux1tm1Ejn/Cys1cpk 3-day-old mice were significantly larger (P ≤ 0.05) than kidneys from Cys1cpk 3-day-old mice in d. e: Blood urea nitrogen (BUN) measurements were collected and represented as mean values ± SE. *BUN in 10-day-old Cux1tm1Ejn/Cys1cpk mice was significantly greater (P ≤ 0.001) than BUN in 10-day-old Cux1tm1Ejn/+/Cys1cpk or Cys1cpk mice.
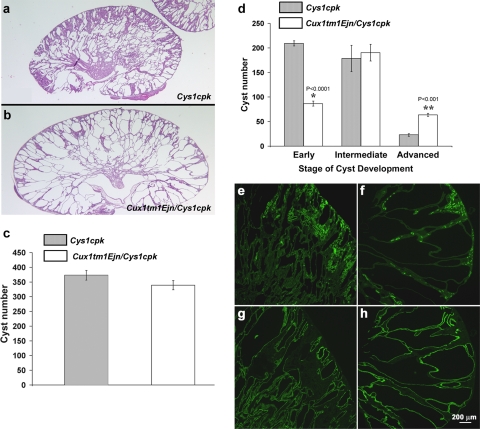
To further analyze the Cux1tm1Ejn/Cys1cpk phenotype, cystic kidneys isolated from Cux1tm1Ejn/Cys1cpk and Cys1cpk mice were examined morphologically (Fig. 2, a and b). Histological analysis showed that the overall number of renal cysts was not different between the Cys1cpk and Cux1tm1Ejn/Cys1cpk mice (Fig. 2c). Rather the size of the cysts appeared to be increased in the kidneys from Cux1tm1Ejn/Cys1cpk mice. Cyst numbers were quantified in regard to the developmental stage of each individual cyst. The developmental stage of the renal cysts in Cux1tm1Ejn/Cys1cpk and Cys1cpk mice was determined by counting the number of cells lining the cysts (see materials and methods). The results showed that kidneys from Cux1tm1Ejn/Cys1cpk mice had an increased number of advanced stage cysts compared with age-matched kidneys from Cys1cpk mice (Fig. 2d). This was associated with a reduced number of early stage cysts in the kidneys from Cux1tm1Ejn/Cys1cpk mice compared with kidneys from age-matched Cys1cpk mice. Moreover, the pattern of cystogenesis characteristic of the Cys1cpk mutation was unchanged. Cys1cpk mice undergo two phases of cystic disease (3, 17). Initially, Cys1cpk mice develop proximal tubule cysts that resolve by postnatal day 7. This is followed by a second phase of cystogenesis, in which cysts develop primarily from collecting ducts, resulting in massive enlargement of the kidneys. In kidneys from both Cux1tm1Ejn/Cys1cpk and Cys1cpk mice, the cysts were primarily restricted to the collecting ducts, with few proximal tubule cysts, although the collecting duct cysts in the Cux1ΔCR1/cpk kidneys were larger than those in the cpk kidneys (Fig. 2, e–h). Our results suggest that the Cux1tm1Ejn gene accelerates cyst growth, but does not induce cyst formation.
Fig. 2.
Characterization of Cux1tm1Ejn/Cys1cpk cystic kidneys. a and b: Representative midsagittal sections of cystic kidneys isolated from 10-day-old Cys1cpk (a) and Cux1tm1Ejn/Cys1cpk (b) mice. c: Overall number of cysts is unchanged in midsagittal sections of kidneys from 10-day-old Cys1cpk and Cux1tm1Ejn/Cys1cpk mice. d: Numbers of cysts present in early, intermediate, and advanced stage of development. Cysts were considered early stage if there were less than 50 cyst-lining epithelial cells, intermediate if there were 51–200 cyst-lining epithelial cells, and advanced stage if greater than 200 cyst-lining cells. *There are significantly less early forming cysts in Cux1tm1Ejn/Cys1cpk. **Significantly more advanced stage well-developed cysts. Each bar represents the mean of 3 different animals of the indicated genotype ± SE, with the exception of the cyst number for Cys1cpk intermediate stage where 5 animals were used. e–h: Cux1tm1Ejn/Cys1cpk cysts are predominately derived from collecting duct origin. Midsagittal sections of kidneys from Cys1cpk (e and g) and Cux1tm1Ejn/Cys1cpk (f and h) mice were labeled for lotus tetragonolobus agglutinin (LTA) to identify proximal tubules (e and f) or dolichus bifluorus agglutinin (DBA) to identify collecting ducts (g and h). Bar = 200 μm.
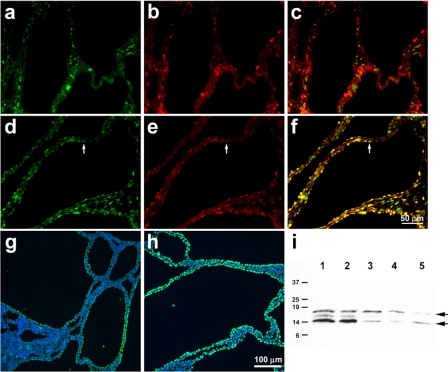
Cell proliferation is a key feature of cyst growth (4, 8, 22, 68). Moreover, there is a direct correlation between levels of cell proliferation and progression of cystic disease (8, 57, 62). Thus, one possibility is that the increased cyst size observed in the kidneys from Cux1tm1Ejn/Cys1cpk mice resulted from increased proliferation of the cyst-lining epithelial cells. In the developing kidney, Cux1 is expressed in the nephrogenic zone where it colocalizes with markers for cell proliferation, but is downregulated in maturing glomeruli and tubules (64). However, in Pkd1 null kidneys, Cux1 is ectopically expressed in the cyst-lining cells, and in normal appearing mature tubule epithelia, where it is associated with cell proliferation (53). Wild-type Cux1 protein was not expressed in the cyst-lining cells of kidneys isolated from 10-day-old Cys1cpk mice (Fig. 3, a–c), as described previously (53). In contrast, Cux1ΔCR1 protein colocalized with PCNA in the cyst-lining cells of kidneys isolated from 10-day-old Cux1tm1Ejn/Cys1cpk mice (Fig. 3, d–f). While there is limited apoptosis in the cysts of 10-day-old Cys1cpk mice, apoptosis is increased at later stages of cyst growth, where it is thought to contribute to cyst progression (8, 53). Moreover, inhibition of apoptosis slows cyst progression in mouse and rat models of PKD (8, 57). Consistent with an acceleration of cyst progression in the kidneys of Cux1tm1Ejn/Cys1cpk mice, we observed an increase in TUNEL labeling in the cyst-lining cells of Cux1tm1Ejn/Cys1cpk mice compared with Cys1cpk mice, and an increase in activated caspase 3 in kidneys from Cux1tm1Ejn/Cys1cpk mice compared with Cys1cpk mice (Fig. 3, g–i).
Fig. 3.
Increased proliferation and apoptosis in cystic kidneys from Cux1tm1Ejn/Cys1cpk mice. a–c: Detection of Cux1(p200) (a) and PCNA (b) in kidneys from 10-day-old Cys1cpk mice. At early stages of cyst development in Cys1cpk mice, Cux1 (p200) is expressed in the interstitial cells (a), but is absent from the cyst-lining cells, where PCNA labeling indicates cell proliferation (b). The merged image (c) shows no overlap of Cux1 (p200) with PCNA. d–f: Detection of Cux1ΔCR1 (d) and PCNA (e) in kidneys from 10-day-old Cux1tm1Ejn/Cys1cpk mice. The mutant Cux1ΔCR1 protein is expressed in both the interstitial cells and the cyst-lining cells (d). The cyst-lining cells are proliferative, as indicated by PCNA labeling (e), and the merged image shows colocalization of Cux1ΔCR1 and PCNA in cyst-lining cells (f). Arrows in d–f demonstrate coexpression of Cux1ΔCR1 protein with PCNA in cystic epithelial cells. g–i: Increased apoptosis in cystic kidneys from 10-day-old Cux1tm1Ejn/Cys1cpk mice. TUNEL labeling is increased in cyst-lining cells of Cux1tm1Ejn/Cys1cpk kidneys (h) compared with Cys1cpk kidneys (g). i: Levels of activated caspase-3 are increased in cystic kidneys from Cux1tm1Ejn/Cys1cpk mice (lanes 1–2) compared with Cys1cpk mice (lanes 3–5). Bar = 50 μm (a–f). Bar = 100 μm (g–h).
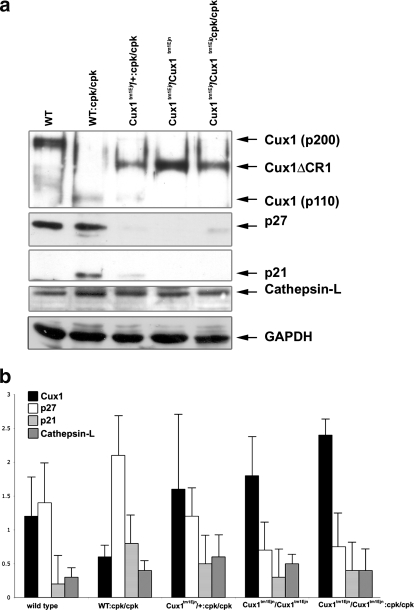
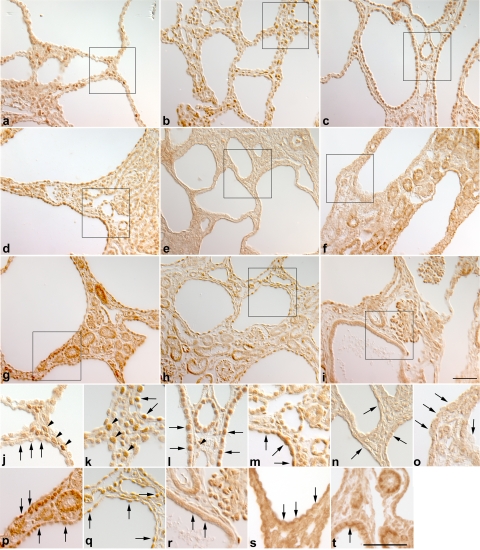
The expanded expression of the Cux1ΔCR1 protein in the cystic kidneys was surprising since this deletion would not be expected to affect the regulatory elements in the Cux1 gene. Moreover, the levels of Cux1tm1Ejn mRNA are not different from that of wild-type Cux1 (p200) mRNA (63). However, the levels of Cux1ΔCR1 protein appear to be elevated in some tissues compared with Cux1 (p200). To determine whether the Cux1ΔCR1 protein was elevated in the kidney, we performed Western blot analysis on kidneys isolated from postnatal day 10 wild-type, Cys1cpk, and Cux1tm1Ejn mice, and Cys1cpk mice carrying one or two mutant Cux1 alleles (Cux1tm1Ejn/+/Cys1cpk or Cux1tm1Ejn/Cys1cpk). Figure 4 shows the expression of Cux1 (p200) in wild-type mice, which is completely processed to the p110 form in Cys1cpk mice. This corresponded to an increase in nuclear cathepsin-L in Cys1cpk mice, compared with wild-type mice. In contrast, the Cux1ΔCR1 protein is not processed in Cys1cpk mice. Moreover, while p21 and p27 are both highly expressed in Cys1cpk mice, they are downregulated in Cys1cpk mice carrying one or two mutant Cux1 alleles (Fig. 4). In addition, Cux1 is not expressed in the cyst-lining cells of Cys1cpk mice (Fig. 5, a and j), which is associated with the ectopic expression of p21 and p27 in the cyst-lining cells (Fig. 5, d, g, m, p). In contrast, the Cux1ΔCR1 protein is ectopically expressed in the cyst-lining cells of Cux1tm1Ejn/Cys1cpk mice (Fig. 5, c and l), and this is associated with reduced expression of p21 and p27 in the cyst-lining cells (Fig. 5, f, i, o, r). Interestingly, Cys1cpk mice heterozygote for the Cux1tm1Ejn mutation (Cux1tm1Ejn/+/Cys1cpk) showed expression of the Cux1/Cux1ΔCR1 protein in cyst-lining cells (Fig. 5, b and k) that was associated with a reduction in p21 expression, but not p27 expression in the cyst-lining cells (Fig. 5, e, h, n, q).
Fig. 4.
Reduced expression of p21 and p27 in the presence of Cux1ΔCR1 protein. a: Western blotting of nuclear extracts (30 μg) isolated from kidneys from 10-day-old WT, Cys1cpk (WT:cpk/cpk), Cux1tm1Ejn/+/Cys1cpk (Cux1tm1Ejn/+:cpk/cpk), Cux1tm1Ejn (Cux1tm1Ejn/Cux1tm1Ejn), and Cux1tm1Ejn/Cys1cpk (Cux1tm1Ejn/Cux1tm1Ejn:cpk/cpk) mice. The Cux1 (p200) protein is highly expressed in the WT kidney but is completely processed to the p110 form in Cys1cpk kidneys. This is associated with an increase in both p21 and p27, and with an increase in nuclear cathepsin-L expression. The Cux1tm1Ejn mutation generates a truncated protein (Cux1ΔCR1) that is not processed to the p110 form and is associated with the downregulation of p21 and p27. b: Relative densitometry of Cux1 (black bars), p27 (white bars), p21 (light gray bars), and cathepsin-L (dark gray bars) in nuclear extracts from 10-day-old WT, Cys1cpk (WT:cpk/cpk), Cux1tm1Ejn/+/Cys1cpk (Cux1tm1Ejn/+:cpk/cpk), Cux1tm1Ejn (Cux1tm1Ejn/ Cux1tm1Ejn), and Cux1tm1Ejn/Cys1cpk (Cux1tm1Ejn/Cux1tm1Ejn:cpk/cpk) mice was obtained by NIH Image J64. Western and densitometry data represent 3 independent experiments.
Fig. 5.
Ectopic expression of Cux1ΔCR1 protein in cystic kidneys results in the downregulation of p21 and p27 in Cux1tm1Ejn/Cys1cpk kidneys. Localization of Cux1 (a, j), Cux1/Cux1ΔCR1 (b, k), Cux1ΔCR1 (c, l), p21 (d–f, m–o), p27 (g–i, p-r), and EGFR (s, t) in kidneys isolated from 10-day-old Cys1cpk (a, d, g, j, m, p, s), Cux1tm1Ejn/+/Cys1cpk (b, e, h, k, n, q), and Cux1tm1Ejn/Cys1cpk (c, f, i, l, o, r, t) mice. a and j: Cux1 protein is restricted to interstitial cells in cystic kidneys from Cys1cpk mice (arrowheads in j) and is not expressed in cyst-lining cells (arrows in j). b and k: In Cux1tm1Ejn/+/Cys1cpk mice, Cux1/Cux1ΔCR1 protein is expressed in interstitial cells (arrowheads in k), with few cysts exhibiting ectopic expression in cyst-lining cells (arrows in k). c and l: Cux1ΔCR1 protein is ectopically expressed in cyst-lining cells in cystic kidneys from Cux1tm1Ejn/Cys1cpk mice (arrows in l), as well as in interstitial cells (arrowheads in l). d–f, m–o: p21 protein is ectopically expressed in cyst-lining cells in Cys1cpk mice (d, and arrows in m), but is downregulated in cyst-lining cells in both Cux1tm1Ejn/+/Cys1cpk and Cux1tm1Ejn/Cys1cpk mice (e, f, and arrows in n and o). g–i, p–r: p27 protein is ectopically expressed in cyst-lining cells in both Cys1cpk and Cux1tm1Ejn/+/Cys1cpk mice (g, h, and arrows in p and q) but is downregulated in cyst-lining cells in Cux1tm1Ejn/Cys1cpk mice (i, and arrows in r). s and t: EGFR protein is apically expressed in some cyst-lining cells in Cys1cpk mice (arrow in s) and Cux1tm1Ejn/Cys1cpk mice (arrow in t). Bar = 50 μm (a-i). Bar = 50 μm (j–t).
The Cux1tm1Ejn phenotype is similar to previously described mouse mutants in the EGFR pathway, including wa-1 (35, 38) and wa-2 (16, 36). The role of EGFR in PKD has been well-described (18, 29, 32, 49, 54). Moreover, blocking EGFR activity, either genetically using the wa-2 mutation, or pharmacologically, results in decreased cyst formation and improved kidney function (49, 55, 56). To determine whether changes in EGFR signaling were disrupted in Cux1tm1Ejn/Cys1cpk mice, we examined the expression of EGFR in Cys1cpk and Cux1tm1Ejn/Cys1cpk mice. Figure 5 shows that no differences in expression were found between Cys1cpk and Cux1tm1Ejn/Cys1cpk mice (Fig. 5, s and t). This is consistent with previous reports suggesting that the Cux1tm1Ejn phenotype does not result from disruption of the EGFR pathway (63).
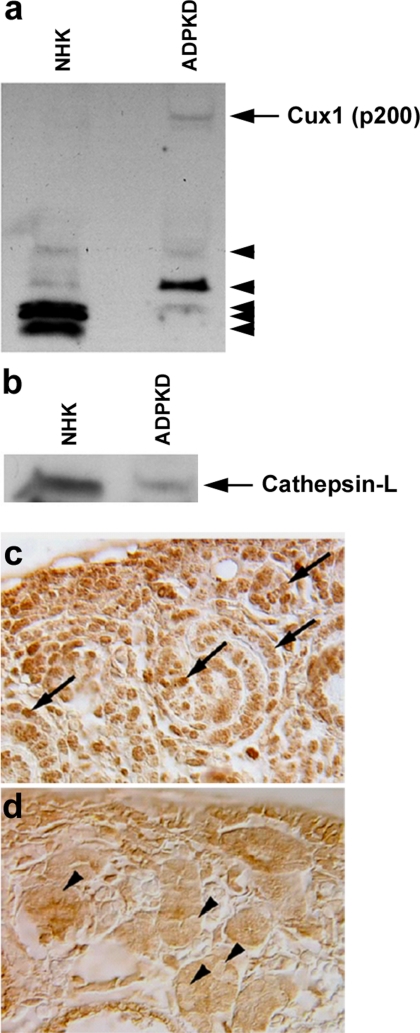
We previously reported differences in Cux1, p21, and p27 expression in mice with ARPKD (Cys1cpk) and ADPKD (Pkd1 null) (53). To determine whether these expression differences might result from differences in the proteolytic processing of Cux1, we evaluated Cux1 expression in epithelial cells isolated from cysts of human ADPKD kidneys or tubules of NHKs. We were unable to detect full-length Cux1 (p200) protein in nuclear extracts from NHK (Fig. 6a). However, there was an accumulation of full-length Cux1 (p200) protein in the nuclear extracts of cells isolated from the cysts of ADPKD kidneys (Fig. 6a). To determine whether this increase in full-length Cux1 (p200) protein was from decreased proteolytic processing, we compared nuclear cathepsin-L levels in NHK and ADPKD cells. Figure 6b shows that nuclear cathepsin-L was significantly reduced in ADPKD cells. When we examined cathepsin-L expression in kidneys isolated from embryonic wild-type and Pkd1 null mice, we found that nuclear cathepsin-L was similarly reduced in Pkd1 null mice (Fig. 6, c and d).
Fig. 6.
Increased Cux1 (p200) is associated with reduced nuclear cathepsin-L expression in human autosomal dominant polycystic kidney disease (ADPKD). a: Western blot for Cux1 in 45 μg nuclear extract isolated from epithelial cells from tubules of normal human kidneys (NHK) or from cysts of human ADPKD kidneys. In NHK cells, most of Cux1 is proteolytically processed (arrowheads). In contrast, in ADPKD cells, some of Cux1 protein remains in the full-length form (arrow). b: Western blot analysis for cathepsin-L in 45 μg nuclear extract from epithelial cells isolated from tubules of NHK or from cysts of human ADPKD kidneys. Cathepsin-L is present in the nuclei of NHK, but less in the nuclei of ADPKD kidneys. c: Cathepsin-L is localized to nuclei in cells of the nephrogenic zone in embryonic day 18.5 (E18.5) WT kidneys (arrows). d: In contrast, cathepsin-L is reduced in the nuclei of cystic kidneys from E18.5 Pkd1 null mice (arrowheads).
DISCUSSION
Cux1 is highly expressed in the nephrogenic zone during normal kidney development where it functions to repress p27 gene expression promoting cell proliferation (30, 64). Transgenic mice expressing the Cux1 (p200) cDNA using the CMV immediate early gene promoter develop multiorgan hyperplasia and exhibit continued cell proliferation in adult kidneys (30). However, these mice do not develop renal cysts, suggesting that cell proliferation alone is insufficient for cystogenesis.
Cux1tm1Ejn mice carry an in-frame deletion of Cux1, encompassing exons 15 and 16 (60). The Cux1tm1Ejn phenotype is similar to previously described mouse mutants in the EGFR pathway (16, 35, 36, 38). The role of EGFR in PKD has been well-described (18, 29, 32, 49, 54). Moreover, blocking EGFR activity, either genetically or pharmacologically, results in decreased cyst formation and improved kidney function (49, 55, 56). To determine whether the Cux1tm1Ejn would similarly alter cyst progression, we crossed the Cux1tm1Ejn mice with Cys1cpk mice to generate Cux1tm1Ejn/Cys1cpk mice. Our results show that an in-frame deletion of Cux1, encompassing exons 15 and 16, modifies the progression of PKD in Cys1cpk mice. However, rather than slowing the progression of cystogenesis, the presence of the Cux1tm1Ejn mutation accelerated cyst growth. Given the similarity between phenotypes of Cux1tm1Ejn mice and mouse mutants in the EGFR pathway, one possibility is that the EGFR pathway is disrupted in the Cux1tm1Ejn/Cys1cpk mice. However, the Cux1tm1Ejn phenotype does not appear to result from a disruption of the EGFR pathway, as no change in the expression of EGFR or TGF-α was found in Cux1tm1Ejn mice (63). Moreover, Cux1 expression was not changed in epidermoid carcinoma cells that overexpress the EGFR (A431 cells) treated with EGF, compared with untreated cells, suggesting that Cux1 is not regulated by EGFR. In addition, EGFR expression was not altered between Cys1cpk and Cux1tm1Ejn/Cys1cpk mice.
We propose an alternate model to explain the acceleration of cyst progression in Cys1cpk mice expressing the Cux1tm1Ejn mutation in which the Cux1ΔCR1 protein is ectopically expressed resulting in the repression of p21 and p27, increased cell proliferation, and a more rapid progression of cystic disease. Several observations are consistent with this model. First, expression analysis suggests that the mutant Cux1ΔCR1 protein was more broadly expressed than the Cux1 (p200) protein in the cystic kidneys isolated from 10-day-old Cux1tm1Ejn/Cys1cpk or Cys1cpk mice, respectively. Second, the ectopically expressed Cux1ΔCR1 protein is associated with increased cell proliferation in cystic kidneys from Cux1tm1Ejn/Cys1cpk mice. Third, while p21 and p27 are upregulated in kidneys isolated from Cys1cpk mice (47), both are downregulated in cystic kidneys from Cux1tm1Ejn/Cys1cpk mice.
Cux1 (p200) represses p21 and p27 promoter activity in a concentration-dependent manner (10, 25). The decreased expression of p21 and p27 in the cystic kidneys expressing the Cux1ΔCR1 protein suggests that this protein can function similar to the Cux1 (p200) protein. It is of interest that p27, but not p21, was ectopically expressed in the cyst-lining cells of Cux1tm1Ejn/+/Cys1cpk mice. Moreover, the kidneys from Cux1tm1Ejn/+/Cys1cpk mice were not significantly enlarged compared with kidneys from Cys1cpk mice. The reduced expression of p21 in the Cux1tm1Ejn/+/Cys1cpk mice, with no significant change in kidney size, suggests that loss of p21 does not contribute significantly to the Cux1tm1Ejn/Cys1cpk phenotype. This is consistent with previous studies showing that p21 null mice do not exhibit a cell proliferation defect (14), while p27 knockout mice develop hyperplasia (15, 28, 42).
The mechanism underlying the elevated levels of the mutant protein observed in cystic kidneys from Cux1tm1Ejn/Cys1cpk mice, compared with the levels of Cux1 (p200) in Cys1cpk mice, is not clear at present. Importantly, the Cux1tm1Ejn mutation does not alter the promoter and, thus, does not change the pattern of expression. This suggests that the ectopic expression of the Cux1tm1Ejn gene results from the Cys1cpk phenotype, while the Cux1ΔCR1 protein accumulation results from some other mechanism.
During S-phase of the cell cycle, a nuclear isoform of cathepsin-L proteolytically processes the Cux1 (p200) at amino acids 643, 747, and 755 to generate the p110 isoform (21). The deletion in the Cux1ΔCR1 protein includes the first cut repeat and the cathepsin-L site at position 643, while the cathepsin-L sites a positions 747 and 755 remain in the mutant protein. While Cux1 (p200) is completely processed to the p110 isoform in kidneys from Cys1cpk mice, the Cux1ΔCR1 protein is not processed in kidneys from Cux1tm1Ejn/Cys1cpk mice, suggesting the mutant Cux1ΔCR1 protein is more stable than the wild-type protein.
In contrast to Cys1cpk mice, nuclear cathepsin-L is reduced in both human ADPKD cells and in Pkd1 null kidneys. This suggests a mechanism in which reduced processing results in the accumulation of Cux1 (p200) leading to downregulation of p21 and p27 and increased cell proliferation in ADPKD. In contrast, the levels of nuclear cathepsin-L were not reduced in kidneys from Cys1cpk mice compared with kidneys from wild-type mice. Thus, in Cys1cpk mice, Cux1 (p200) would not escape proteolytic processing, and therefore would not accumulate. However, deletion of the first cathepsin-L cleavage site in Cux1ΔCR1 reduces proteolytic processing resulting in Cux1ΔCR1 protein accumulation.
It is of interest that reduced activity of cathepsin-L has previously been reported in the Han:SPRD rat model of PKD (52) and in human ADPKD cells (25). In both, the reduced activity was not a result of decreased gene expression, but of abnormal targeting of the enzyme. It is similarly possible that reduction of the nuclear isoform of cathepsin-L in human ADPKD cells and in kidneys from Pkd1 null mice is the result of abnormal targeting.
GRANTS
This work was supported by National Institutes of Health Grants RO1-DK-58377 (G. B. Vanden Heuvel) and P50-DK-57301 (R. L. Maser, G. B. Vanden Heuvel, D. P. Wallace), and by the Polycystic Kidney Disease Foundation (G. B. Vanden Heuvel and D. P. Wallace).
Acknowledgments
We thank R. Barkley, E. Roach, P. Simone, C. Carlton, and G. Reif for expert technical assistance. We thank Dr. C. Sorenson for critically reading the manuscript and Drs. J. Grantham, J. Calvet, L. Guay-Woodford, D. Quarles, and D. Abrahamson for many helpful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alcalay NI, Vanden Heuvel GB. Pathophysiological roles of Cux1: regulation of cell proliferation and differentiation in the kidney. Frontiers of Bioscience In press. [DOI] [PMC free article] [PubMed]
- 2.Andres V, Nadal-Ginard B, Mahdavi V. Clox, a mammalian homeobox gene related to Drosophila cut, encodes DNA-binding regulatory proteins differentially expressed during development. Development 116: 321–334, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Avner ED, Sweeney WE Jr, Young MC, Ellis D. Congenital murine polycystic kidney disease. II. Pathogenesis of tubular cyst formation. Pediatr Nephrol 2: 210–218, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109: 157–168, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Blochlinger K, Bodmer R, Jan LY, Jan YN. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev 4: 1322–1331, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer R, Barbel S, Sheperd S, Jack JW, Jan LY, Jan YN. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell 51: 293–307, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci USA 98: 12174–12179, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444: 949–952, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cadieux C, Harada R, Paquet M, Cote O, Trudel M, Nepveu A, Bouchard M. Polycystic kidneys caused by sustained expression of CUX1 isoform p75. J Biol Chem 283: 13817–13824, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin Nephrol 21: 107–123, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Combs HL, Shankland SJ, Setzer SV, Hudkins KL, Alpers CE. Expression of the cyclin kinase inhibitor, p27kip1, in developing and mature human kidney. Kidney Int 53: 892–896, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Coqueret O, Berube G, Nepveu A. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J 17: 4680–4694, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley BD, Grantham JJ, Muessel MJ, Kraybill AL, Gattone VH 2nd. Modification of disease progression in rats with inherited polycystic kidney disease. Am J Kidney Dis 27: 865–879, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82: 675–684, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85: 733–744, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Fowler KJ, Walker F, Alexander W, Hibbs ML, Nice EC, Bohmer RM, Mann GB, Thumwood C, Maglitto R, Danks JA, Chetty R, Burgess AW, Dunn AR. A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc Natl Acad Sci USA 92: 1465–1469, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattone VH, Calvet JP, Cowley BD Jr, Evan AP, Shaver TS, Helmstadter K, Grantham JJ. Autosomal recessive polycystic kidney disease in a murine model. A gross and microscopic description. Lab Invest 59: 231–238, 1988. [PubMed] [Google Scholar]
- 18.Gattone VH, Kuenstler K, Lindemann G, Lu X, Cowley B, Rankin C, Calvet J. Renal expression of a transforming growth factor-alpha transgene accelerates the progression of inherited, slowly progressive polycystic kidney disease in the mouse. J Lab Clin Med 127: 214–222, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Goulet B, Watson P, Poirier M, Leduy L, Berube G, Meterissian S, Jolicoeur P, Nepveu A. Characterization of a tissue-specific CDP/Cux isoform, p75, activated in breast tumor cells. Cancer Res 62: 6625–6633, 2002. [PubMed] [Google Scholar]
- 21.Goulet B, Baruch A, Moon NS, Poirier M, Sansregret LL, Erickson A, Bogyo M, Nepveu A. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol Cell 14: 207–219, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Grantham JJ 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol 3: 1841–1857, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Guay-Woodford LM Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol 285: F1034–F1049, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Guay-Woodford LM, Wright CJ, Walz G, Churchill GA. Quantitative trait loci modulate renal cystic disease severity in the mouse bpk model. J Am Soc Nephrol 11: 1253–1260, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Hartz PA, Wilson PD. Functional defects in lysosomal enzymes in autosomal dominant polycystic kidney disease (ADPKD): abnormalities in synthesis, molecular processing, polarity, and secretion. Biochem Mol Med 60: 8–26, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D'Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109: 533–540, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85: 721–732, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Lakshmanan J, Eysselein V. Hereditary error in epidermal growth factor prohormone metabolism in a rat model of autosomal dominant polycystic kidney disease. Biochem Biophys Res Commun 197: 1083–1093, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Ledford AW, Brantley JG, Kemeny G, Foreman TL, Quaggin SE, Igarashi P, Oberhaus SM, Rodova M, Calvet JP, Vanden Heuvel GB. Deregulated expression of the homeobox gene Cux-1 in transgenic mice results in downregulation of p27(kip1) expression during nephrogenesis, glomerular abnormalities, and multiorgan hyperplasia. Dev Biol 245: 157–171, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lievens PM, Tufarelli C, Donady JJ, Stagg A, Neufeld EJ. CASP, a novel, highly conserved alternative-splicing product of the CDP/cut/cux gene, lacks cut-repeat, and homeo DNA-binding domains, and interacts with full-length CDP in vitro. Gene 197: 73–81, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Lowden D, Lindemann G, Merlino G, Barash B, Calvet J, Gattone VH 2nd. Renal cysts in transgenic mice expressing transforming growth factor-α. J Lab Clin Med 124: 386–394, 1994. [PubMed] [Google Scholar]
- 33.Lu W, Shen X, Pavlova A, Lakkis M, Ward CJ, Pritchard L, Harris PC, Genest DR, Perez-Atayde AR, Zhou J. Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum Mol Genet 10: 2385–2396, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet 17: 179–181, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73: 263–278, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 8: 399–413, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Maitra U, Seo J, Lozano MM, Dudley JP. Differentiation-induced cleavage of Cutl1/CDP generates a novel dominant-negative isoform that regulates mammary gene expression. Mol Cell Biol 26: 7466–7478, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann GB, Fowler KJ, Gabriel A, Nice EC, Williams RL, Dunn AR. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell 73: 249–261, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Mochizuki T, Wu G, Hayashi T, Xenophontos S, Veldhuisen B, Saris J, Renolds D, Cai Y, Gabow P, Pierides A, Kimberling W, Breuning M, Deltas C, Peters D, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Moon NS, Premdas P, Truscott M, Leduy L, Berube G, Nepveu A. S phase-specific proteolytic cleavage is required to activate stable DNA binding by the CDP/Cut homeodomain protein. Mol Cell Biol 21: 6332–6345, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mrug M, Li R, Cui X, Schoeb TR, Churchill GA, Guay-Woodford LM. Kinesin family member 12 is a candidate polycystic kidney disease modifier in the cpk mouse. J Am Soc Nephrol 16: 905–916, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85: 707–720, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays 26: 844–856, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Nepveu A Role of the multifunctional CDP/Cut/Cux homeodomain transcription factor in regulating differentiation, cell growth and development. Gene 270: 1–15, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Neufeld EJ, Skalnik DG, Lievens PM, Orkin SH. Human CCAAT displacement protein is homologous to the Drosophila homeoprotein, cut. Nat Genet 1: 50–55, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Ong AC, Wheatley DN. Polycystic kidney disease–the ciliary connection. Lancet 361: 774–776, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Onuchic L, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schöneborn S, Mrug M, Sweeney W, Avner E, Zerres K, Guay-Woodford L, Somlo S, Germino G. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple IPT domains and PbH1 repeats. Am J Hum Genet 70: 1305–1317, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol 12: 938–943, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Richards WG, Sweeney WE, Yoder BK, Wilkinson JE, Woychik RP, Avner ED. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clin Invest 101: 935–939, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romio L, Fry AM, Winyard PJ, Malcolm S, Woolf AS, Feather SA. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J Am Soc Nephrol 15: 2556–2568, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Sansregret L, Nepveu A. The multiple roles of CUX1: insights from mouse models and cell-based assays. Gene 412: 84–94, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Schaefer L, Han X, Gretz N, Schaefer RM. Alterations of cathepsins B, H and L in proximal tubules from polycystic kidneys of the Han:SPRD rat. Kidney Int 50: 424–431, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Sharma M, Brantley JG, Alcalay NI, Zhou J, Heystek E, Maser RL, Vanden Heuvel GB. Differential expression of Cux-1 and p21 in polycystic kidneys from Pkd1 null and cpk mice. Kidney Int 67: 432–442, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Sweeney W, Avner E. Functional activity of epidermal growth factor receptors in autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol 275: F3887–F3894, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Sweeney W, Futey L, Frost P, Avner E. In vitro modulation of cyst formation by a novel tyrosine kinase inhibitor. Kidney Int 56: 406–413, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Sweeney W, Chen Y, Nakanishi K, Frost P, Avner E. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int 57: 33–40, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc Natl Acad Sci USA 102: 6954–6959, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The American PKD1 Consortium. Analysis of the genomic sequence for the autosomal dominant polycystic kidney disease (PKD1) gene predicts the presence of a leucine-rich repeat. Hum Mol Genet 4: 575–582, 1995. [DOI] [PubMed] [Google Scholar]
- 59.The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77: 881–894, 1994. [DOI] [PubMed] [Google Scholar]
- 60.The International Polycystic Kidney Disease Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell 8: 289–298, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Thomson RB, Mentone S, Kim R, Earle K, Delpire E, Somlo S, Aronson PS. Histopathological analysis of renal cystic epithelia in the Pkd2WS25/− mouse model of ADPKD. Am J Physiol Renal Physiol 285: F870–F880, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Tufarelli C, Fujiwara Y, Zappulla DC, Neufeld EJ. Hair defects and pup loss in mice with targeted deletion of the first cut repeat domain of the Cux/CDP homeoprotein gene. Dev Biol 200: 69–81, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Vanden Heuvel GB, Bodmer R, McConnell KR, Nagami GT, Igarashi P. Expression of a cut-related homeobox gene in developing and polycystic mouse kidney. Kidney Int 50: 453–461, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Vanden Heuvel GB, Quaggin SE, Igarashi P. A unique variant of a homeobox gene related to Drosophila cut is expressed in mouse testis. Biol Reprod 55: 731–739, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Ward C, Hogan M, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham J, Bacallao R, Ishibashi M, Milliner D, Torres V, Harris P. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Wu G, D'Agati V, Cai Y, Markowitz G, Park J, Reynolds D, Maeda Y, Le T, Hou H, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Yoder B, Hou X, Guay-Woodford L. The polycystsic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are colocalized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Yoon SO, Chikaraishi DM. Isolation of two E-box binding factors that interact with the rat tyrosine hydroxylase enhancer. J Biol Chem 269: 18453–18462, 1994. [PubMed] [Google Scholar]