Abstract
There is increasing evidence that mammalian urinary tract epithelial cells utilize membrane channels and transporters to transport solutes across their apical (luminal) and basalateral membranes to modify solute concentrations in both cell and urine. This study investigates the expression, localization, and regulation of the ROMK (Kir 1.1) potassium channels in rat and dog ureter and bladder tissues. Immunoblots of homogenates of whole ureter, whole bladder, bladder epithelial cells, and bladder smooth muscle tissues in both rat and dog identified ∼45- to 50-kDa bands characteristic of ROMK in all tissues. RT-PCR identified ROMK mRNA in these same tissues in both animal species. ROMK protein localized by immunocytochemistry was strongly expressed in the apical membranes of the large umbrella cells lining the bladder lumen and to a lesser extent in the cytoplasm of epithelial cells and smooth muscle cells in the rat bladder. ROMK protein and mRNA were also discovered in cardiac, striated, and smooth muscle in diverse organs. There was no difference in immunoblot expression of ROMK abundance in bladder homogenates (whole bladder, epithelial cell, or muscle cell) or ureteral homogenates between groups of rats fed high- or low-potassium diets. Although the functional role of ROMK in urinary tract epithelia and smooth muscle is unknown, ROMK may participate in the regulation of epithelial and smooth muscle cell volume and osmolality, in the dissipation of potassium leaked or diffused from urine across the epithelial cell apical membranes or tight junctions, and in net or bidirectional potassium transport across urinary tract epithelia.
Keywords: epithelial sodium channel, calponin
mammalian urinary tract epithelia (urothelia) has long been held to serve solely as a storage conduct for urine made by the kidney and, in particular, to prevent modification of urine by inhibiting net flux of water and solutes across the epithelial cell luminal membrane (7). The barrier(s) preventing transepithelial flux of urine constituents have been shown to include: the lumenal membrane (containing unique uroplakin proteins) of the large (“umbrella”) cells lining the urinary tract lumen (12), tight junctions adjoining the umbrella cells, and a glycosaminoglycans layer overlying and adjacent to the lumenal membrane (13, reviewed in Ref. 17). Evidence for the effectiveness of these barriers, which have been thought to be passive in nature and not to utilize or require membrane transporters, comes from Ussing chamber studies documenting very low urothelial permeability to water, ions, and nonelectrolytes and very high transepithelial cell resistance in stretched bladders and apical membrane endosomes obtained from rabbits under basal conditions (2, 24).
However, although the permeabilities of urothelia for water and various urinary solutes may be low under basal conditions, urothelial permeabilities in animals subjected to dietary, physiological (e.g., water loading/water restricted), or other stress could be altered. Furthermore, urothelial cells are routinely subjected to multiple stresses that might alter or promote transepithelial water and solute movement, including prolonged exposure of epithelial cells to enormous (and fluctuating) concentration gradients of pH, osmolarity, and numerous urinary solutes and constituents, including some [urokinase (19; reviewed in Ref. 17) and glycosaminoglycans (13, 26)] that might directly effect membrane transporters and/or permeability. Epithelial cell permeabilities may also be altered by local mechanical factors, including intermittently high intraluminal hydrostatic pressures and alternating stretch and contraction phenomena as the bladder fills and empties, and potentially by systemic factors including circulating hormones and cytokines.
Given these considerations, it may not be surprising that net urothelial flux of multiple ionic and nonionic solutes, including urea (9, 10, 11, 16, 26, 34), sodium (9, 10, 11, 16, 27, 34, 35), calcium (26), and potassium (16, 27, 34, 35), has been described using a variety of in vivo and in vitro techniques in many animal species. In these experiments, fluxes usually occurred in the direction of the concentration gradient, but fluxes were influenced as well by urine pH (8), bladder volume (9), and hydrostatic pressure (35).
Although simple diffusion processes are likely responsible for some component(s) of the reported fluxes, the mechanism(s) whereby individual net solute fluxes occur are generally unknown. It is noteworthy, however, that investigations using electrophysiological methodologies demonstrated an amiloride-sensitive sodium channel as early as 1964 (18, 38). This channel was subsequently identified as the epithelial sodium channel (ENaC) and localized in or under the urothelia cell lumenal membrane (29). Ussing chamber studies have shown that hydrostatic pressure increased urothelial mucosal-to-serosal sodium reabsorption via ENaC and also induced potassium secretion in mucosal bathing solution via an unidentified stretch-activated nonselective cation channel (35). In other studies, we have demonstrated the presence of aquaporins (AQP)-2 and -3 and urea transporter-B in urothelial basalateral membrane in both rats and dogs (30–32). More recently, Sun and coworkers (33) discovered a channel in cultured human bladder epithelial cells with a strong inward-rectifying potassium current and conductance characteristics of the Kir 2.1 potassium channel. They also corroborated the presence of both Kir 2.1 and a large-conductance calcium-activated potassium channel (BK, or Maxi-K) presence in cultured human urothelial cells by RT-PCR and Western blot analysis (33). The cellular location of these channels was not reported.
Taken together, these findings suggest that mammalian urinary tract epithelial cells may contain a number of channels and transporters known to be present in their nephron counterparts. Given the importance of extracellular and intracellular potassium concentrations to cellular function, the wide range of potassium concentrations in urine bathing the epithelial cells, the findings of net potassium flux across urothelia in several mammalian species (see above), and considering that the majority of the urothelial transporters (AQP-2, AQP-3, ENaC, Maxi-K) identified to date are prominently present in the collecting duct (which develops embryologically from the same tissue as the lower urinary tract), we reasoned that additional distal nephron potassium transporters might be present in urothelial cells. Herein, we report the presence and cell localization of ROMK (Kir 1.1) in rat and dog urothelia and urinary tract smooth muscle cells (and in other tissues containing smooth muscle cells) using Western blot, RT-PCR, and immunolocalization techniques and the impact of dietary potassium on ROMK abundance in rat bladder, ureter, and renal cortex tissues.
METHODS
All research reported herein adheres to Guiding Principles in the Care and Use of Animals and was approved by The Johns Hopkins University Animal Care and Use Committee.
Rat experiments.
The animals used in these studies were female Wistar rats (Harlan, Indianapolis, IN) weighing ∼225 grams and maintained on an ad libitum intake of chow (Quality Lab Products) containing 14% protein until the day of experimental procedure, or the start of one of the experimental diets.
Diets and resulting urine characteristics.
Before experimental procedures, animals were either maintained on ad libitum standard chow or assigned to one of two treatment or dietary (Quality Lab Products) groups (all groups n = 6): 1) high (5%)-potassium diet for 7–10 days [mean urine potassium concentration = 430 ± 24 (SE) meq/l]; 2) low (0%)-potassium diet for 7–10 days [mean urine potassium concentration = 15 ± 5 (SE) meq/dl].
Preparation for immunoblotting.
To obtain tissue samples, rats were anesthetized with intraperitoneal Inactin, and a midline abdominal incision was made. For immunoblotting, ureters and bladders, renal cortex, inner medulla (including papilla), and outer medulla (and in additional studies, pieces of aorta, heart, stomach, small and large intestine, quadriceps muscle, and renal, carotid, and femoral arteries) were rapidly removed, rinsed in PBS, minced, and placed in an ice-cold isolation buffer solution composed of 250 mM sucrose and 10 mM triethanolamine adjusted to pH 7.6 with 1 N HCl, as well as the protease inhibitors leupeptin (1 μg/ml) and phenylmethylsulfonyl fluoride (0.1 mg/ml).
All minced tissues were homogenized in ice-cold isolation solution using a Tissumizer homogenizer (Tekmar, Cincinnati, OH). Tissues were homogenized with five bursts of five strokes of a microsaw tooth generator. Homogenates were centrifuged at 4°C at 3,000 g for 10 min to separate incompletely homogenized tissue. Aliquots of the supernatant were obtained for measurement of total protein concentration using a Pierce bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL) A quantity of 5× Laemmli buffer was added to the remainder of the supernatant in a ratio of one part buffer to four parts homogenate, and samples were then heated to 60°C for 15 min to solubilize proteins, separated into aliquots, and stored at −80°C until analyzed.
In groups of rats receiving low (0%)- and high (5%)-potassium diets (n = 6, each group), ureters, bladders, and renal cortex tissues were removed from each rat and processed as above. In additional groups of rats receiving low- and high-potassium diets (n = 6, each group), bladder epithelial cells were scraped off with a scalpel, the cells were rinsed in a microcentrifuge tube with ice-cold isolation buffer solution totaling 100 μl, samples were vortexed, and aliquots were obtained for protein concentration and addition of Laemmli buffer. The remaining bladder tissue for each rat was then rinsed with PBS, rescraped two times to remove any residual epithelial cells, rinsed, then blotted dry and homogenized as bladder muscularis and serosa.
Antibodies.
The following two previously described polyclonal affinity purified antibodies were used as probes: one raised in chickens against a purified COOH-terminal 21-amino-acid (AA) sequence [LC35 (14)] and a second widely reported commercial antibody raised in rabbits against a COOH-terminal 49 AA sequence overlapping with the sequence used for the chicken antibodies (APC-001; Alomone Labs). In experiments designed to demonstrate the lack of smooth muscle cell contamination of epithelial cell scrapings, we used a mouse monoclonal antibody against calponin (calponin 1; sc-58707; Santa Cruz Biotechnology) an actin- and tropomyosin-binding protein found in smooth muscle cells. Secondary antibodies were species-specific goat anti-chicken or goat or donkey anti-rabbit antibodies.
Electrophoresis and immunoblotting of membranes.
SDS-PAGE was carried out on minigels of 10% polyacrylamide. The proteins were transferred to nitrocellulose membranes electrophoretically. After blocking the membranes with 5% nonfat dry milk in phosphate buffer solution, the primary antibody was applied overnight, usually at a 1:3,000 dilution of antibody in phosphate buffer solution containing 0.2% BSA. The blots were exposed for 1 h to secondary antibody (donkey anti-rabbit immunoglobin G conjugated with horseradish peroxidase; Amersham Pharmacia Biotech) or rabbit anti-chicken IgG conjugated with horseradish peroxidase (Sigma). Blots were developed with enhanced chemiluminescence agents (Amersham Pharmacia Biotech or Pierce Biotechnology) before exposure to X-ray film to visualize sites of antigen antibody reaction. Where appropriate, controls were carried out using antibody preabsorbed overnight with the immunizing peptide.
For immunoblot comparisons of high- to low-dietary-potassium animals control minigels were run before Western blotting and were Coomassie stained to confirm equality of loading of each lane. For this purpose, several representative bands in each sample lane were quantified by densitometry and compared with analogous bands of other samples. Densitometry of Coomassie-stained gels and immunoblots was performed with a Molecular Dynamics Densitometer using ImageQuant, version 5.0, software. Before comparisons, dose (μg sample loaded)-response (intensity of bands by densitometry) curves were obtained to ensure that loading doses fell in the linear response range. Data in Figs. 1–8 are representative of three or more experiments.
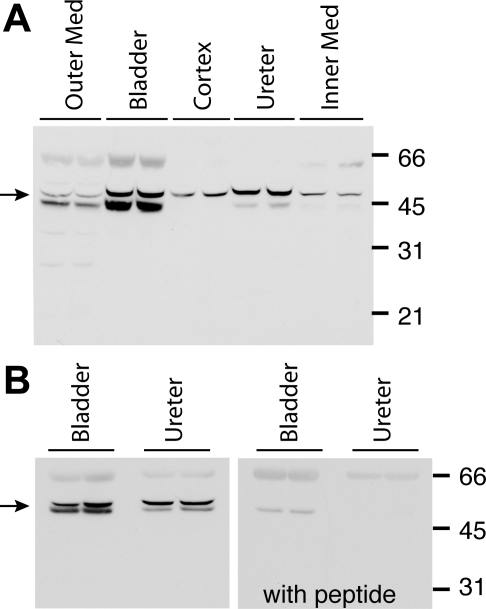
Fig. 1.
A: immunoblot using chicken antibody to ROMK in homogenates of outer medulla, urinary bladder, renal cortex, ureter and inner medulla of Wistar rats. Quantities of 15 μg of protein were loaded in each gel lane. The expected ROMK band is indicated by the arrow. B: immunoblot using chicken antibody to ROMK in homogenates of rat bladder, and ureter (left) ROMK signal at ∼48 kDa, but not the 67-kDa band, was ablated when ROMK chicken antibody was preabsorbed with its immunizing peptide (right).
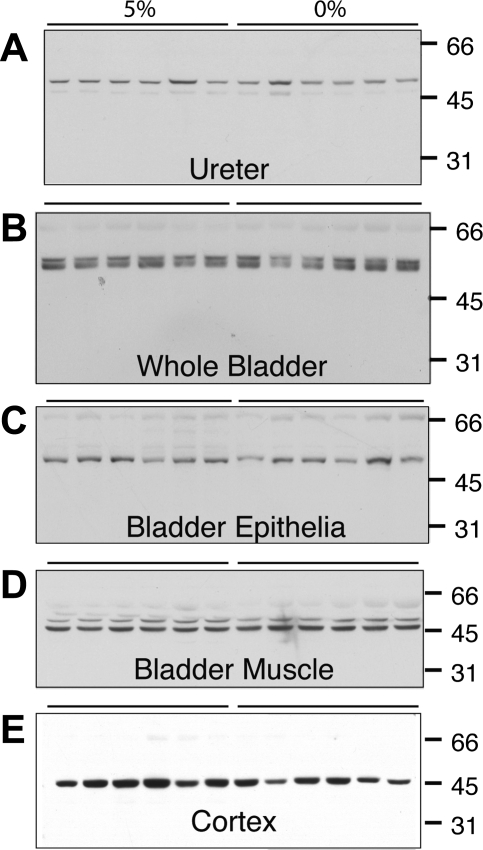
Fig. 8.
Representative immunoblots of ROMK using chicken antibody in homogenates of rat whole ureter, whole bladder, bladder epithelial cells, and bladder serosa and muscularis and renal cortex in rats fed 5% potassium diet or 0% potassium diet. Quantities of 10, 15, 17.5, and 12.5 μg were loaded on the gel lanes for whole ureter, whole bladder, bladder epithelial cells, and bladder muscle, respectively. There were no differences in ROMK expression in bladder or ureteral tissues between the dietary groups. There was a 31% reduction in ROMK expression in renal cortex in rats receiving 0% potassium diet compared with that in rats receiving 5% potassium diet (P = 0.02).
Statistics.
Densitometry data were tabulated as means ± SE. Statistical comparisons were made by using unpaired Student's t-test.
Procedures for immunocytochemistry.
Bladders and ureters of anesthetized rats were fixed for immunolocalization by immersion of the tissues in 4% freshly prepared paraformaldehyde in PBS for 1 h. Tissues were then immersed in a cryprotectant solution of 10% EDTA in 0.1 M Tris buffer, pH 7.4, for 1 h at 4°C and frozen on dry ice. Antibodies were immunolocalized on 8-μm frozen sections as previously described (30). Sections were incubated overnight at 4°C with primary antibodies diluted to 10 μl/ml. Secondary antibodies were species-specific goat anti-rabbit antibodies (Jackson Immunoresearch Laboratories, West Grove, PA) coupled to Alexa 488 and Alexa 568, respectively (Molecular Probes, Eugene, OR). Tissues thus treated were examined using standard and confocal immunofluorescent microscopy. Controls were carried out using antibody preabsorbed overnight with the immunizing peptide. Data in Figs. 1–8 are representative of three or more experiments.
Light microscopy.
For purposes of light microscopic orientation for immunocytochemistry, bladder and ureters were removed from a control rat and fixed in buffered formalin. Organs were embedded in paraffin blocks and sectioned at 3–5 μm; sections were affixed to glass slides stained with hematoxylin and eosin and examined by standard microscopy.
Dog experiments.
Dogs were six healthy male beagle dogs and one male mixed breed/beagle weighing 18–35 lbs maintained on ad libitum water and standard dog chow until the day of experimental procedure.
Procedure for obtaining dog tissue samples.
Dogs were anesthetized with gas anesthesia and monitored with an electrocardiogram and pulse oxymetry. An intravenous solution of lactated Ringer solution was infused (infusion rate varied), producing a mild diuresis. After bone marrow aspirations were obtained (for a different research protocol), the animals were killed with pentobarbital sodium. Immediately upon death, midline abdominal and skin incisions were made, and urine was obtained by needle puncture of the bladder. Bladder, ureteral, and quadriceps muscle tissues were obtained within 2–3 min, and tissues were subsequently treated identically to rat tissues for both immunoblotting and RT-PCR. In some dogs, samples of bladder epithelial cells were obtained for immunoblotting or RT-PCR by carefully scraping the epithelial cells away from the underlying stroma and remainder of the bladder or by cutting bladder smooth muscle tissues from the serosal side of the bladder, with all tissues separately processed for immunoblotting and RT-PCR. Confirmation of tissue separation was made by light microscopic examination of the tissues.
RT-PCR for ROMK.
Using aseptic techniques, rat bladder epithelial cells, bladder muscle tissue, renal outer medulla, cortex, ureter, heart, aorta, large and small intestine, liver and quadriceps muscle tissues, as well as dog bladder epithelial cells and bladder muscle cells were collected [as well as fibroblasts from an established cell culture line (PS120) (37) used as a negative control], submerged in RNAlater (Ambion), incubated overnight at 4°C, and stored at −20°C. Total RNA was isolated by using Trizol Reagent (Invitrogen). Two-step RT-PCR was performed using an iscript cDNA Sythesis kit (Bio-Rad) and Advantage 2 PCR kit (Clontech Laboratories). PCR primers for ROMK (chosen to identify multiple ROMK isoforms, including 1, 2, 3, and 6) were as follows 5′-TACATCTGGGTGTCGTCCGTTTCA-3′ (antisense) and 5′-CATCACTCCTGAAGGCGAGACCAT-3′ (sense). Primers were also designed for a “housekeeping” gene that encodes a ubiquitous protein, glyceraldehyde-3-phosphate dehydrogenasae (GAPDH), to be used as a control. Expected product sizes were 474 bp for ROMK and 309 bp for GAPDH.
RESULTS
Immunoblots of bladder, ureter, renal, and other tissues.
Immunoblot results for ROMK in homogenates of rat whole bladder, ureter, and renal cortex and medulla and in homogenates of bladder epithelial cells and bladder smooth muscle in rats and dogs using ROMK antibody raised in chickens and in rabbits are shown in Figs. 1–3.
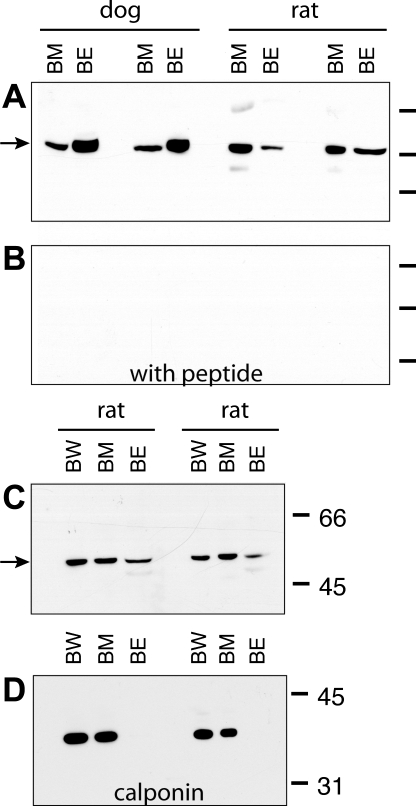
Fig. 3.
A: immunoblot using chicken antibody to ROMK in homogenates of dog (n = 2) bladder smooth muscle (and serosal) tissue and scraped epithelial cells and rat (n = 2) bladder muscle (and serosal) tissue and scraped epithelial cells. B: ROMK signal was ablated when ROMK antibody was preabsorbed with its immunizing peptide. C: immunoblot using chicken antibody to ROMK is homogenates of rat whole bladder, bladder muscle, and bladder epithelia. D: immmunoblot using antibody to calponin, a marker of smooth muscle cells, in the same nitrocellulose membrane used in C. Calponin was not identified in the epithelial cell scrapings. BW, whole bladder; BM, bladder muscle; BE, bladder epithelial cells.
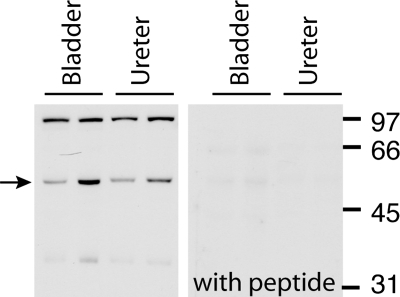
ROMK antibody raised in chicken detected a band specific for ROMK in homogenates of renal outer medulla [as was found previously with the same antibody (4)], renal cortex and inner medulla, and in whole bladder and ureter (Fig. 1A). This ∼45- to 50-kDa band often appeared as a doublet as described previously by Ecelbarger et al. (4) and may represent the glycosylated ROMK species. A fainter 66- to 70-kDa band was also seen on some blots. Preabsorption of the ROMK chicken antibody with the immunizing peptide ablated the ∼45- to 50-kDa band in all tissues (as shown in bladder and ureter in Fig. 1B). To determine if ROMK might be similarly detected using another antibody source, we subjected the same rat bladder, ureter, and renal medulla and cortex tissue to Western blotting using ROMK antibody raised in rabbits. This antibody detected several strong bands, including a band at ∼48–54 kDa (analogous to the 45- to 50-kDa band detected by the chicken antibody) and at ∼98–100 kDa as well as a less intense band at ∼34 kDa (shown for bladder and ureter, Fig. 2, left). In all tissues all bands ablated when rabbit ROMK antibody was preabsorbed with the immunizing peptide (Fig. 2, right). Detection of bands other than the 45- to 50-kDa band, particularly higher-molecular-weight bands, have been previously noted in immunoblots using rabbit antibodies raised to similar ROMK COOH-terminal peptide sequences (4, 13, 14, 20, 23, 39). Most authors agree that bands present at ∼40–50 kDa most likely represent mature ROMK (3, 4, 14, 39), and different authors have suggested that higher-molecular-weight bands might represent a ROMK dimmer or ROMK-protein complex (39) or a cross-reacting protein unrelated to ROMK (4).
Fig. 2.
Immunoblot using rabbit antibody to ROMK in homogenates of rat bladder and ureter (left). Quantities of 10–20 μg of protein were loaded in each gel lane. The expected ROMK band is indicated by the arrow. The 98- to 100-kDa band may represent a dimer. ROMK signal was ablated when rabbit ROMK antibody was preabsorbed with its immunizing peptide (right).
To examine whether ROMK was similarly present in dog bladder and ureter tissues, and to localize the sites of ROMK within the bladder in both rat and dog species, we performed immunoblots using chicken antibodies to ROMK in homogenates of dog and rat bladder epithelial cells carefully scraped from the underlying stromal tissue and homogenates of bladder muscle (and serosal) tissue carefully separated from stromal tissue in both dog and rats.
Immunoblots using chicken antibody showed bands at ∼45–50 kDa in both rat and dog whole bladder homogenates, as well as in homogenates of both the scraped epithelial cells and the muscularis bladder tissue (Fig. 3A). Preabsorption of the ROMK chicken antibody with the immunizing peptide ablated the bands in both rat and dog tissue (Fig. 3B). To examine whether the epithelial cell scrapings were contaminated with smooth muscle tissue, we performed Western blot analysis of rat whole bladder, bladder smooth muscle, and scraped epithelial cells, first probing for ROMK using chicken antibody and then reprobing the same nitrocellulose membrane with antibody to calponin 1 (a smooth muscle cell marker). Homogenates of whole bladder, bladder muscle, and epithelial cell scrapings demonstrated characteristic ROMK bands at 48–50 kDa (Fig. 3C). Antibody to calponin showed the expected bands at ∼34 kDa in whole bladder and bladder muscle homogenates but not in epithelial cell homogenates (Fig. 3D). In immunoblots using rabbit antibody bands at ∼48 kDa, bands at ∼98–100, and ∼34 kDa also were present in dog whole bladder and ureter homogenates, and in homogenates of scraped epithelial cells from dog bladder and bladder muscle and serosal tissues (data not shown).
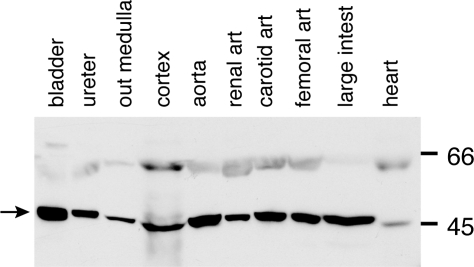
Because ROMK was unexpectedly present in rat and dog bladder smooth muscle tissue, we also examined homogenates of selected other tissues to determine if ROMK might be similarly present in other tissues containing smooth, cardiac, or striated muscle. In Western blots using the chicken antibody to ROMK, typical ∼45- to 50-kDa bands were again seen in urinary tract and renal tissues and also in homogenates of aorta, heart, renal, carotid, and femoral arteries and large intestine (Fig. 4A). When the antibody was preabsorbed with its immunizing peptide, all ∼45- to 50-kDa bands in these tissues were ablated (data not shown). When rabbit antibody was used in homogenates of a similar panel of rat tissues, there was tissue heterogeneity as regards the intensity of the expressed bands at different molecular weights, with aorta, heart, and quadriceps muscle tissues showing stronger bands at ∼98–100 kDa and fainter bands at ∼45–50 kDa (data not shown).
Fig. 4.
Immunoblot using chicken antibody to ROMK in homogenates of rat whole bladder (24), ureter (20), outer medulla (45), renal cortex (219), aorta (60), renal artery (30), carotid artery (42), femoral artery (36), large intestine (188), and heart (100). Quantities of protein (μg) loaded in each lane are indicated in parentheses.
RT-PCR for ROMK.
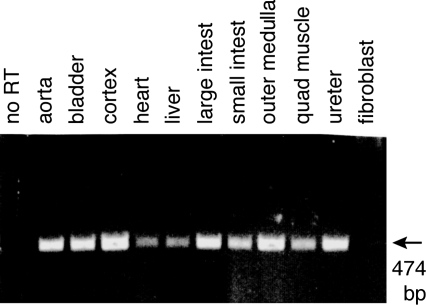
To support our findings of ROMK protein in urothelial and other tissues, we performed RT-PCR for ROMK mRNA in rat tissues, including bladder and ureter, renal cortex and outer medulla, aorta, heart, liver, large and small intestine, quadriceps muscle, and fibroblasts from a cell culture line. As shown in Fig. 5. RT-PCR yielded the expected 474-bp product in all tissues except the fibroblast cells, confirming the presence of mRNA for ROMK in these tissues. Omitting the reverse transcriptase from the RT reaction solution confirmed that the mRNAs were free from contaminating genomic DNA. RT-PCR yielded the expected 309 bp for GAPDH in all tissues, including the fibroblast cells (data not shown). Additional RT-PCR experiments similarly identified ROMK mRNA in rat and dog epithelial cells carefully scraped from bladders and from bladder muscularis/serosa obtained by carefully dissecting these layers from otherwise untouched dog and rat bladders (data not shown).
Fig. 5.
Expression of ROMK mRNA using RT-PCR in homogenates of rat aorta, whole bladder, renal cortex, heart, liver, large intestine, small intestine, renal outer medulla, quadriceps muscle, ureter, and fibroblasts from cell culture. A single expected 474-bp was detected in all tissues except the fibroblast cells. In contrast, RT-PCR for the mRNA encoding the gene for the ubiquitous protein glyceraldehyde-3-phosphate dehydrogenase found the expected 309-bp product in all tissues, including fibroblasts (data not shown). No PCR product was obtained when RT was omitted.
Localization of ROMK in bladder tissue.
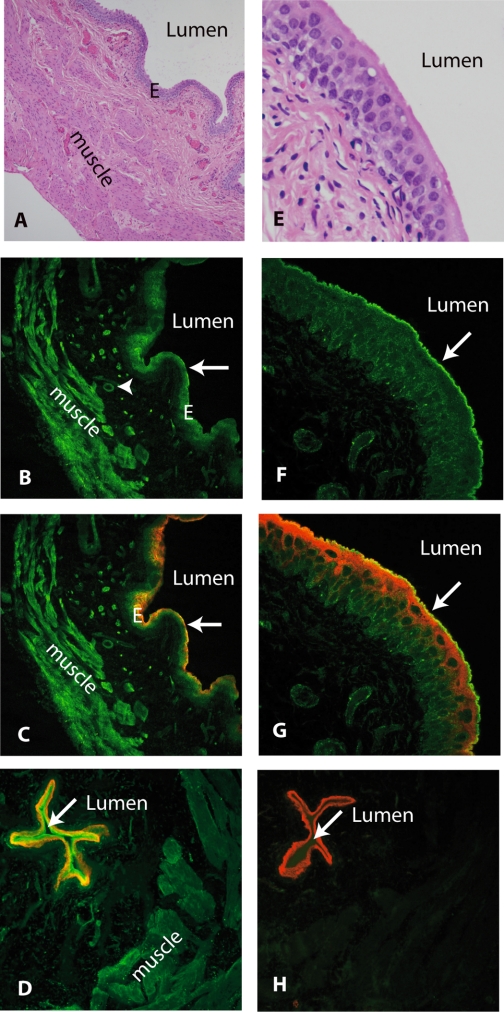
To further localize the site(s) of expression of ROMK, immunocytochemical studies of rat bladder were performed using chicken antibody, as shown in Fig. 6. For purposes of orientation, hemotoxylin- and eosin-stained sections of bladder are shown at low magnification (A) and high magnification (E). Labeling for ROMK (green) was strongly positive in bladder epithelia and both longitudinal and circumferential smooth muscle and arterial smooth muscle cells as shown in B (low power). ROMK antibody colocalized (C, yellow-orange) in epithelial cells with red-staining antibodies to uroplakin [which is present only in epithelia cells as shown in C (low power)]. Under high-power confocal microscopy (F), ROMK (green) is seen to lightly stain the cytoplasm and to strongly stain the lumenal membrane of the large umbrella cells lining the bladder lumen. When sections were simultaneously stained with antibody to uroplakin, ROMK antibody (green) and uroplakin (red) were seen to colabel (yellow) on the lumenal membrane (high power, G).
Fig. 6.
Immunocytochemical localization of ROMK using chicken antibody in rat bladder. For purposes of orientation, light microscopic views of hematoxylin- and eosin-stained tissues are shown in A (low power) and E (high power). In B (low power), ROMK (green) is localized in bladder smooth muscle (“muscle”), vascular smooth muscle (arrowhead), and epithelial cells (arrow). ROMK colocalized (yellow-orange) with red-staining uroplakin antibodies localized to the epithelial cells lining the bladder lumen (C). In F (high power), ROMK (green) is localized in epithelial cells, particularly on the umbrella cell lumenal membrane (arrow) where it colocalizes (G, yellow) with red-staining uroplakins. In D, ROMK (green) also colabels with aquaporin-3 (red) in epithelial cells lining rat bladder lumen. ROMK signal was ablated when antibody to ROMK was preabsorbed with its immunizing peptide, leaving only aquaporin-3 staining the epithelia (H). In D and H, rat bladder was sectioned near its dome, resulting in a smaller lumen.
To confirm specificity of the antibody for ROMK, we performed similar immunocytochemistry experiments using chicken antibody preabsorbed with its immunizing peptide. In Fig. 6D, rat epithelial cells are colabeled with chicken anti-ROMK antibody (green, strong in luminal membrane of epithelial cells) and rabbit anti-AQP-3 (red, stronger in “basal” layer of epithelial cells) in a section of rat bladder sectioned near the “dome” of the bladder (resulting in a small “luminal” area). When chicken ROMK antibody was preincubated with its immunizing peptide, the ROMK signal ablated from both bladder muscle cells and epithelial cells while epithelial cells remain stained red by AQP-3 (shown in Fig. 6H).
Similarly, rabbit ROMK antibody stained epithelial and to a lesser extent smooth muscle cells, and the signal was ablated when rabbit ROMK antibody was preincubated within its immunizing peptide (data not shown).
Localization of ROMK in other tissue.
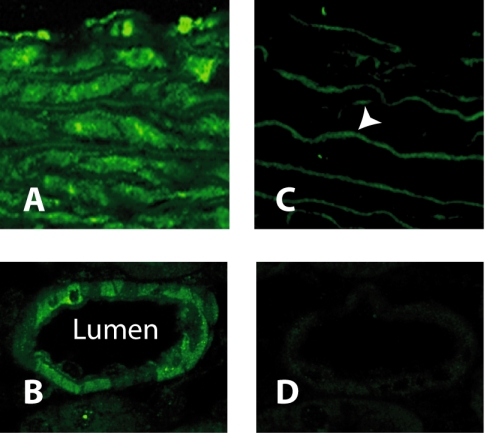
To localize ROMK in vascular tissues, we performed immunocytochemistry assays using chicken antibody to ROMK in cross sections of both rat aorta and interlobular artery of the kidney using high-power confocal microscopy (Fig. 7, A and B). Smooth muscle cells in both tissues stained for ROMK, and the signal was ablated when the antibody was preabsorbed with the immunizing peptide, except for aortic elastic laminas which display nonspecific autofluorescence (Fig. 7C).
Fig. 7.
Immunolocalization of ROMK in blood vessels. ROMK (green) is localized in rat aorta in A. Preabsorption of the antibody with immunizing peptide (C) blocks labeling of muscle, except for the elastic laminas (arrowhead), which display nonspecific autofluorescence. B shows that ROMK (green) is also localized in the wall of the renal interlobular artery. Preabsorption of the antibody with immunizing peptide (D) blocks labeling of smooth muscle.
Effect of dietary potassium on ROMK abundance in bladder, ureter, and renal cortex tissues.
Representative immunoblot results for ROMK in homogenized ureter, whole bladder, scraped bladder epithelial cells, bladder muscle and serosa, and renal cortex are shown in Fig. 8 for groups (n = 6) of rats fed 5% potassium diet or 0% potassium diet. There was no apparent difference in ROMK expression between rats fed 5% potassium diet and 0% potassium diet in any of the bladder or ureter tissue comparisons. Semiquantitive densitometry revealed no significant differences in ROMK abundance between dietary groups in any of the four bladder or ureter tissue groups (all differences = 24% or less between dietary groups, all P > 0.05). However, there was a 31% decrease (P = 0.02) in ROMK abundance in renal cortex in rats fed a 0% potassium diet compared with rats fed 5% dietary potassium, similar to results previously reported (3, 22).
DISCUSSION
In this study, we used two previously described antibodies against ROMK [one raised in chickens (4) and the other raised in rabbits (Alomone; see Ref. 20)], in immunoblots of homogenized dog and rat tissues and demonstrate for the first time that the potassium channel ROMK (Kir 1.1) is present in the urinary tract of both species. Both antibodies identified a band, or doublet bands, at or just above 45 kDa characteristic of glycosylated ROMK (4, 14, 39). Observed species and tissue differences in exact band location and size may be due, in part, to cellular or tissue heterogenicity in the molecular weight of ROMK species or isoform, differences in protein glycosylation, and/or dimer or heteromultimeric complex formation as suggested by several authors (4, 21, 14, 39). We further demonstrate that ROMK protein is localized to bladder smooth muscle cells and epithelial cells in both rats and dogs by performing immunoblots and immunocytochemical assays utilizing both rabbit and chicken antibodies. ROMK antibodies labeled the bladder epithelial cells and in particular strongly labeled the apical membrane of the umbrella cells lining the bladder lumen as well as the bladder circumferential and longitudinal and arterial smooth muscle cells. Finally, we report the presence of ROMK mRNA in bladder smooth muscle cells and epithelial cells of both species using RT-PCR. Because ROMK protein has not been previously reported in muscle cells, immunoblots and RT-PCR were performed and demonstrated both ROMK protein and mRNA in multiple organs and blood vessels containing smooth muscle as well as in cardiac and striated (quadriceps) muscle. Immunocytochemical assay confirmed that ROMK was present in vascular smooth muscle cells and in the cytoplasm and sarcolema of rat quadriceps muscle (data not shown). Thus these data in various rat tissues containing smooth muscle cells support the findings of Kondo and coworkers (15) who found ROMK 6 (Kir 1.1f) isoform mRNA in many tissues containing muscle, including heart, colon, small intestine, liver, and skeletal muscle. Because distributions, but not electrophysiological properties, are different between ROMK isoforms in the kidney, those workers suggested that ROMK 6 might be involved in potassium secretion in many tissues (15). Although the function of ROMK in these cells is not known, it is possible that ROMK may play a role in regulation of intracellular potassium concentration, maintenance of resting membrane potential, and even regulation of vascular smooth muscle tone. It is interesting that ROMK knockout mice have low blood pressure. Although this hypotension was suggested to be due to polyuria and volume depletion (21), it is possible that loss of smooth muscle cell ROMK may result in reduced vascular tone in resistance blood vessels. Furthermore, whether ROMK expression in muscle cells is regulated by dietary sodium and potassium and by mineralocorticoids, as is the case for renal ROMK (1, 36), is unknown.
Finally, our data show that dietary potassium has no apparent effect on ROMK abundance in rat bladder and ureter tissues. In contrast, we found that restriction of dietary potassium reduced ROMK abundance in renal cortex by 31% compared with rats fed 5% potassium diets. Our findings in renal cortex support previous reports that restriction of dietary potassium reduced ROMK protein abundance, compared with control diet, in both cortical and medullary renal tissues (22), an effect ascribed to increased endocytosis and degradation of ROMK protein in cortical collecting duct cells (3).
The function(s) of potassium channels in lower urinary tract are not clear, and our study was not designed to assess ROMK function. In the kidney, ROMK channels represent the major potassium secretory pathway for regulated potassium excretion in the connecting segment and cortical collecting duct [in contrast to the Maxi-K channel, which appears to mediate tubular flow-regulated potassium secretion (28)]. In addition, ROMK channels play critical roles in salt transport by the Na-K-2Cl transporter in the thick ascending limb by providing the pathway for apical potassium recycling as well as generating the lumen-positive potential that drives the paracellular reabsorption of significant amounts of sodium and potassium (6).
Previous studies that examined net in vivo potassium flux in bladder and ureter have demonstrated potassium reabsorption down its concentration gradient from lumen (urine or artificial test solutions) to blood (8, 16, 27, 34). Although the mechanism(s) of potassium reabsorption may, in part, be secondary to passive diffusion and or leak across the apical cell membrane or through tight junctions, in recent electrophysiological studies, Sun and coworkers (33) showed a strongly rectifying potassium current with conductive characteristics of the Kir 2.1 channel as well as the Maxi-K channel in normal cultured human bladder cells (33), and, in Ussing chamber experiments with rabbit bladder, Wang et al. (35) demonstrated that increased mucosal hydraulic pressure led to increased sodium reabsorption (probably through ENaC) as well as potassium secretion, likely through a stretch-activated nonselective cation channel. Although none of these channels had the characteristics of ROMK channels, these findings support the hypothesis that multiple potassium channels and transporters are present in urothelia as is the case for renal epithelia. Thus, taken as a whole, these data suggest that potassium ions can be reabsorbed or secreted by urothelia depending on ambient conditions and that ROMK may play a role in such transport. ROMK and other epithelial cell potassium transporters may, therefore, work to disperse potassium leaked, diffused, or transported across the apical membrane from urine; regulate epithelial cell potassium concentration, osmolarity, and cell volume; and maintain appropriate transmembrane electrical potential. Furthermore, transport of potassium and other ions may play an important role in sensory transduction by coupling changes in hydrostatic pressure, for example, to other urothelial cell functions such as ATP-mediated cell signaling (5, 35). Finally, it is possible that ROMK and other urothelial transporters play a role in regulating the composition of extracellular fluid by modifying potassium concentration of urine produced by the kidney. That this paradigm has precedent in mammalian urinary tract is demonstrated by the example of the American black bear, which during its hibernation reabsorbs its entire daily production of urine and thereby has no obligate loss of water or solute (including potassium) during its 3- to 4-mo hibernation (25).
GRANTS
This work was supported in part by grants from The National Kidney Foundation of Maryland (D. Spector) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-32839 (J. B. Wade) and DK-55881(E. J. Weinman).
Acknowledgments
We thank David Kurniawan and Richard Coleman for photographic assistance, Patricia King for manuscript assistance, Ziyun Wang for technical assistance, Dr. Karin Blakemore and Mariya Gendelman for dog tissues, and Dr. Tung-Tien Sun for uroplakin antibodies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beesley AH, Hornby D, White SJ. Rapid Report Regulation of distal nephron K+ channels (ROMK) mRNA expression by aldosterone in rat kidney. J Physiol 509.3: 629–634, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang A, Hammond TG, Sun TT, Zeidel ML. Permeability properties of the mammalian bladder apical membrane. Am J Physiol Cell Physiol 267: C1483–C1492, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Chu PY, Quigley R, Babich V, Huang CL. Dietary potassium restriction stimulates endocytosis of ROMK channel in rat cortical collecting duct. Am J Physiol Renal Physiol 285: F1179–F1187, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, Kim GH, Knepper MA, Liu J, Tate M, Welling PA, Wade JB. Regulation of potassium channel Kir 1.1 (ROMK) abundance in the thick ascending limb of Henle's Loop. J Am Soc Nephrol 12: 10–18, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson DR, Kenndy Ian, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes: a possible sensory mechanism? J Physiol 505: 503–511, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert SC, Andreli TE. Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl absorption. J Genet Physiol 87: 567–590, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks RM The mammalian urinary bladder: an accomodating organ. Biol Rev 50: 215–246, 1975. [DOI] [PubMed] [Google Scholar]
- 8.Hlad CJ Jr, Nelson R, Holmes JH. Transfer of electrolytes across the urinary bladder in the dog (Abstract). Am J Physiol 184: 406, 1956. [DOI] [PubMed] [Google Scholar]
- 9.Hohlbrugger G Changes of hypo- and hypertonic sodium chloride induced by the rat urinary bladder at various filling stages. Eur Urol 13: 83–89, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Hohlbrugger G The vesical blood-urine barrier: A relevant and dynamic interface between renal function and nervous bladder control. J Urol 154: 6–15, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Hohlbrugger G, Lentsch P, Pfaller K, Madersbacher H. Permeability characteristics of the rat urinary bladder in experimental cystitis and after overdistension. Urol Int 40: 211–216, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Hu Ping Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates uothelial permeability. Am J Physiol Renal Physiol 283: F1200–F1207, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Hurst RE, Rhodes SW, Adamson PB, Parsons CL, Roy JB. Functional and structural characteristics of the glycosaminoglycans of the bladder luminal surface. J Urol 138: 433–437, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Kohda Y, Ding W, Phan E, Housini I, Wang J, Star RA, Huang CL. Localization of the ROMK potassium channel to the apical membrane of distal nephron in rat kidney. Kidney Int 54: 1214–1223, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Kondo C, Isomoto S, Matsumoto S, Yamada M, Horio Y, Yamashita S, Takemura-Kameda K, Matsuzawa Y, Kurachi Y. Cloning and functioning expression of a novel isoform of ROMK inwardly rectifying ATP-dependent K+ channel, ROMK6 (Kir l.1f) FEBS Lett 399: 122–126, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Levinsky NG, Berliner RW. Changes in composition of the urine in ureter and bladder at low urine flow. Am J Physiol 196: 549–553, 1959. [DOI] [PubMed] [Google Scholar]
- 17.Lewis SA Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 278: F867–F874, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Lewis SA, Diamond JM. Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol 28: 1–40, 1976. [DOI] [PubMed] [Google Scholar]
- 19.Lewis SA, Clausen C. Urinary Proteases degrade epithelial radium channels. J Membr Biol 122: 77–78, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's Syndrome. J Biol Chem 277: 37871–37880, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Mennett PA, Frindt G, Silver RB, Palmer LG. Potassium restriction downregulates ROMK expression in rat kidney. Am J Physiol Renal Physiol 278: F916–F924, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Mennett PA, Wade JB, Ecelbarger CA, Palmer LG, Frindt G. Localization of ROMK channels in the rat kidney. J Am Soc Nephrol 8: 1823–1830, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol Renal Fluid Electrolyte Physiol 271: F886–F894, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Nelson RA, Jones JD, Wahner HW, McGill DB, Code Cr. Nitrogen metabolism in bears: urea metabolism in summer starvation and in winter sleep and role of urinary bladder in water and nitrogen conservation. Mayo Clinic Proc 50: 141–146, 1975. [PubMed] [Google Scholar]
- 26.Parsons CL, Boychuk D, Jones S, Hurst R, Callahan H. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol 143: 139–142, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport A, Nicholson TF, Yendt ER. Movement of electrolytes across the wall of the urinary bladder in dogs. Am J Physiol 198: 191–194, 1960. [DOI] [PubMed] [Google Scholar]
- 28.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72: 566–573, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Smith PR, Mackler SA, Weiser PC, Brooker DR, Ahn YJ, Harte BJ, McNulty KA, Kleyman TR. Expression and localization of epithelial sodium channel in mammalian urinary bladder. Am J Physiol Renal Physiol 274: F91–F96, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Spector DA, Wade JB, Dillow R, Steplock DA, Weinman EJ. Expression, localization, and regulation of aquaporin-1 to -3 in rat urothelia. Am J Physiol Renal Physiol 282: F1034–F1042, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Spector DA, Yang Q, Liu J, Wade JB. Expression, localization, and regulation of urea transporter B in rat urothelia. Am J Physiol Renal Physiol 287: F102–F108, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Spector DA, Yang Q, Wade JB. High urea and creatinine concentrations and urea transporter B in mammalian urinary tract tissues. Am J Physiol Renal Physiol 292: F467–F474, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Chen M, Lowentritt BH, Zijl SV, Koch KR, Keay S, Simard JM, Chai TC. EGF and HB-EGF modulate inward potassium current in human bladder urothelial cells from normal and interstitial cystitis patients. Am J Physiol Cell Physiol 292: C106–C114, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Walser BL, Yagil Y, Jamison RL. Urea flux in the ureter. Am J Physiol Renal Fluid Electrolyte Physiol 255: F244–F249, 1988. [DOI] [PubMed] [Google Scholar]
- 35.Wang ECY, Lee JM, Johnson JP, Kleyman TR, Bridges R, Apodaca G. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol 285: F651–F663, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Babilonia E, Sterling H, Jin Y, Wang WH. Mineralocorticoids decrease the activity of the apical small-conductance K channel in the cortical collecting duct. Am J Physiol Renal Physiol 289: F1065–F1071, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Weinman EJ, Wang Y, Wang F, Greer C, Steplock D, Shenolikar S. A C-terminal PDZ motif in NHE3 binds NHERF-1 and enhances cAMP inhibition of sodium-hydrogen exchange. Biochemistry 42: 12662–12668, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Wickham JEA Active transport of sodium ion by the mammalian bladder epithelium. Invest Urol 2: 145–153, 1964. [PubMed] [Google Scholar]
- 39.Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert S. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol Renal Physiol 273: F739–F748, 1997. [DOI] [PubMed] [Google Scholar]