Abstract
Adenosine-activated renovascular dilatation in Sprague-Dawley (SD) rats is mediated by stimulating adenosine2A receptors (A2AR), which is linked to epoxyeicosatrienoic acid (EET) synthesis. The A2AR-EET pathway is upregulated by high salt (HS) intake in normotensive SD rats. Because this pathway is antipressor, we examined the role of the A2AR-EET pathway in Dahl salt-sensitive (SS) rats. Male Dahl salt-resistant (SR) and SS rats were fed either HS (8.0% NaCl) or normal salt (NS; 0.4% NaCl) diet for 7 days. On day 8, isolated kidneys were perfused with Krebs-Henseleit buffer containing indomethacin and NG-nitro-l-arginine methyl ester and preconstricted with phenylephrine. Bolus injections of the stable adenosine analog 2-chloroadenosine (2-CA; 0.1–20 μg) elicited dose-dependent dilation in both Dahl SR and SS rats. Dahl SR rats fed a HS diet demonstrated a greater renal vasodilator response to 10 μg of 2-CA, as measured by the reduction in renal perfusion pressure, than that of Dahl SR rats fed a NS diet (−104 ± 6 vs. −77 ± 7 mmHg, respectively; P < 0.05). In contrast, Dahl SS rats did not exhibit a difference in the vasodilator response to 2-CA whether fed NS or HS diet (96 ± 6 vs. 104 ± 13 mmHg in NS- and HS-fed rats, respectively). In Dahl SR but not Dahl SS rats, HS intake significantly increased purine flux, augmented the protein expression of A2AR and the cytochrome P-450 2C23 and 2C11 epoxygenases, and elevated the renal efflux of EETs. Thus the Dahl SR rat is able to respond to HS intake by recruiting EET formation, whereas the Dahl SS rat appears to have exhausted its ability to increase EET synthesis above the levels observed on NS intake, and this inability of Dahl SS rats to upregulate the A2AR-EET pathway in response to salt loading may contribute to the development of salt-sensitive hypertension.
Keywords: epoxyeicosatrienoic acids, kidney, salt-sensitive hypertension
salt sensitivity, as defined by blood pressure elevation in response to a dietary salt load, is not only a causative factor for a subgroup of humans with essential hypertension but also has been reported to be an independent cardiovascular risk factor in patients with hypertension (32). Therefore, understanding the mechanisms that contribute to the development of salt sensitivity and identifying potential therapeutic targets for the management of salt-sensitive hypertension should provide novel approaches to treat elevated blood pressure. Cytochrome P-450 (CYP) epoxygenases play a critical role in moderating salt sensitivity by producing epoxyeicosatrienoic acids (EETs). Capdevila and colleagues (5, 28) established the importance of EETs in antagonizing the pressor effects of high salt (HS) intake. Blood pressure did not increase in rats receiving a HS diet unless EET synthesis was either inhibited or deficient. Thus increased production of natriuretic-vasodilator EETs is a decisive component of the kidney's adaptive response to preventing elevation of blood pressure when challenged with HS intake (8, 30, 41).
Adenosine, a product of ATP metabolism, has multiple effects on kidney function; it plays a critical role in the regulation of renal vascular tone and reactivity and, in addition, affects tubular transport. Adenosine modulates both afferent and efferent arteriolar tone (33), thus regulating renal blood flow (3, 37) and glomerular filtration rate (34). Furthermore, adenosine influences vascular tone and reactivity of the medullary vasa recta and, consequently, medullary blood flow (1, 38, 47). Adenosine also affects tubular transport at several segments of the nephron. Adenosine enhances NaCl reabsorption in the proximal tubule by stimulating adenosine A1 receptors (4); it also reduces NaCl reabsorption in the thick ascending limb of the loop of Henle (TALH) (2, 15) and in the collecting duct (42).
Adenosine actions are initiated by its binding to purinergic receptors (AR); four AR subtypes have been described: A1, A2A, A2B, and A3 (13). Adenosine-induced renovascular dilation in Sprague-Dawley (SD) rats is mediated via activation of A2AR, which we have linked to stimulation of EET synthesis (7, 23). In SD rats, HS intake augmented renovascular responses to a nonselective adenosine receptor agonist, 2-chloroadenosine (2-CA), associated with increased renal protein expression of the A2AR and CYP2C23, a salt-inducible epoxygenase that promotes increased renal efflux of EETs and the hydrolysis products of EETs, dihydroxyeicosatrienoic acids (DHTs). To extend our studies, we have determined the participation of the A2AR-EET pathway in the Dahl salt-sensitive (SS) model of hypertension. The pathophysiology of hypertension in Dahl SS rats has been under extensive investigation. Many mechanisms have been proposed to contribute to the development of hypertension in Dahl SS rats, including a deficiency of 20-hydroxyeicosatetraenoic acid (20-HETE) production in the TALH (18), an inability to increase renal epoxygenase activity in response to dietary salt intake (27, 28), and an inability to produce or release eicosanoid precursors from phospholipid stores in response to dietary salt (22). We studied Dahl salt-resistant (SR) and SS rats, a well-established model of salt-sensitive hypertension, with the expectation of demonstrating abnormalities in the A2AR-epoxygenase pathway in the Dahl SS rat. This proved to be the case, since when challenged with 2-CA, Dahl SS rats fed a HS diet were unable to increase renal EET release above EET levels observed in Dahl SS rats fed a normal salt (NS) diet. The Dahl SR rat on a HS intake, however, did increase renal EET levels when challenged with the adenosine receptor agonist.
MATERIALS AND METHODS
Male Dahl SS and SR rats weighing 200–260 g (6–7 wk old; Harlan Sprague Dawley) were used in accordance with National Institutes of Health guidelines. The New York Medical College Institutional Animal Care and Use Committee approved all experimental protocols. Rats were placed on either NS (0.4% NaCl) or HS (8.0% NaCl) diet for 7 days, and on day 8, rats were anesthetized with pentobarbital sodium (60 mg/kg ip). Systolic blood pressure (SBP) was monitored by tail-cuff plethysmography (XBS1100; Kent Scientific, Torrington, CT).
Isolated perfused kidney.
The right kidney was prepared for perfusion as described previously (23). After laparotomy, the right renal artery was cannulated with a 21-gauge needle; the kidney was then removed and perfused with warmed (37°C), gassed (95% O2-5% CO2) Krebs-Henseleit buffer at a flow rate of 7 ml/min using a Harvard Apparatus peristaltic pump. The composition of the Krebs-Henseleit buffer was (in mM) 118 NaCl, 4.7 KCl, 1.19 KH2PO4, 1.19 MgSO4, 1.9 CaCl2, 25 NaHCO3, and 5.5 glucose. NG-nitro-l-arginine methyl ester (l-NAME; 200 μM), a nitric oxide synthase (NOS) inhibitor, and indomethacin (Indo; 10 μM), a nonselective cyclooxygenase (COX) inhibitor, were included in the Krebs-Henseleit buffer to eliminate any potential interaction of either NO (35) or COX (10) on CYP-derived arachidonic acid (AA) metabolite levels. In preliminary experiments, we found that the renal vascular response to adenosine in both Dahl SR and SS rats was independent of NO release or COX metabolites and that the responses to neither 2-CA nor sodium nitroprusside (SNP), a NO donor, changed over the course of the experiment (140 min).
The basal renal perfusion pressures (RPP) of Dahl SR and SS rats were not different whether on NS (96 ± 8 vs. 95 ± 7 mmHg, respectively) or HS diet (71 ± 9 vs. 77 ± 3 mmHg, respectively). Once a stable RPP was obtained, pressure was further increased by ∼100 mmHg by infusing phenylephrine (10−7 M) to amplify detection of vasodilator responses. Renal effluent was collected during responses to bolus injections of the stable adenosine analog 2-CA (0.01–20 μg) and SNP (50 ng), in the absence and presence of a selective epoxygenase inhibitor, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH; 12 μM; synthesized by Dr. John R. Falck, University of Texas Southwestern Medical Center) (40) and a selective A2AR antagonist, 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol (ZM 241385; 1 μM; Tocris Cookson, Ellisville, MO) (36); aliquots were stored frozen for later analysis.
Release of CYP-AA metabolites.
Aliquots (10 ml) of effluent collected during responses were processed for the measurement of CYP-AA metabolites. One nanogram of deuterated (D2) 20-HETE, 1 ng each of D8 8,9-EET, 11,12-EET, and 14,15-EET (Biomol, Plymouth Meeting, PA), and 1 ng each of D8 8,9-DHT, 11,12-DHT, and 14,15-DHT (synthesized from D8 EETs by Dr. Houli Jiang, New York Medical College) were added as internal standards. The eicosanoids were extracted and separated by reverse-phase high-pressure liquid chromatography (RP-HPLC) and derivatized and analyzed by gas chromatography-mass spectrometry as previously described (23).
Western immunoblot analysis.
After removal of the right kidney, the left kidney of each rat was excised and hemisected, and the cortex and medulla were separated, snap frozen in liquid nitrogen, and stored at −80°C for later analysis. Tissues were placed in a tube with 0.1 ml of cell lysis buffer (Cell Signaling Technology, Danvers, MA) containing 10 μl/ml of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and 1 mg/ml phenylmethylsulfonyl fluoride and homogenized in a tight glass homogenizer with a Teflon pestle. The homogenate was centrifuged at 14,000 rpm at 4°C for 10 min, and the supernate was recovered. Protein concentrations were determined with a detergent-compatible protein assay (DC protein assay kit; Bio-Rad, Hercules, CA) according to the procedures described by the manufacturer, and samples were stored at −20°C. Samples (15 μg/well) were mixed with an appropriate volume of Laemmli sample buffer (Bio-Rad) and boiled for 5 min. Proteins were separated on a 10% SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane. Membranes were placed in blocking solution (LI-COR Bioscience, Lincoln, NE) at room temperature (RT) for 1 h, followed by incubation overnight at 4°C with the first antibody [anti-A1R antibody (Chemicon, Temecula, CA), anti-A2AR antibody, anti-A2BR antibody (Chemicon), anti-CYP2C23 antibody (gift from Dr. J. Capdevila, Vanderbilt University), anti-CYP2C11 antibody (Oxford Biomedical Research, Oxford, MI), or anti-β-actin antibody (Sigma-Aldrich)]. The membranes were then washed and incubated at RT for 1 h with horseradish peroxidase-conjugated donkey anti-rabbit IgG secondary antibody (Amersham, Arlington Heights, IL). Membranes were washed, and protein expression was evaluated using infrared fluorescence (Odyssey; LI-COR Bioscience). The intensity (densitometric units) ratio of adenosine receptors or CYP2C isozymes to β-actin on the same membrane was calculated and used for quantitative comparison.
Measurement of urinary purines.
Twenty-four-hour urine samples were collected for measurement of purine levels. Urinary adenosine levels were measured with fluorescence detection following the protocol of Jackson et al. (19). Briefly, 200 μl of urine were mixed with 20 μl of 9-d-arabinofuranosyladenine (40 μM; internal standard) and 1 ml of 0.5 M ammonium sulfate (pH 9.3). Samples were extracted on a C18 Sep-Pak cartridge (Varian, Walnut Creek, CA), and adenosine was then eluted with 2 ml of 10% methanol in 10 mM phosphoric acid. Forty microliters of 0.5 M acetate buffer (pH 4.8) and 40 μl of 50% chloroacetaldehyde in water were added to 1.5 ml of eluant, after which samples were heated at 80°C for 1 h. Forty microliters of each sample were injected into a RP-HPLC (Hewlett Packard, Series 1050), and the effluent was monitored with a Waters model 470 scanning fluorescent detector (excitation and emission wavelengths were set at 275 and 420 nm, respectively). The mobile phase consisted of 95.5% citrate-phosphate buffer (0.014 M citric acid and 0.017 M Na2HPO4) and 4.5% acetonitrile; the flow rate was 1.2 ml/min. The area under each peak for adenosine and internal standard was calculated, and the concentration of adenosine was quantitated by comparing the ratio of peak areas to a standard curve.
Urinary purine levels were measured with UV absorption detection according to the protocol of Mi and Jackson (31). Twenty microliters of each sample were injected into a RP-HPLC (Hewlett Packard, Series 1050), and the effluent was monitored by UV absorbance (254 nm). Inosine, hypoxanthine, and xanthine were quantified as the area under the chromatographic peak, and the concentration in the sample was calculated from corresponding standard curves for each substance.
Analysis of data.
All data are means ± SE. Statistical analysis was performed using one-way analysis of variance, followed by the Newman-Keuls test when multiple comparisons were made (i.e., dose-response curves to 2-CA in NS- vs. HS-fed rats, before and after inhibitor) or by t-tests. Paired analysis (paired t-test) was used when comparisons were made of data obtained from the same experimental preparation (i.e., total EET and DHT release in response to 2-CA before and after inhibitor, in the same kidney). Unpaired analysis (unpaired t-test) was used when comparisons were made of data obtained from different experimental preparations (i.e., kidneys of HS vs. NS groups). A P value <0.05 was considered statistically significant.
RESULTS
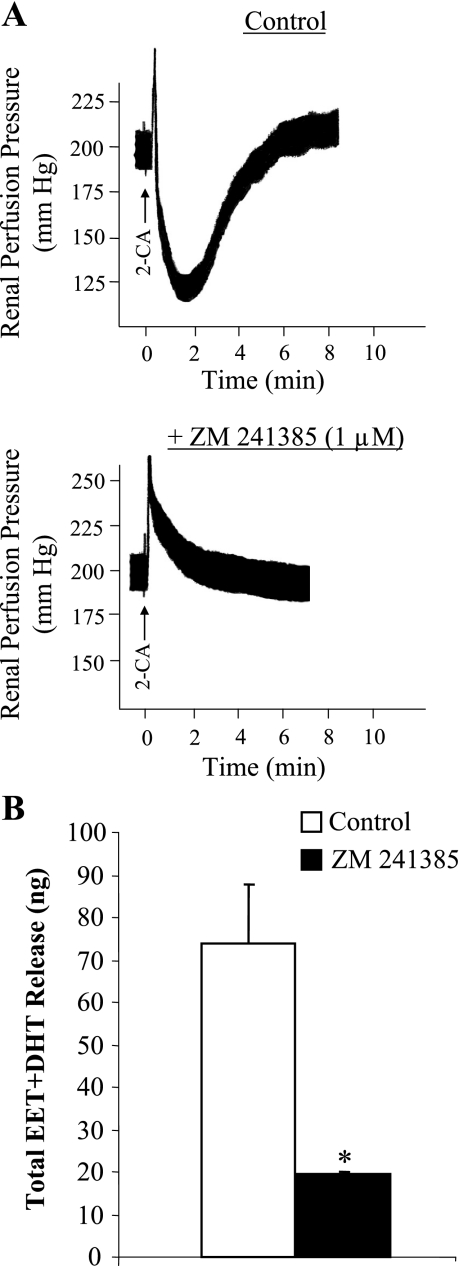
SBP increased from 123 ± 4 mmHg on NS diet to 156 ± 2 mmHg (P < 0.05) after 7 days of HS diet in Dahl SS rats, whereas HS challenge did not affect the SBP of Dahl SR rats (122 ± 4 mmHg on NS diet and 125 ± 6 mmHg after 7 days of HS diet). Four groups of Dahl rats (n = 5–9 in each group), two SS and two SR, were studied for changes in RPP in response to 2-CA in the isolated, Krebs-Henseleit-perfused kidney. Intra-arterial bolus injections of 2-CA (0.01–20 μg) into the isolated kidney of Dahl SR and SS rats elicited biphasic responses: a transient vasoconstriction followed by a prolonged dilation (Fig. 1A). Because 2-CA is a nonselective agonist of all four adenosine receptors, we determined whether 2-CA-induced vasodilation of isolated, perfused Dahl rat kidneys was mediated by stimulating A2ARs, thereby increasing EET synthesis, as we have reported for SD rats (23). To assess the involvement of A2AR in 2-CA-induced vasodilation of isolated, perfused kidneys obtained from Dahl SR and SS rats, we determined responsiveness to 2-CA in the absence and presence of ZM 241385 (1 μM), a selective A2AR antagonist (Fig. 1A). Administration of 10 μg of 2-CA elicited renal vasodilation in the NS-fed Dahl SR rats that was abolished by ZM 241385, resulting in a transient vasoconstriction. The abrogated renal vasodilator response was accompanied by reduced total EET and DHT release (74 ± 14 vs. 20 ± 1 ng before and after ZM 241385, respectively; Fig. 1B). Similar results were found in HS-fed Dahl SR rats and SS rats on both NS and HS intake (data not shown), confirming that 2-CA-induced vasodilation of the isolated, perfused kidney from either Dahl SR or SS rats was mediated solely by A2AR activation and EETs irrespective of salt diet.
Fig. 1.
Effect of adenosine2A receptor (A2AR) inhibition on the responsiveness of isolated, perfused Dahl salt-resistant (SR) rat kidneys to 10 μg of 2-chloroadenosine (2-CA) under normal salt (NS; 0.4% NaCl) intake. A: vascular response to 10 μg of 2-CA was assessed before and after A2AR blockade by 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol (ZM 241385; 1 μM). B: total release of epoxyeicosatrienoic acids (EETs) plus dihydroxyeicosatrienoic acids (DHTs) in response to 10 μg of 2-CA was compared in the absence and presence of ZM 241385 (1 μM). Data are means ± SE; n = 3. *P < 0.05 vs. control.
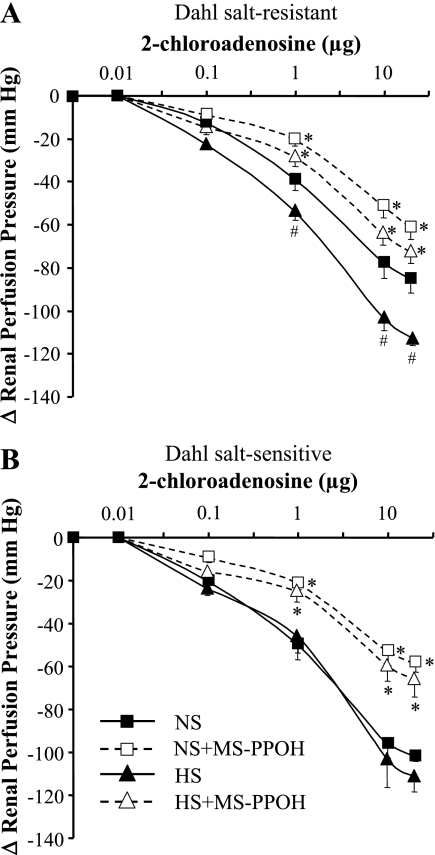
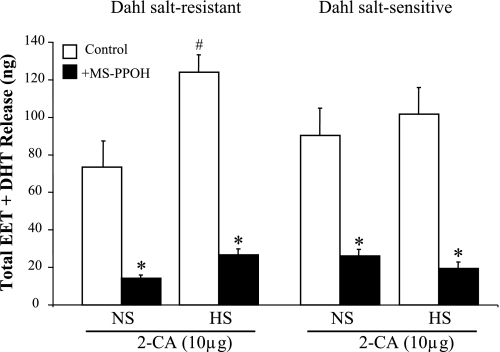
The dilator responses to 2-CA were dose dependent; 2-CA (0.01–20 μg) was given by bolus in ascending doses of 0.01, 0.1, 1, 10, and 20 μg and then repeated after inhibition of epoxygenases with MS-PPOH (12 μM) infusion for 15 min (Fig. 2). Compared with NS intake, renovasodilator responses to 2-CA were enhanced in Dahl SR rats with salt loading; at 10 μg of 2-CA, the drop in RPP increased from −77 ± 7 to −104 ± 6 mmHg in NS- and HS-fed Dahl SR rats (P < 0.05), respectively (Fig. 2A). Because salt loading increases renal epoxygenase activity (5, 23), we assessed the role of EETs in the enhanced dilator response to 2-CA seen in kidneys from HS-fed Dahl SR rats. Epoxygenase activity was determined as a measurement of total EET and DHT release before and after the addition of MS-PPOH. As shown in Fig. 3, in Dahl SR rats salt loading significantly increased renal EET and DHT release compared with NS intake in response to 10 μg of 2-CA (124 ± 9 vs. 74 ± 14 ng, respectively). Furthermore, in the Dahl SR rats fed a HS diet, inhibition of EET synthesis with MS-PPOH, which greatly reduced the EET component, resulted in a diminished renal vasodilator effect of 2-CA that did not differ from the 2-CA dose-response curve to NS in Dahl SR rats (Fig. 2A).
Fig. 2.
Dose-response curves to 2-CA (0.01–20 μg) in kidneys from Dahl SR (A) and salt-sensitive (SS) (B) rats fed either NS or high-salt (HS; 8.0% NaCl) diet for 7 days. Kidneys were isolated, perfused with Krebs-Henseleit buffer containing NG-nitro-l-arginine methyl ester (200 μM) and indomethacin (10 μM), and preconstricted with phenylephrine (10−7 M). Dose-response curves were obtained in the absence and presence of N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH; 12 μM). Data are means ± SE; n = 5–9. *P < 0.05 vs. control (i.e., HS vs. HS + MS-PPOH). #P < 0.05 NS vs. HS.
Fig. 3.
Renal release of cytochrome P-450 (CYP) epoxygenase metabolites in response to 10 μg of 2-CA in Dahl SR and SS rats. Renal release of total EETs + DHTs in response to 10 μg of 2-CA was compared in NS- and HS-fed Dahl SR and SS rats in the absence and presence of MS-PPOH (12 μM). Data are means ± SE; n = 4–5. *P < 0.05 vs. control (i.e., HS vs. HS + MS-PPOH). #P < 0.05 NS vs. HS.
In Dahl SS rats, in contrast to the Dahl SR strain, the decline in RPP was identical in Dahl SS rats fed either NS or HS diets; that is, the dose-response curves to 2-CA did not differ irrespective of salt intake in Dahl SS rats (Fig. 2B). Moreover, after inhibition of epoxygenase activity with MS-PPOH, the dose-response curves in Dahl SS rats were reduced similarly, suggesting that the EET component in the renal vasodilator response to 2-CA in the Dahl SS rat was similar with either NS or HS intake. This interpretation is supported by the quantity of total EETs (EET + DHT) released by 10 μg of 2-CA (Fig. 3) in Dahl SS rats. Dahl SS rats released similar amounts of total EETs in response to 2-CA whether fed NS or HS diets (90 ± 14 vs. 102 ± 14 ng, respectively). These effects of 2-CA on total EETs released can be correlated with the dose-response curves to 2-CA in Dahl SS rats, which are virtually superimposable irrespective of dietary salt intake (Fig. 2B). Thus the Dahl SR rat is able to respond to HS intake by recruiting EET formation, whereas the Dahl SS rat appears to have exhausted its ability to increase EET synthesis above the levels observed on NS intake. Furthermore, the EET-to-DHT ratio did not change substantially in Dahl SR rats fed NS (1:9) compared with that in HS-fed rats (2:8) or in Dahl SS rats (1:9) irrespective of salt diet.
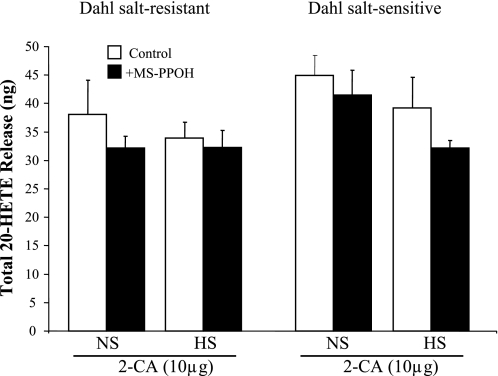
Renal dilator responses to SNP were not different between Dahl SR and SS rats and were unaffected by salt loading (data not shown). In contrast to total EET and DHT release, levels of 20-HETE were affected by neither salt intake nor MS-PPOH in both Dahl SR and SS rats (Fig. 4). Levels of 20-HETE were comparable in Dahl SR and SS rats irrespective of salt diet.
Fig. 4.
Renal release of 20-hydroxyeicosatetraenoic acid (20-HETE) in response to 10 μg of 2-CA in Dahl SR and SS rats. Renal release of 20-HETE in response to 10 μg of 2-CA was compared in NS- and HS-fed Dahl SR and SS rats in the absence and presence of MS-PPOH (12 μM). Data are means ± SE; n = 4–5.
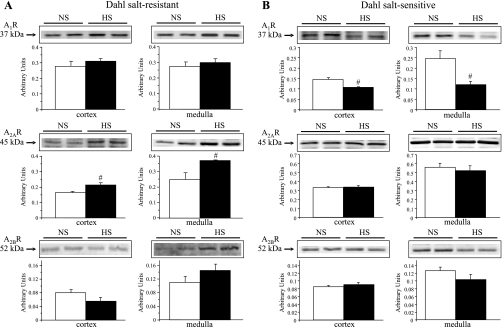
We then examined the effect of salt loading on the renal protein expression of the adenosine receptors A1R, A2AR, and A2BR, as well as the epoxygenase isozymes CYP2C23 and CYP2C11, in Dahl SR and SS rats. Representative Western blots and densitometric analyses are depicted in Figs. 5 and 6. As we have shown with SD rats (23), salt loading significantly increased the renal protein expression of A2AR in both cortex and medulla of Dahl SR rats but not in those of Dahl SS rats (Fig. 5, A and B). Expression of A1R and A2BR was not significantly affected by salt intake in Dahl SR rats; in Dahl SS rats, A1R expression was reduced in both cortex and medulla, whereas A2BR was unaffected.
Fig. 5.
Renal homogenate protein expression of adenosine receptors (ARs) in Dahl SR (A) and SS (B) rats. Representative Western blots and densitometric analysis compare protein expression of ARs in cortical and medullary homogenates in NS- vs. HS-fed Dahl SR and SS rats. The bands were quantitated, and protein expression was normalized with β-actin to control for variations in sample loading. Open bars represent NS rats; filled bars represent HS rats. Data are means ± SE; n = 3–4. #P < 0.05 NS vs. HS.
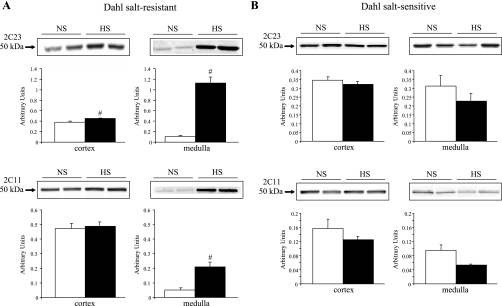
Fig. 6.
Renal homogenate protein expression of CYP2C isozymes in Dahl SR (A) and SS (B) rats. Representative Western blots and densitometric analysis compare protein expression of CYP2C23 and CYP2C11 in cortical and medullary homogenates in NS- vs. HS-fed Dahl SR and SS rats. The bands were quantitated, and protein expression was normalized with β-actin to control for variations in sample loading. Open bars represent NS rats; filled bars represent HS rats. Data are means ± SE; n = 3–4. #P < 0.05 NS vs. HS.
In agreement with other studies (16, 46), HS intake significantly increased the renal protein expression of CYP2C23 and CYP2C11 in Dahl SR rats (Fig. 6A). Increased protein levels of CYP2C23 were detected in both cortex and medulla, with a sixfold increase in expression observed in the medulla of HS- compared with NS-fed Dahl SR rats. An increase in the protein expression of CYP2C11 was detected only in the medulla of Dahl SR rats. In contrast to Dahl SR rats, salt loading failed to upregulate the renal protein expression of the CYP2C isozymes in Dahl SS rats (Fig. 6, A and B). In fact, medullary protein levels of both CYP2C23 and CYP2C11 showed a tendency to be reduced by HS intake in Dahl SS rats.
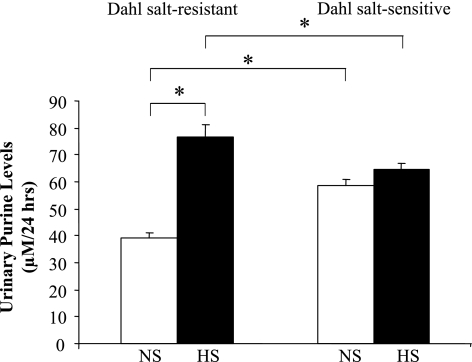
Although urinary adenosine levels were unaffected by salt intake in either Dahl SR or SS rats (data not shown), total purine levels (adenosine, hypoxanthine, xanthine, and inosine), an index of increased flux through the adenosine pathway, were significantly increased in Dahl SR rats fed HS compared with NS diet (77 ± 2 vs. 39 ± 2 μM/24 h, respectively, P < 0.05; Fig. 7). Urinary purine levels were not affected by salt intake in Dahl SS rats; however, under conditions of NS intake, levels of urinary purines were higher in Dahl SS rats compared with Dahl SR rats (59 ± 4 vs. 39 ± 2 μM/24 h, respectively, P < 0.05).
Fig. 7.
Urinary purine levels after 7 days of HS diet in Dahl SR and SS rats. Levels of urinary purines (i.e., adenosine, hypoxanthine, xanthine, and inosine) were measured in Dahl SR and SS rats on NS diet and after 7 days of HS diet. Data are means ± SE; n = 5–7. *P < 0.05.
DISCUSSION
We have reported that adenosine-activated renovascular dilatation in SD rats is mediated by stimulating the A2AR, which is linked to increasing EET synthesis, with the latter mediating adenosine actions on the renal vasculature. The adenosine-epoxygenase pathway is upregulated by HS intake in normotensive SD rats (23). Because this pathway is antipressor, we examined the role of the adenosine-epoxygenase pathway in Dahl SS hypertensive rats, a genetic model of salt-sensitive hypertension. To define the renovascular responses to adenosine in Dahl SS and SR rats, we used a stable analog of adenosine, 2-CA, that is inactivated by neither adenosine deaminase/kinase nor rapid removal by nucleoside carriers. Dahl SS rats challenged with HS intake, in contrast to Dahl SR rats, failed to respond to 2-CA-elicited renal vasodilation above the levels produced by 2-CA in Dahl SS rats on NS intake. Thus the renal vasodilator dose-response curves to 2-CA (Fig. 2) and the efflux of EETs in response to 2-CA (Fig. 3) did not differ in Dahl SS rats, irrespective of salt intake, whether fed NS or HS. Moreover, differences in the renal vasodilator effect of 2-CA demonstrated by Dahl SS and SR rats on either NS or HS intake were entirely accounted for by their ability to release EETs, because inhibition of epoxygenase activity with MS-PPOH eliminated differences in renal vasodilator responses to 2-CA observed in Dahl SR rats on NS vs. HS intake, as well as differences in the renal vasodilator responses to 2-CA occurring between Dahl SR rats and Dahl SS rats (Fig. 2). Thus inhibiting EET synthesis obliterated all differences in renal vasodilator responses between NS- and HS-fed Dahl SS rats produced by 2-CA as well as those between Dahl SR and SS rats, demonstrating the crucial role of EETs as mediators of renovascular responses in activating the renal adenosine system.
In contrast to that of Dahl SR rats, salt loading of Dahl SS rats failed to increase the renal protein expression of either CYP2C23 or CYP2C11 (Fig. 6B). Rather, levels of medullary CYP2C23 and CYP2C11 tended to decrease with HS intake in Dahl SS rats. The functional significance of this reduction in CYP2C protein is unknown, because the renovascular responsiveness of Dahl SS kidneys to 2-CA mediated by EETs was not decreased with HS feeding compared with NS intake.
Plasma adenosine concentrations have been reported to be higher in Dahl SS rats than in SR rats on NS intake and were positively correlated with blood pressure (44). In our study, total purines, which reflect increased flux through the adenosine pathway, were also higher in Dahl SS rats than in Dahl SR rats on NS intake, suggesting that an abnormality in adenosine signaling contributes to the development of hypertension in Dahl SS rats, as shown by the inability to upregulate the A2AR in Dahl SS rats fed HS (Fig. 5B). HS intake did increase urinary purine excretion in Dahl SR rats in contrast to the unresponsiveness to additional increases of urinary purine excretion that occurred in Dahl SS rats on HS intake (Fig. 7). Since renal purine excretion increased in Dahl SR rats on HS intake (Fig. 7), the upregulation of A2AR was unexpected. Adenosine receptors are G protein-coupled receptors, which typically exhibit desensitization. However, an increased A2AR expression in the face of increased purine levels in Dahl SR rats does not conform to typical G protein-coupled receptor pharmacology and instead displays a feed-forward mechanism. In agreement with other studies (48), the renal expression of A1R decreased with HS intake in both cortex and medulla of Dahl SS rats but not in Dahl SR rats. Spontaneously hypertensive rats develop tolerance to chronic administration of a selective A1R agonist, but not a selective A2AR agonist, over the course of 21 days (9). Based on these observations, it is likely that the four adenosine receptors are regulated in a subtype-specific manner, as has been shown with other receptors such as angiotensin II receptors (39). Although the functional relevance remains unknown, HS intake also has been shown to increase the expression of A3R (48).
It is well documented that hypertensive Dahl SS rats exhibit altered responses to vasoactive stimuli (11, 26). In hypertensive Dahl SS rats fed a HS diet, vasoconstrictor responses to norepinephrine were increased while relaxations in response to acetylcholine were depressed (26). The basis of the attenuated endothelium-dependent vasodilation in Dahl SS rats has been related to the inability of the Dahl SS rat to moderate the renin-angiotensin system (RAS), since the RAS affects microvessel reactivity (14, 25). Deficiency of NO also has been shown to contribute to impaired endothelium-dependent vasodilation in Dahl SS rats, possibly reflecting increased activity of arginase, which competes with NOS for l-arginine (21). However, we could not detect involvement of either NO or a COX product in 2-CA-induced renal vasodilation under our experimental conditions in Dahl SS rats because NOS and COX were inhibited in both Dahl SR and SS rats before dose-response curves to 2-CA were conducted. Thus, in the presence of l-NAME and Indo, 2-CA-induced renal vasodilatation was inhibited by MS-PPOH, under conditions of either NS or HS intake, in both Dahl SR and SS rats. Because the response was not completely abolished by MS-PPOH, EETs may not be the sole mediator of 2-CA-induced dilation, as shown in SD rats (23). For example, carbon monoxide, a product of heme degradation by heme oxygenase, may mediate epoxygenase-independent vasodilatation in response to adenosine (24).
The rapid hydrolysis of EETs to biologically inactive DHTs by soluble epoxide hydrolase (sEH) is the dominant pathway for renal EET metabolism (45). Total EET and DHT release were measured as an index of epoxygenase activity. Renal efflux of EETs and DHTs in response to 2-CA was greater in Dahl SR rats fed a HS diet than in those fed a NS diet. Epoxygenase inhibition abolished the HS-induced increase in EET + DHT release in SR rats, indicating that de novo synthesis of EETs, rather than release of preformed EETs from storage in phospholipids (6), was responsible for the enhanced renovascular response. Because we did not observe any significant change in the EET-to-DHT ratios with salt loading, it is unlikely that the increased EET levels seen with HS intake in SR rats can be accounted for by decreased sEH activity. In view of the antagonistic effects of 20-HETE on EET-induced renal vasodilatation (17), we also examined the effects of HS diet on 2-CA elicited renal release of 20-HETE. Salt loading did not affect 20-HETE renal release in response to 2-CA (Fig. 4), thereby eliminating the potential contribution of changes in 20-HETE production to the altered renal vasodilator effects of 2-CA in Dahl SS and SR rats.
Deficient medullary TALH 20-HETE production associated with deficient cortical EETs has been proposed by Ma et al. (27) to contribute to hypertension in Dahl SS rats. Because 20-HETE is an endogenous inhibitor of the Na+-K+-2Cl− cotransporter in the TALH (12), a 20-HETE deficiency in the outer medulla of Dahl SS rats may promote increased TALH sodium and chloride reabsorption with elevation of blood pressure (18). In contrast to our findings, the production of 20-HETE and EETs by renal cortical microsomes actually fell in Dahl SS rats fed a HS diet (27). Furthermore, we did not find differences in renal 20-HETE production by Dahl SR and SS rats, which reflects critical differences in the assays between our study and that of Ma et al. (27). Namely, we measured renal efflux of CYP-AA metabolites from the isolated, perfused kidney in response to an adenosine analog, whereas they measured the synthesis of EETs and 20-HETE by renal microsomes, which is an index of CYP450 enzyme synthetic capability rather than an index of the response of CYP450 products to experimental conditions such as changes in salt intake.
Since all four adenosine receptor subtypes are expressed within the kidney (20) and 2-CA is a nonselective agonist of the adenosine receptors, it was necessary to define which adenosine receptor mediated the 2-CA-induced vasodilation by synthesizing EETs. The dilator response to 2-CA was abolished in both Dahl SR and SS rats in the presence of the selective A2AR antagonist ZM 241385. Thus 2-CA-induced vasodilation of the rat isolated, perfused kidney is solely mediated via activation of A2AR. Because activation of A2BR dilates renal arteries in a NO-dependent manner (29), we eliminated this pathway as a component of the response to 2-CA by inhibiting NOS.
Our findings suggest a ceiling imposed on both Dahl SR and SS rats that limited the ability of Dahl SS rats to recruit epoxygenase activity as indicated by renal EET release/efflux. Several factors that relate to the adenosine-epoxygenase system have been identified in our study that may explain the inability of HS intake to mobilize increased EET production in the Dahl SS rat in response to HS intake; namely, 1) purine levels reflecting activity of the adenosine pathway were fixed at levels found in NS-fed Dahl SS rats (Fig. 7); 2) protein expression of the preeminent 2C isozyme, 2C23, responsible for increased EET synthesis in HS-fed rats, was unresponsive to HS feeding in Dahl SS rats (Fig. 6B); and 3) expression of the relevant A2AR (Fig. 5B) linked to epoxygenase activity was also unresponsive to HS intake in the Dahl SS rat.
Thus the inability of Dahl SS rats to upregulate the adenosine-epoxygenase pathway may contribute to the development of salt-sensitive hypertension. Because salt sensitivity is an important characteristic of a subgroup of humans with essential hypertension and other forms of salt-dependent hypertension that occur in African-Americans, diabetics, and the aged (43), identification of potential targets for the management of salt-sensitive hypertension may be of therapeutic benefit. The adenosine-A2AR-EET pathway may be an important therapeutic target for managing salt-sensitive hypertension.
GRANTS
This research was supported in part by National Institutes of Health Grants DK-69687, HL-25394, and GM-31278 and by the Pharmaceutical Research and Manufacturers of America Foundation.
Acknowledgments
We thank Jing Li, Jianjin Wang, Anabel Doumad, and Yanna Li for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agmon Y, Dinour D, Brezis M. Disparate effects of adenosine A1- and A2-receptor agonists on intrarenal blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 265: F802–F806, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Beach RE, Good DW. Effects of adenosine on ion transport in rat medullary thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 263: F482–F487, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Berthold H, Just A, Kirchheim HR, Osswald H, Ehmke H. Renal haemodynamic responses to exogenous and endogenous adenosine in conscious dogs. J Physiol 510: 321–330, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Batuman V, Puschett DB, Puschett JB. Effect of KW-3902, a novel adenosine A1 receptor antagonist, on sodium-dependent phosphate and glucose transport by the rat renal proximal tubular cell. Life Sci 55: 839–845, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Wei S, Yan J, Karara A, Jacobson HR, Falck JR, Guengerich FP, DuBois RN. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J Biol Chem 267: 21720–21726, 1992. [PubMed] [Google Scholar]
- 6.Carroll MA, Balazy M, Margiotta P, Huang DD, Falck JR, McGiff JC. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol Regul Integr Comp Physiol 271: R863–R869, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol 291: F155–F161, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Carroll MA, Garcia MP, Falck JR, McGiff JC. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J Pharmacol Exp Ther 260: 104–109, 1992. [PubMed] [Google Scholar]
- 9.Casati C, Monopoli A, Dionisotti S, Zocchi C, Bonizzoni E, Ongini E. Repeated administration of selective adenosine A1 and A2 receptor agonists in the spontaneously hypertensive rat: tolerance develops to A1-mediated hemodynamic effects. J Pharmacol Exp Ther 268: 1506–1511, 1994. [PubMed] [Google Scholar]
- 10.Cheng MK, McGiff JC, Carroll MA. Renal arterial 20-hydroxyeicosatetraenoic acid levels: regulation by cyclooxygenase. Am J Physiol Renal Physiol 284: F474–F479, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Drenjancevic-Peric I, Greene AS, Kunert MP, Lombard JH. Arteriolar responses to vasodilator stimuli and elevated Po2 in renin congenic and Dahl salt-sensitive rats. Microcirculation 11: 669–677, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science 251: 799–802, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001. [PMC free article] [PubMed] [Google Scholar]
- 14.Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW Jr. Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1317–R1323, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gu R, Wang J, Zhang Y, Li W, Xu Y, Shan H, Wang WH, Yang B. Adenosine stimulates the basolateral 50 pS K channels in the thick ascending limb of the rat kidney. Am J Physiol Renal Physiol 293: F299–F305, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest 104: 751–760, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol 127: 1399–1405, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension 33: 419–423, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Jackson EK, Mi Z, Koehler MT, Carcillo JA Jr, Herzer WA. Injured erythrocytes release adenosine deaminase into the circulation. J Pharmacol Exp Ther 279: 1250–1260, 1996. [PubMed] [Google Scholar]
- 20.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283: F41–F51, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 288: R1057–R1062, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RA, Pfeffer JM, Mitch WE, Smith TW. Plasma nonesterified fatty acids in the Dahl rat. Response to salt loading. Hypertension 10: 198–203, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Liclican EL, McGiff JC, Pedraza PL, Ferreri NR, Falck JR, Carroll MA. Exaggerated response to adenosine in kidneys from high salt-fed rats: role of epoxyeicosatrienoic acids. Am J Physiol Renal Physiol 289: F386–F392, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Lin CH, Lo WC, Hsiao M, Tseng CJ. Interaction of carbon monoxide and adenosine in the nucleus tractus solitarii of rats. Hypertension 42: 380–385, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 284: H1124–H1133, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Luscher TF, Raij L, Vanhoutte PM. Endothelium-dependent vascular responses in normotensive and hypertensive Dahl rats. Hypertension 9: 157–163, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 267: R579–R589, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2414–2420, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin PL, Potts AA. The endothelium of the rat renal artery plays an obligatory role in A2 adenosine receptor-mediated relaxation induced by 5′-N-ethylcarboxamidoadenosine and N6-cyclopentyladenosine. J Pharmacol Exp Ther 270: 893–899, 1994. [PubMed] [Google Scholar]
- 30.McGiff JC, Ferreri NR. Eicosanoids and the kidney. In: The Kidney: Physiology and Pathophysiology, edited by Alpern R, Hebert S. New York: Elsevier Academic, 2008, p. 359–384.
- 31.Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J Pharmacol Exp Ther 273: 728–733, 1995. [PubMed] [Google Scholar]
- 32.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350: 1734–1737, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Nishiyama A, Inscho EW, Navar LG. Interactions of adenosine A1 and A2a receptors on renal microvascular reactivity. Am J Physiol Renal Physiol 280: F406–F414, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Osswald H, Spielman WS, Knox FG. Mechanism of adenosine-mediated decreases in glomerular filtration rate in dogs. Circ Res 43: 465–469, 1978. [DOI] [PubMed] [Google Scholar]
- 35.Oyekan AO, Youseff T, Fulton D, Quilley J, McGiff JC. Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride. J Clin Invest 104: 1131–1137, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PW, Jones G, Coll MG. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol 115: 1096–1102, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren Y, Garvin JL, Liu R, Carretero OA. Possible mechanism of efferent arteriole (Ef-Art) tubuloglomerular feedback. Kidney Int 71: 861–866, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Silldorff EP, Pallone TL. Adenosine signaling in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol 280: R854–R861, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Wang DH, Du Y, Yao A, Hu Z. Regulation of type 1 angiotensin II receptor and its subtype gene expression in kidney by sodium loading and angiotensin II infusion. J Hypertens 14: 1409–1415, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther 284: 966–973, 1998. [PubMed] [Google Scholar]
- 41.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol 124: 719–727, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Y, Sun P, Wang Z, Yang B, Carroll MA, Wang WH. Adenosine inhibits ENaC via cytochrome P-450 epoxygenase-dependent metabolites of arachidonic acid. Am J Physiol Renal Physiol 290: F1163–F1168, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Yamada K, Goto A, Ishii M, Yoshioka M, Sugimoto T. Plasma adenosine concentrations are elevated in Dahl salt-sensitive rats. Experientia 51: 227–229, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Zeldin DC, Wei S, Falck JR, Hammock BD, Snapper JR, Capdevila JH. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch Biochem Biophys 316: 443–451, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension 41: 709–714, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Zou AP, Nithipatikom K, Li PL, Cowley AW Jr. Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol Regul Integr Comp Physiol 276: R790–R798, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Zou AP, Wu F, Li PL, Cowley AW Jr. Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension 33: 511–516, 1999. [DOI] [PubMed] [Google Scholar]