Abstract
The epithelial tight junction (TJ) was first described ultrastructurally as a fusion of the outer lipid leaflets of the adjoining cell membrane bilayers (hemifusion). The discovery of an increasing number of integral TJ and TJ-associated proteins has eclipsed the original lipid-based model with the wide acceptance of a protein-centric model for the TJ. In this review, we stress the importance of lipids in TJ structure and function. A lipid-protein hybrid model accommodates a large body of information supporting the lipidic characteristics of the TJ, harmonizes with the accumulating evidence supporting the TJ as an assembly of lipid rafts, and focuses on an important, but relatively unexplored, field of lipid-protein interactions in the morphology, physiology, and pathophysiology of the TJ.
Keywords: lipid rafts, lipid leaflets, claudins, hemifusion
we begin with a brief review of the evolution of a protein-centric tight junction (TJ) model and discuss some issues that cannot be satisfactorily reconciled with a protein-only model. We then focus on the evidence and the need for a lipid-protein hybrid model and conclude with two speculative models solely for the purpose of initiating a dialogue that is expected to escalate.
EVOLUTION OF A PROTEIN-CENTRIC MODEL FOR THE TJ
Descriptive Model of the TJ
The TJ forms an ultrastructural, circumferential belt around an epithelial or endothelial cell, separating the plasma membrane into an apical and a basolateral domain. The belt from one cell binds belts from adjacent cells, forming cell sheets, which act as selective barriers separating the extracellular fluid into different compartments, e.g., separating fluid in the luminal compartment of the intestine or the renal tubule from fluid in the interstitial compartment, i.e., the internal environment (99). As a boundary between the apical and the basolateral plasma membrane domains, the TJ demarcates the well-characterized asymmetric distribution of protein and lipid molecules in these two domains, thereby maintaining polarity in the two-dimensional plane of the plasma membrane. In addition, the TJ also congregates polarity-regulating protein complexes that participate in polarized membrane trafficking and serves as a spatial landmark for vesicle docking (68, 116), suggesting that it also regulates polarity in the three-dimensional space of the cytosol.
In ultrathin-section electron microscopy, the TJ appears as a linear fusion (23) or a series of focal fusions (23, 99) of the exoplasmic leaflets from lipid bilayers of adjacent cells. Freeze-fracture replica electron microscopy demonstrates that the TJ belt is a network of continuous, anastomosing intramembranous strands, each of which is the product of linear polymerization of discrete particles (91). These observations form the basis for the present model for the TJ: each intramembranous TJ strand from one cell “binds” laterally with a paired TJ strand from an adjacent cell, thereby obliterating the intervening extracellular space and giving rise to the fusion between juxtaposed exoplasmic lipid leaflets (99).
Molecular Structure of the TJ: Protein Model vs. Lipid Model
The protein model proposes that a linear array of transmembrane protein particles polymerize to form a TJ strand and that a network of these strands adhere with paired strands from apposing cells to obliterate the intervening intercellular space. The lipid model, which originates from the initial observation of exoplasmic lipid leaflet fusion (23), proposes that the fusion of these lipid leaflets is associated with a transformation of the lamellar configuration (phase) of the participating bilayers into a nonlamellar paired, inverted hexagonal (HII) phase of water-containing cylinders (strands) (14, 44, 73, 82; see below).
At least 50 cytoplasmic plaque and integral transmembrane TJ proteins have been identified (6). Using a new approach for TJ isolation and purification from the human intestinal epithelial line T84, Tang (96) catalogued 914 proteins on the basis of proteomic and bioinformatic analyses. Occludin (26), junctional adhesion molecule 1 (JAM-1) (66) [subsequently renamed JAM-A (77)], and the claudins (75) were the first TJ integral proteins identified. Transfection studies in mouse L fibroblasts suggest that claudins are the transmembrane proteins that constitute the TJ strands (27). These observations provide strong support for the protein model (98, 99) and have led to the dominance of a protein-centric view of the TJ (6, 29, 96). They also have rendered the concept of a lipid-only model untenable.
PROTEIN MODEL: UNRESOLVED ISSUES
Exoplasmic Lipid Leaflets Are Functionally Linked Across the TJ
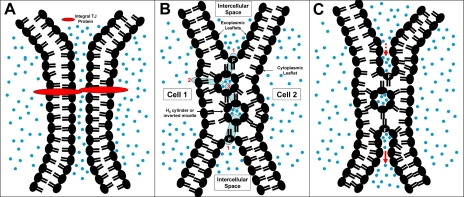
The protein model calls for the transinteractions, across the intercellular space, of integral TJ proteins from apposing cells (Fig. 1A). Such a model would not permit movements of exoplasmic leaflet lipids (or proteins) from one cell to its neighboring cells. A lipid model, on the other hand, would allow such translocations through the fusion region (“F” in Fig. 1, B and C) of the exoplasmic leaflets. The observation that Forssman antigen, an endogenous glycolipid found in exoplasmic lipid leaflets, did not traverse from one cell to its neighboring cells supports a protein model (79, 103, 106).
Fig. 1.
Tight junction (TJ) models. A: a protein model, in which extracellular components of integral TJ proteins (red) from adjacent cells fuse to form TJ barrier. B and C: speculative lipid-protein hybrid models formed by hemifusion of plasma membrane bilayers from 2 adjacent cells (cells 1 and 2) and formation of HII cylinders (TJ strands) or inverted micelles (TJ particles) or a combination of both. In B, protein channels or transporters (blue cylinders) insert into and populate this nonlamellar lipid infrastructure, forming a series of linked aqueous compartments across the TJ. In C, proteins (not shown) participate in trans-TJ transport through a process similar to “endosytosis-transcytosis-exocytosis.” In lipid-protein hybrid models, exoplasmic leaflet of 1 cell is linked to exoplasmic leaflet of the neighboring cell through a fusion region (F); in a protein-only model, no such linkage exists. Blue dots denote aqueous environment.
Others, however, suggested that the bulky head group of Forssman antigen prevented its passage across the TJ, because the HII phase (which is the basis for TJ assembly in the lipid model) is characterized by a region with compressed lipid head groups and, therefore, imposes a size-dependent restriction. Indeed, studies based on fluorescence recovery after photobleaching clearly demonstrate that lipid probes with the less bulky phosphatidylcholine head group and labeled with dye within the hydrophobic moiety diffused readily and rapidly from one cell to another linked by an intact TJ (30). The translocation of dipicrylamine, a lipid-soluble anion, from cell to cell across the TJ has also been demonstrated in a double whole cell clamp experiment in hepatocytes (100). A recent study even calls into question the postulated size-restriction property of the TJ. Thus lipid rafts (LRs), including the large protein molecule cholera toxin B (CT-B) subunit bound to the ganglioside GM1, can also translocate from one cell to another across the TJ (51). These observations are difficult to reconcile with a protein-only model for the TJ.
Known TJ Proteins Lack Adhesive Activities and Do Not Mediate Membrane Bridging or Hemifusion
Current consensus focuses on claudins as the backbone of the TJ strands, with most cells expressing more than two claudin species (99). Different claudin species cis-heteropolymerize to form a TJ strand in one cell, and trans-adhere, homotypically or heterotypically, with claudins in a “paired” strand from an adjacent cell. Adhesion between the extracellular components of the apposing claudin molecules constitutes the TJ barrier. In terms of claudin polymerization, the mechanism remains uncertain. Umeda and associates (101) reported that TJ-associated cytoplasmic proteins zonula occludens (ZO-1 and ZO-2) are instrumental in mediating claudin polymerization and in determining the cellular site where this polymerization would occur. Piontek and associates (83), on the other hand, demonstrated a ZO-1-independent, extracellular transinteraction of claudin molecules across the intercellular space as the basis for their polymerization and the formation of TJ strands.
In terms of adhesion, exogenously expressed claudins exhibited adhesive activities (measured by cell-cell aggregation) much weaker than those observed between transfected endothelial (E)-cadherins (48). The authors noted aggregation in more “compacted forms in which individual cells could hardly be detected” of E-cadherin-transfected L fibroblasts than the claudins. Although the extracellular domain of the E-cadherin molecule from adjacent cells does adhere to form the adherens junction, it neither obliterates the intervening extracellular space [∼200 Å wide (23)] nor exerts a barrier function.
It may be argued that the relatively weak protein-protein transinteraction is a desirable feature for dynamic and transient cell-cell interactions (15, 102), and stronger binding forces to achieve more stable cell-cell adhesions can be generated by molecular oligomerization into zippers and lattices within larger cytoskeleton-adhesion complexes (31). However, the TJ morphology calls for the approximation of the apposing bilayers to within fusion distances. This would require the displacement of water between the apposing hydrophobic surfaces of the interacting plasma membranes and is a thermodynamically unfavorable process. Overcoming this high activation energy barrier requires specialized membrane-bridging and membrane-fusing proteins (9, 41, 107). To our knowledge, neither membrane-bridging nor membrane-fusing activities have been reported in the claudins or any of the known TJ proteins.
Although occludin does not induce TJ strand formation, it is recruited to the TJ, and “claudins together with occludin copolymerize to form the TJ strands in situ” (98). Others proposed that homodimerization of occludin, the claudins, and the junctional adhesive molecules serves as a common structural feature in TJ assembly (4). Although the claudins have only modest adhesive activities, the occludin is virtually devoid of adhesive activities when transfected into L fibroblasts (48, 104). Moreover, there is no evidence for adhesion between the extracellular components of the claudins and the occludin or among any other transmembrane TJ proteins. How do these proteins form a seal that separates the two extracellular aqueous compartments? We are not aware of any biological precedence in which an extended protein-only layer seals off one aqueous compartment from another. The coparticipation of lipids in the assembly of a TJ seal could provide a solution to this apparent dilemma.
TJ Strand: Not All Proteins
In freeze-fracture preparations, the TJ strand is visualized as a linear array of 10-nm particles, spaced at a center-to-center distance of 18 nm (i.e., the particles are separated by a distance of 8 nm) (1). Although there is undisputed evidence for the localization of the claudins to the TJ strands in ultrastructural studies, the precise identity of these freeze-fracture particles is uncertain, inasmuch as similar particles have also been observed in freeze-fracture studies of protein-free, lipid model membranes (109). It is possible that these “lipidic particles,” rather than the protein particles, are the basic units that form the TJ strand. Alternatively, they may fill in the space between the protein particles to form a lipid-protein hybrid TJ strand. The presence of phospholipids in the TJ strands has been demonstrated in freeze-fracture studies (45; see below).
Claudins Reconstitute TJ-Like Strands, not TJ Strands
Furuse and associates (27) first reported the ground-breaking observation that claudin-1 or claudin-2 can reconstitute TJs in cultured fibroblasts. However, it is important to point out that the structures described were not bona fide TJ strands but, rather, “TJ-like” strands. The authors clearly pointed out that the strands they demonstrated “were not zonula but puncta or fascia occludens, i.e., they did not surround individual cells continuously,” and they were not localized to the most apical region of the lateral membrane. Using green fluorescent protein-claudin-1 fusion protein, Sasaki et al. (86) elegantly visualized claudin strands in live L fibroblasts. However, as the authors pointed out, green fluorescent protein fusion aborts the normal binding of claudin-1 to ZO-1. It is known that claudins that dissociate from ZO-1 migrate diffusely into the lateral membrane (39, 71) or the submembranous lysosomal pool (76). Indeed, Sasaki et al. stressed that the claudin strands they observed were not necessarily representative of TJ strands per se. In both studies, the puncta occludens were readily visualized in live cells at light-microscopic resolutions, whereas visualization of bona fide TJs or ZOs requires electron-microscopic resolutions.
Solution: A Lipid-Protein Hybrid TJ
Because neither the lipid-centric nor the protein-centric model fully accounts for the known structure and function of the TJ, we propose a third possibility: a lipid-protein hybrid complex in which, similar to the plasma membrane, proteins function within a lipid infrastructure. Similar to the cell membrane, the TJ separates and regulates two distinct aqueous compartments through proteinaceous conductive pathways across a lipidic seal. The concept of a lipid-protein hybrid TJ is not new, but it has been neglected. Pinto da Silva and Kachar (82) postulated in 1982 that, in the TJ, the intramembranous HII cylinders were stabilized by integral transmembrane proteins. We propose that the TJ proteins by themselves, and in combination with the lipids, serve, in addition, functional roles of homeostatic regulation of polarity, barrier function, and cell growth and differentiation.
EVIDENCE AND NEED FOR A LIPID-PROTEIN HYBRID MODEL FOR THE TJ
Three examples support and call for the participation of lipids in TJ structure and function.
Role of Lipid Bilayer Hemifusion in TJ Assembly
We have mentioned that the TJ was first described as fusions between the exoplasmic leaflets from adjacent cells (23). Fusion between the exoplasmic leaflets, leaving the two corresponding cytoplasmic leaflets intact and, thereby, no mixing of the aqueous content between the two participating cells, is known as hemifusion (8). An appreciation of the genesis and the structural basis of hemifusion calls for a brief review of the concept of lipid polymorphism.
Lipid polymorphism.
The familiar phospholipid bilayer is a lamellar structure consisting of lipid molecules arranged in two parallel leaflets (Fig. 2B). All plasma membrane lipid molecules are amphiphilic, i.e., molecules with both hydrophilic (water-loving), polar head groups and hydrophobic (water-fearing), nonpolar hydrocarbon tails/chains. The hydrocarbon tails in each leaflet minimize contact with water by aligning themselves tightly together in the center of the bilayer, forming a hydrophobic core, while the hydrophilic head groups face the aqueous extracellular or intracellular compartments. The concept of lipid polymorphism is based on the well-established observation that, in addition to this classical bilayer configuration, hydrated lipids can also adopt nonbilayer configurations/phases (13).
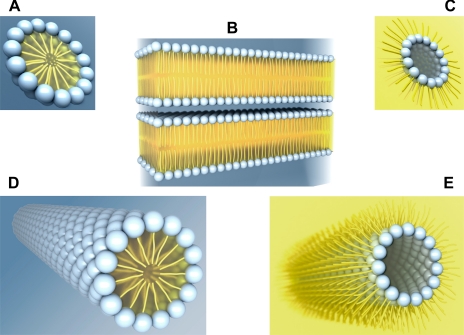
Fig. 2.
Lipid polymorphism. Schematic illustration of a micelle (A), bilayers (B), an inverted micelle (C), and a hexagonal (HI, D) or an inverted hexagonal (HII, E) cylinder. Each phospholipid molecule consists of a hydrophilic (water-loving) head group (blue spheres) facing an aqueous environment (blue background) and 1 or 2 hydrophobic (water-fearing) carbon tails (yellow) facing a lipid environment (yellow background).
Examples of nonlamellar structures in lipid-water systems include the micelle (Fig. 2A), the inverted micelle (Fig. 2C), the hexagonal HI cylinder (Fig. 2D), and the inverted hexagonal HII cylinder (Fig. 2E). A micelle is a spheroidal structure with a hydrophilic shell of polar head groups and a hydrophobic core of hydrocarbon chains (oil-in-water). An inverted micelle is structurally the reverse of a micelle, with a hydrophobic shell and an aqueous interior core (water-in-oil). The hexagonal phases correspond to two-dimensional arrays of hexagonally coordinated cylinders in which the lipid acyl chains are oriented inward (HI) or outward (HII). A lipid molecule, viewed with the polar head group pointing upward, exhibits one of three shapes: a cone, a cylinder, or an inverted cone. Lipid molecules with one hydrocarbon tail (lysophospholipids) have a cross-sectional profile with the head group broader than the hydrocarbon tail, i.e., the shape of an inverted cone, and demonstrate a preference to form a micelle and an HI cylinder (Fig. 2, A and D, respectively). Lipid molecules with two hydrocarbon tails (especially with tails containing unsaturated bonds and, therefore, with “kinks” that resist tight packing) have a reversed head group-to-hydrocarbon tail ratio, i.e., the shape of a cone, and tend to form an inverted micelle and an HII cylinder (Fig. 2, C and E, respectively). Finally, lipid molecules with approximate cylindrical shape pack into lamellar bilayers (Fig. 1B) (13).
Lipid polymorphism, hemifusion, and TJ.
Lamellar-to-HII phase transition is the fundamental process in the generation of hemifusions (8, 65). Kachar and Reese (44), on the basis of freeze-fracture studies, proposed that TJ strands are pairs of lipid HII cylinders sandwiched between linearly fused exoplasmic membrane leaflets from adjacent cells (Fig. 1B). In some freeze-fracture replicas, in place of these intramembranous cylinders, linear rows of particles were observed. In an earlier study, Verkleij et al. (109) reported that the bilayer-to-HII transition of cardiolipin (lipids without protein) mediated by the addition of Ca2+, occurred through an intermediate phase of particle (∼70 Å diameter) formation. The 32P-NMR spectrum of these particles is consistent with the notion that these lipidic particles are inverted micelles. To confirm that inverted micelles can form intramembranous particles, freeze fracture of model membranes prepared from an equimolar mixture of lecithin and cardiolipin in the presence of Ca2+ demonstrated particles with a mean diameter of 100 Å (10 nm) on one fracture face and complementary 70-Å-diameter pits on the opposite fracture face (109). The water-containing inverted micelle and the HII cylinders are characterized by inverted molecular organization, and the HII phase is postulated to represent a one-dimensional extension of the spherical water-containing, inverted micellar particles (108). As mentioned earlier, the presence of phospholipids in the TJ strands has been demonstrated in freeze-fracture studies (45).
The role of the lamellar-to-HII phase transition in TJ assembly and disassembly has also been studied from a functional point of view. Examination of the influence of basic amino acids, Ca2+, protamine, and protons on transepithelial resistance (TER) of confluent Madin-Darby canine kidney (MDCK II) monolayers has led to the conclusion that TJ permeability reflects a lipid phase equilibrium in which the lamellar phase corresponded to an opened, disassembled state and the HII phase to a closed, assembled state of the junction complex (35, 36). Taken together, these observations support an important role for lipids in TJ assembly and function.
In search of a TJ membrane bridging and hemifusion molecule.
As mentioned earlier, membrane fusion is an energetically unfavorable process that is accomplished in nature by special fusion molecules. Soluble N-ethylmaleimide-sensitive factor attachment protein receptors mediate intracellular membrane fusion events and appear to be involved in all steps of the secretory pathway (40). In an extracellular environment, enveloped and nonenveloped viral transmembrane proteins mediate fusion between the viral membrane and the host cell plasma membrane (20, 97). Several proteins are implicated in developmental cell-cell fusion; among these proteins, syncytin (72) and fusogen epithelial fusion failure-1 (84) have been shown to fuse cells in culture.
Common to all known fusion processes is hemifusion, an intermediate stage prior to full fusion that is defined as the establishment of a direct continuity between the two previously separate, lipid bilayer-enclosed aqueous compartments (8). The TJ, however, appears to represent a form of stable hemifusion, in that it does not progress to complete fusion but is otherwise a structurally and functionally dynamic complex. Other reported examples of stable hemifusion include that between the cortical granules and the plasma membrane of a sea urchin egg before fertilization (112) and that between synaptic vesicles and their target membrane in neurons (117).
Annexin A2 heterotetramer: a membrane-bridging protein at the TJ.
Annexin A2 heterotetramer (AnxA2t) is an important intracellular membrane-bridging protein (18, 63, 88) that anchors secretory vesicles/granules to each other or to the cytoplasmic face of the plasma membrane in preparation for vesicular fusion and vesicle-plasma membrane fusion (secretion), respectively (78, 87, 88). AnxA2t is made up of two copies each of annexin A2 (AnxA2) and p11 (an 11-kDa calmodulin-related protein also known as S100A10) (110). In in vitro studies, the addition of exogenous AnxA2 or AnxA2t to suspensions of isolated chromaffin granules (18, 54) or liposomes (54) induced aggregation and junction formation between these lipid bilayer-encased structures without progression to complete fusion. AnxA2- or AnxA2t-induced junction formation between liposomes (devoid of other proteins) provides persuasive evidence for its role in junction assembly between lipid bilayers.
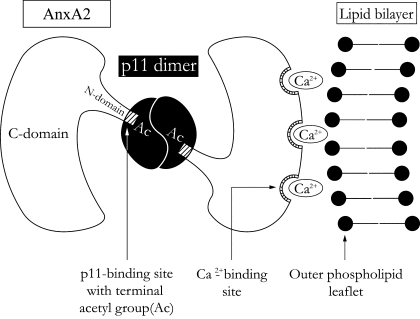
A proposed molecular mechanism for bridging the external leaflets from adjacent lipid bilayers is depicted in Fig. 3. An AnxA2 molecule consists of a COOH-terminal protein core domain (C-domain) and an NH2-terminal domain (N-domain). The protein molecule is in the shape of a slightly curved disk with a convex and a concave face (60). Ca2+-binding sites are located on the convex face, while the NH2 and COOH termini are located on the concave face. Each AnxA2 subunit binds to the outer phospholipid leaflet of one lipid bilayer along its convex face and to the p11 dimer along its concave face. The AnxA2-outer phospholipid leaflet binding is mediated through a Ca2+-bridging mechanism in which a Ca2+ molecule links the Ca2+-binding site of the AnxA2 subunit to the negatively charged phospholipid leaflet (47, 95).
Fig. 3.
Annexin A2 heterotetramer (AnxA2t)-mediated junction formation [modified from Lambert et al. (54)]. AnxA2-p11 complex (AnxA2t) forms a symmetrical bridge between 2 lipid bilayers (only 1 of the 2 participating lipid bilayers is shown at right). The 2 annexin A2 (AnxA2) subunits, each binding to a separate bilayer, are, in turn, linked by a p11 dimer. [Modified from Lee et al. (55).]
A possible problem with the application of this model to the TJ is that the exoplasmic lipid leaflets of the plasma membrane are made up predominantly of phosphatidylcholine and sphingomyelin and exhibit no net charge (90). However, recent studies indicate that, in addition to the Ca2+-bridging mechanism, AnxA2 and AnxA2t also bind directly to cholesterol of the lipid membrane (2, 28, 118). We shall point out that the TJ is an assembly of lipid rafts, which are cholesterol- and sphyingolipid-enriched membrane structures. Moreover, in these cholesterol-rich membrane microdomains, Kunzelmann-Marche and associates (49) reported externalization of phosphatidylserine (negatively charged) from the cytoplasmic leaflet to the exoplasmic leaflet of the plasma membrane. This lipid asymmetry is dependent on lipid raft (LR) integrity and is lost with its disruption. Thus, at the TJ, AnxA2t may link the adjacent exoplasmic leaflets through cholesterol and Ca2+ bridges. AnxA2t may also bind cell surface heparan sulfate glycosaminoglycan (46).
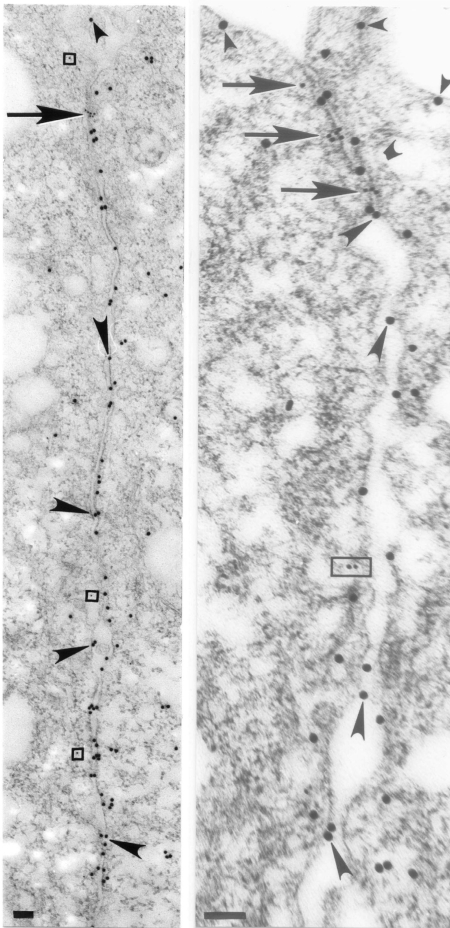
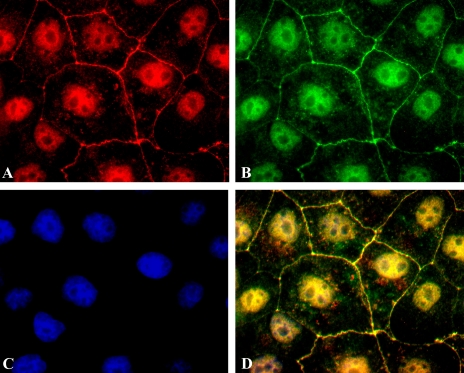
We have reported structural and functional evidence in support of the notion that AnxA2t assembles TJs in a similar fashion in MDCK II monolayers (55). Figure 4 (56) illustrates the immunofluorescent colocalization of AnxA2t (using the AnxA2 subunit as a marker) with the TJ protein claudin-1. We have also reported colocalization of AnxA2t with ZO-1 and with occludin (55). Immuno-electron microscopy confirms the presence of ZO-1 (Fig. 5, left) and occludin (Fig. 5, right) at the apical location of the lateral intercellular space and their ultrastructural juxtaposition with AnxA2 subunits of AnxA2t. A synthetic peptide that reversibly disrupts the binding between AnxA2 and p11 caused TJ disassembly in MDCK II monolayers, as reflected by a 90% reduction in TER (55).
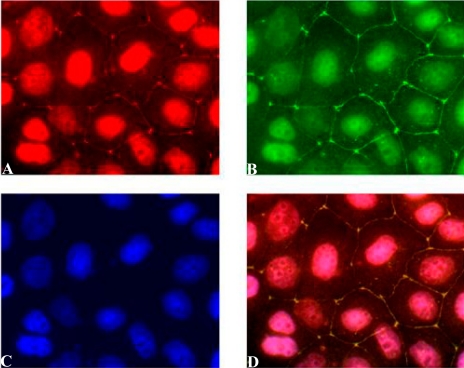
Fig. 4.
AnxA2t colocalizes with the TJ protein claudin-1. An immediately preconfluent Madin-Darby canine kidney (MDCK II) monolayer was triple-stained for AnxA2 subunit (A, red) of AnxA2t, TJ protein, claudin-1 (B, green), and the nucleus (C, blue). Nuclear staining was accomplished using 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR). Merged image (D) shows TJ colocalization of AnxA2 and claudin-1 (yellow). [Triple staining of the nucleus (purple) is discussed in Lee et al. (56).] Magnification ×1,000. [From Lee et al. (56).]
Fig. 5.
Ultrastructural immunogold localization of AnxA2t and TJ proteins in MDCK II monolayers. AnxA2 subunit of AnxA2t (larger, 20-nm gold particles) is distributed in juxtaposition to the TJ proteins (smaller, 10-nm gold particles, arrows) zonula occludens-1 (ZO-1, left) and occludin (right). AnxA2 labels also extend beyond the TJ, along the length of the lateral plasma membrane and the apical, luminal membrane (small arrowheads). ZO-1 particles (arrow, left) aggregate in an area where apposing membranes seem to merge and appear mostly on the cytoplasmic side of the plasma membrane. Cytoplasmic ZO-1 labeling is sometimes observed outside the TJ (enclosed by squares). reflecting the general phenomenon of internalization of TJ proteins (115). Occludin particles (arrows, right) have a similar distribution, consistent with the use of antibody generated against a fusion protein consisting of the cytoplasmic COOH-terminal 150 amino acids of human occludin. Basolateral cytoplasmic labeling of occludin (enclosed by rectangle) is consistent with prior reports and represents the less phosphorylated form of this molecule, distributed in detergent-soluble lipid domains (81, 85). Although most AnxA2 labeling is distributed along the cytoplasmic side of the plasma membrane, it is also seen along the exoplasmic side of the plasma membrane and in the intercellular space (large arrowheads). Scale bars, 0.1 μm. [From Lee et al. (56).]
Note the cytoplasmic and exoplasmic distribution of AnxA2t along the length of the apical and lateral plasma membrane beyond the TJ (Fig. 5). This is consistent with its other known biological actions, which include Ca2+-dependent exocytosis, endocytosis, and cell-cell adhesion (28, 47, 110). On the cytoplasmic side of the plasma membrane, AnxA2t has been observed as 7- to 8-nm (70- to 80-Å) to 80-nm (800-Å) ultrastructural strands that link secretary granules to each other or to the plasma membrane (78, 88). Our observation of the basal extension of extracellular AnxA2t beyond the TJ raises the possibility that AnxA2t may also link lateral plasma membranes from apposing cells and, thereby, regulate the dimension and configuration of the intercellular space basal to the TJ. Indeed, Yamada and associates (114) reported the involvement of AnxA2t in the formation of the E-cadherin-based adherens junctions in MDCK cells. Hansen and associates (32) also reported that AnxA2 (subunit of AnxA2t) localizes rapidly to cell-cell contacts in E-cadherin-driven junction-forming MDCK cells. However, neither study addressed the junction-forming action of AnxA2t or AnxA2. Yamada et al. (114) proposed that AnxA2t mobilizes E-cadherin; Hansen et al. (32) proposed that AnxA2 mobilizes Rac-1 complexes, to nascent cell-cell contacts.
The assembly and the molecular architecture of the TJ lipid complex are expected to be much more complex than AnxA2t bridging of the plasma membrane from adjacent epithelial or endothelial cells. Molecules other than AnxA2t may be involved in this process. Also, in our study, MDCK II monolayers did not exhibit equal sensitivity and sustainability to the action of AnxA2t inhibitory peptide (55). It is reasonable to expect that, given that the sealing of the intercellular space is a matter of life and death, nature has built redundancies into this critical biological process. Also, it has been demonstrated by cryo-electron microscopy that AnxA2t forms a junction between two apposing lipid bilayers but does not actually mediate the merger of the two exoplasmic leaflets into one single leaflet (54). Lambert et al. (54) noted that the distance separating the two bilayers at the junction was 90 ± 3 Å, which corresponds to the thickness of the AnxA2t molecule. In this context, it is of interest to recall that Farquhar and Palade (23) noted that the ultrastructural appearance of the TJ was an “extreme narrowing of the intercellular ‘gap'” to a distance of 70–90 Å. Actual fusion of the apposing exoplasmic lipid leaflets into a single merged line was not consistently seen (23). We anticipate that additional agents and macromolecules may participate in the bridging and hemifusion of lipid bilayers in the TJ assembly. As an example, physical and functional interactions between AnxA2 and SNAP-23 have been reported recently (111).
TJ Exhibits Structural and Functional Characteristics of LRs
There is persuasive evidence supporting the notion that the TJ is an LR assembly. LRs are detergent-insoluble, sphingolipid- and cholesterol-enriched membrane microdomains that sort and concentrate signaling and trafficking proteins (89). Individual LRs in living, resting cells are small and unstable and are below the resolution of an optical microscope, i.e., <250–300 nm (50). However, these nanostructures can coalesce into larger, stable raft clusters (50, 69). The multimerization of native rafts and their associated proteins generates adhesion complexes (31, 33) that function as signaling and trafficking platforms (89). LRs resist solubilization by nonionic detergents at low temperatures and, because of their low buoyant density, can be isolated by density gradient ultracentrifugation.
There are many functional similarities between the TJ and the LR. The TJ (68) and the LR (89) participate in a host of cellular processes, e.g., membrane trafficking, protein sorting, signal transduction, and regulation of cell growth and differentiation. The TJ (99), similar to the LR (33), plays a pivotal role in the assembly of cell-cell adhesion complexes and the recruitment and remodeling of the actin cytoskeleton. LR-adhesion complexes play important roles in tissue organization and organ formation in embryonic development and in adult tissue remodeling (31, 33). They undergo cycles of assembly and disassembly of cell-cell contacts in a precise spatiotemporal pattern that fashion cell aggregates into three-dimensional tissues. When a tissue becomes fully formed and differentiated, its integrity and function are maintained by a more stable form of cell-cell adhesion and signaling complex, the TJ (99). These observations raise the possibility that the TJ adhesion complex represents a larger and more stable form of LR assembly.
Cholesterol is primarily located in cell membranes, where it is mostly localized with sphingomyelin and glycophospholipids in LRs. Prior to, and in parallel with, the development of the LR concept, an important correlation between membrane cholesterol and TJ structure and function has been recognized. The presence and the accumulation of cholesterol in TJ-forming domains, detected as cholesterol-filipin complexes, have been reported in ultrastructural studies of rat hepatocytes (24). The observation that confluence-induced junction complex formation in endothelial cells is paralleled by a dramatic increase in membrane cholesterol also supports the notion that TJ assembly is associated with cholesterol-rich LR mobilization (11).
Experimental modulation in cell membrane cholesterol by methyl-β-cyclodextrin (MβCD) in MDCK monolayers exhibited biphasic changes in TER (25). The early rise in TER was not associated with changes in the staining pattern for occludin and ZO-1. Ultrastructurally, the number of TJ strands and the dimensions of the intercellular space remained unchanged, although the number of TJ particles in the E fracture face was significantly higher in the MβCD-treated monolayers. The late fall in TER was associated with increased mannitol flux, reduced TJ staining for occludin and ZO-1, actin redistribution, and a reduction in the number of TJ strands in freeze-fracture replicas. The early rise in TER was attributed to a change in the interaction between TJ particles and the underlying cytoskeletal elements and the late fall in TER to the disassembly of the TJ network. An alternate interpretation of these observations is that cholesterol depletion and associated changes in the lipid environment can first modify TJ protein function in the absence of overt structural changes, leading to an increase in TER (see Lipid Complements Protein in TJ Signaling and Trafficking). More sustained cholesterol depletion culminated in structural disruption of the TJ LR, TER reduction, and loss of barrier function. Indeed, in a more recent study from the same laboratory (64), the initial, transient rise in TER with membrane cholesterol extraction was attributed to changes in phosphorylation of occludin and/or other TJ proteins, thus supporting the thesis that cholesterol depletion and associated changes in the lipid environment can modify TJ structure and function.
Nusrat and associates (80), on the basis of observations in T84 intestinal epithelial cells, first proposed that the TJ is LR membrane microdomains. We (56) and others (64) confirmed the localization of TJ proteins in LR preparations from a different epithelium, MDCK II monolayers. In addition, we have provided direct visualization of LRs in the TJ (Fig. 6 ). LRs were visualized using an Alexa Fluor dye conjugate of CT-B, which binds to the pentasaccharide chain of the plasma membrane ganglioside GM1, an endogenous LR marker (42). Lambert and associates (52, 53), using the Caco-2 cell line, also confirmed that cholesterol depletion with MβCD led to displacement of occludin, claudin-3, claudin-4, claudin-7, and JAM-A out of the detergent-resistant LRs, along with increases in TJ permeability. Using neutral-pH, detergent-free fractionation of LRs from rat cerebral microvessels, McCaffrey et al. (70) confirmed the association of occludin, claudin-5, and ZO-1 with these plasma membrane domains. They further demonstrated that, in their preparations, which are more reflective of the native TJ in vivo, occludin and claudin-5 are incorporated into the TJ as preformed homodimers. In retrospect, the early observation that TJ strands were resistant to detergent extraction (93, 94) may have provided the first clue to the LR nature of the TJ complex.
Fig. 6.
TJ protein claudin-2 colocalizes with lipid raft marker GM1. An immediately preconfluent MDCK II monolayer was triple-stained for claudin-2 (A, red), GM1 (B, green), and the nucleus (C, blue). Merged image (D) shows TJ colocalization of claudin-2 and GM1 (yellow). Differences in staining intensity of the 3 labels account for nuclear staining in merged image (D), which assumed a gold, rather than a purplish, hue, as in Fig. 4D. [See Lee et al. (56) for discussion of nuclear staining of claudin-2 and GM1.] Magnification ×1,500. [From Lee et al. (56).]
More recent studies have implied that the TJ is the destination for LRs from other plasma membrane domains (12) and the pathway through which LRs translocate from one cell to another in confluent epithelia (51). The first study (12) demonstrated that transepithelial group B coxsackievirus transmission is initiated through viral association with decay-accelerating factor (DAF) in the apical membrane. DAF and other GPI-anchored membrane proteins are known to concentrate in LRs. These DAF LRs, in association with the infecting virus, are then translocated into the TJ, where the virus interacts with coxsackievirus and adenovirus receptor (CAR, a recognized TJ protein), culminating in its cellular entry and the release of viral RNA. In the second study (51), LR CT-B bound to extracellularly facing ganglioside GM1 in one cell was shown to translocate to contiguous cells. This transfer was most likely mediated through the TJ, since it was inhibited by pretreatment with poly-l-lysine and polyethylenimine. These elegant studies strongly support the thesis that LRs congregate in, and traffic through, the TJ complex.
Wosik et al. (113) reported that angiotensin II activation of type 1 angiotensin receptors restricts passage of tracers across the TJ in cultured human blood-brain barrier endothelial cells through the phosphorylation of occludin and its mobilization to LR membrane microdomains. This observation suggests that TJ function is related to the presence of phosphorylated occludin in an LR environment. The disruption of epithelial barrier function by the toxins of Clostridium difficile, the etiological agent of pseudomembranous colitis, has been attributed to the parallel redistribution of TJ proteins out of membrane raft microdomains (81). These toxins are known to inactivate the Rho family of GTPases (10), suggesting a role of Rho proteins in LR localization of TJ proteins. In relation to inflammatory bowel disease, interferon-γ and tumor necrosis factor-α have been shown to disrupt TJs in the intestinal T84 cell line as a result of alterations in the lipid composition and fatty acyl substitutions of phospholipids in lipid microdomains (58).
Lipid Complements Protein in TJ Signaling and Trafficking
In the context of the TJ as an LR assembly discussed above, the known functional role of the TJ as a signaling and trafficking complex is hardly surprising. Inasmuch as there is persuasive evidence supporting LRs in the mediation and regulation of cell signaling through facilitation of lipid-protein/protein-protein interactions (74), the time has come to incorporate lipids in elucidating the structure and function of the TJ.
Lipids are known to serve as ligands that activate signal transduction pathways, as mediators of these signaling pathways, and as the substrates of lipid kinases and lipid phosphatases (22). The protein-focused TJ model (6, 29, 96) can be rendered more comprehensive with the additional consideration of lipids. A lipid-protein hybrid TJ resembles a cell membrane, acting not only as a regulated barrier, but also as a platform for lipid-protein interactions, which serve a variety of important cellular functions. In addition to participation as components of signal transduction pathways, lipids can serve as anchorage for proteins and as sources for cleavage products, which, in turn, serve as ligands and substrates in multiple additional signaling and trafficking cascades. Some examples of the importance of lipid-protein interaction in TJ structure and function are emerging.
A direct lipid modification of TJ proteins is supported by the report that the claudins are palmitoylated (64, 105). In the case of claudin-14, inhibition of palmitoylation reduces its localization to, and association with, detergent-resistant membrane domains, paralleled by a decrease in TJ barrier function (105). Palmitoylation, i.e., the addition of palmitate to a Cys residue, is known to influence protein trafficking, stability, and aggregation (61). In this context, palmitoylation is known to increase the affinity of integral membrane proteins for LRs (89). Parenthetically, occludin is not palmitoylated (64), raising the possibility that it may undergo alternative posttranslational lipid modifications.
Stankewich et al. (92) reported another example of lipid modification of protein function. Lovastatin induced a 30% reduction in cholesterol in MDCK cells and led to a more rapid development and the attainment of a higher electrical resistance in Ca2+-induced TJ assembly. This was attributed to a reduction in the synthesis of isoprenoids and levels of prenylated proteins. More recently, a statin-mediated reduction in TJ permeability across primary cultures of endothelial cells has been reported (38). No change in membrane expression and localization of occludin, JAM-A, vascular endothelial cadherin, ZO-1, and ZO-2 was observed, and the decrease in blood-brain barrier permeability was also attributed to the abrogation of the isoprenylation pathways and the associated modulation of the activity of small GTPases. RhoA- and Rac-dependent phosphorylation of TJ proteins is known to regulate TJ barrier function (5, 37).
A number of studies have reported modulation of TJ barrier function by lipids and fatty acids. Exposure of T84 human colorectal cancer cell monolayers to alkylphospholipids, synthetic analogs of lysophosphatidylcholine, led to a rapid, reversible, and dose-dependent reduction in resistance and increases in paracellular permeability to [3H]mannitol (57). Phospholipid derivatives (62) and ω-3 polyunsaturated fatty acids (19) as enhancers of paracellular permeability have also been reported in Caco-2 monolayers. In another study, oxidized l-α-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine, a component of minimally modified LDL, increased the diffusive flux of 10-kDa dextran in a dose-dependent manner across bovine aortic endothelial cells (16). Treatment of human aortic endothelial cells with triglyceride-rich lipoprotein lipolytic products has also been shown to decrease TER and TJ barrier function (21).
On the other hand, several polyunsaturated fatty acids, such as γ-linolenic and eicosapentaenoic acids, have been found to increase TER and reduce paracellular permeability in endothelial monolayers (43). γ-Linolenic acid also increased TER and reduced paracellular permeability in human breast cancer cell lines (67). In experimental colitis, n-3 polyunsaturated fatty acids attenuated TJ disruption and improved histological score (59). The apparent discordant effect of some fatty acids on TJ permeability in different studies may represent variation in the effect of changes in the lipid environment on protein function in different tissues or under different experimental conditions.
Lipids, as an important functional component of the TJ, are again highlighted in a recent study (7). Of the seven groups of lipids studied, four groups, sphingosines, alkylglycosides, oxidized lipids, and ether lipids, exhibited the ability to reduce TER by up to 95%, in association with increases in transepithelial permeation, at noncytotoxic concentrations. Immunofluorescent staining of ZO-1, occludin, and claudin-4 suggests that these functional modulations occurred in the absence of structural changes in TJ morphology.
SPECULATIVE LIPID-PROTEIN HYBRID TJ MODELS
Although there is good evidence supporting the presence and the role of lipids in the TJ, there is little information on the structural relationship between the lipids and the proteins in this junction complex. Central to the hemifusion model is the implication that there is an intralipid aqueous compartment (IAC) between the two fused exoplasmic leaflets (Fig. 1, B and C). This compartment consists of an assembly of inverted micelles (particles) or a network of HII water cylinders (strands) or a combination of both. It may be conjectured that proteinaceous conductive pathways, e.g., channels and pumps, can link the intercellular space with this IAC (Fig. 1B, 1), intracellular space with IAC (Fig. 1B, 2), and IAC with IAC (Fig. 1B, 3), in each case linking two aqueous compartments across the equivalent of a lipid bilayer and, together, forming an intercellular apical-basolateral aqueous conduit. DiBona (17), after collating a large series of data examining TJ perturbations mediated by changes in osmotic or electrical gradients, concluded that the primary components of the TJ structure in freeze-fracture studies were lipidic in nature and that the junction complex functioned as a series of aqueous compartments.
The ionic composition of the fluid in these relatively small IACs can be readily modified by the activities of protein channels and pumps, with little or no change in their physical dimension or configuration, thereby altering ionic conductivity without affecting passage of uncharged molecules across the TJ. This can provide an alternate explanation for the observations on the unanticipated opposite changes in TER and paracellular permeability of uncharged molecules (3, 34, 71). Structurally, the protein components of the TJ have, in addition, been proposed to provide stability to the lipid infrastructure (82).
Figure 1C represents another conjecture for communication between the apical and basolateral compartments of the lateral intercellular space across the TJ. In this case, trans-TJ transport is mediated through processes similar to a coupled “endocytosis” (Fig. 1C, dashed arrow) and “exocytosis” (Fig. 1C, solid arrow). Tang (96) highlighted ultrastructural evidence for “vesicles fusing/budding at the tight junction membrane domain, secreted substances encased between the TJ kisses, endocytosis of TJ double membranes.” The two conjectures need not be mutually exclusive and are only examples of a myriad of other possibilities. We anticipate, with certainty, many surprises as the lipid-protein hybrid model evolves.
GRANTS
This work was supported in part by National Institutes of Health Grant 1RO1 DK/HD-51948, the Department of Veterans Affairs Merit Review Board, and the UCLA Council on Research/Faculty Research Grant Program (2006–2007).
REFERENCES
- 1.Anderson JM Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci 16: 126–130, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ayala-Sanmartin J, Henry JP, Pradel LA. Cholesterol regulates membrane binding and aggregation by annexin 2 at submicromolar Ca2+ concentration. Biochim Biophys Acta 1510: 18–28, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci 63: 505–514, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol 287: C327–C335, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cereijido M, Contreras RG, Flores-Benitez D, Flores-Maldonado C, Larre I, Ruiz A, Shoshani L. New diseases derived or associated with the tight junction. Arch Med Res 38: 465–478, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chen-Quay SC, Eiting KT, Li AW, Lamharzi N, Quay SC. Identification of tight junction modulating lipids. J Pharm Sci. In press. [DOI] [PubMed]
- 8.Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell 123: 375–382, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chernomordik LV, Zimmerberg J, Kozlov MM. Membranes of the world unite! J Cell Biol 175: 201–207, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciesla WP, Bobak DA. Clostridium difficile toxins A and B are cation-dependent UDP-glucose hydrolases with differing catalytic activities. J Biol Chem 273: 16021–16026, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Corvera S, DiBonaventura C, Shpetner HS. Cell confluence-dependent remodeling of endothelial membranes mediated by cholesterol. J Biol Chem 275: 31414–31421, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124: 119–131, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Cullis PR, de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta 559: 399–420, 1979. [DOI] [PubMed] [Google Scholar]
- 14.Cullis PR, Hope MJ. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature 271: 672–674, 1978. [DOI] [PubMed] [Google Scholar]
- 15.Davis SJ, van der Merwe PA. The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today 17: 177–187, 1996. [DOI] [PubMed] [Google Scholar]
- 16.DeMaio L, Rouhanizadeh M, Reddy S, Sevanian A, Hwang J, Hsiai TK. Oxidized phospholipids mediate occludin expression and phosphorylation in vascular endothelial cells. Am J Physiol Heart Circ Physiol 290: H674–H683, 2006. [DOI] [PubMed] [Google Scholar]
- 17.DiBona DR Functional analysis of tight junction organization. Pflügers Arch 405 Suppl 1: S59–S66, 1985. [DOI] [PubMed] [Google Scholar]
- 18.Drust DS, Creutz CE. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature 331: 88–91, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Duraisamy Y, Lambert D, O'Neill CA, Padfield PJ. Differential incorporation of docosahexaenoic acid into distinct cholesterol-rich membrane raft domains. Biochem Biophys Res Commun 360: 885–890, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Earp LJ, Delos SE, Park HE, White JM. The many mechanisms of viral membrane fusion proteins. Curr Top Microbiol Immunol 285: 25–66, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am J Physiol Heart Circ Physiol 292: H2745–H2753, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Eyster KM The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ 31: 5–16, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 17: 375–412, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feltkamp CA, Van der Waerden AW. Junction formation between cultured normal rat hepatocytes. An ultrastructural study on the presence of cholesterol and the structure of developing tight-junction strands. J Cell Sci 63: 271–286, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Francis SA, Kelly JM, McCormack J, Rogers RA, Lai J, Schneeberger EE, Lynch RD. Rapid reduction of MDCK cell cholesterol by methyl-β-cyclodextrin alters steady state transepithelial electrical resistance. Eur J Cell Biol 78: 473–484, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143: 391–401, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev 82: 331–371, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778: 729–756, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Grebenkamper K, Galla HJ. Translational diffusion measurements of a fluorescent phospholipid between MDCK-I cells support the lipid model of the tight junctions. Chem Phys Lipids 71: 133–143, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Gumbiner BM Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84: 345–357, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Hansen MD, Ehrlich JS, Nelson WJ. Molecular mechanism for orienting membrane and actin dynamics to nascent cell-cell contacts in epithelial cells. J Biol Chem 277: 45371–45376, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris TJ, Siu CH. Reciprocal raft-receptor interactions and the assembly of adhesion complexes. Bioessays 24: 996–1003, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa H, Fujita H, Katoh H, Aoki J, Nakamura K, Ichikawa A, Negishi M. Opposite regulation of transepithelial electrical resistance and paracellular permeability by Rho in Madin-Darby canine kidney cells. J Biol Chem 274: 20982–20988, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Hein M, Madefessel C, Haag B, Teichmann K, Post A, Galla HJ. Implications of a non-lamellar lipid phase for the tight junction stability. II. Reversible modulation of transepithelial resistance in high and low resistance MDCK-cells by basic amino acids, Ca2+, protamine and protons. Chem Phys Lipids 63: 223–233, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Hein M, Post A, Galla HJ. Implications of a non-lamellar lipid phase for the tight junction stability. I. Influence of basic amino acids, pH and protamine on the bilayer-hexagonal II phase behaviour of PS-containing PE membranes. Chem Phys Lipids 63: 213–221, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins AM, Li D, Mrsny RJ, Walsh SV, Nusrat A. Modulation of tight junction function by G protein-coupled events. Adv Drug Delivery Res 41: 329–340, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Ifergan I, Wosik K, Cayrol R, Kebir H, Auger C, Bernard M, Bouthillier A, Moumdjian R, Duquette P, Prat A. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol 60: 45–55, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Ikari A, Hirai N, Shiroma M, Harada H, Sakai H, Hayashi H, Suzuki Y, Degawa M, Takagi K. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J Biol Chem 279: 54826–54832, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem 68: 863–911, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol 147: 447–461, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang WG, Bryce RP, Horrobin DF, Mansel RE. Regulation of tight junction permeability and occludin expression by polyunsaturated fatty acids. Biochem Biophys Res Commun 244: 414–420, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Kachar B, Reese TS. Evidence for the lipidic nature of tight junction strands. Nature 296: 464–466, 1982. [DOI] [PubMed] [Google Scholar]
- 45.Kan FW Cytochemical evidence for the presence of phospholipids in epithelial tight junction strands. J Histochem Cytochem 41: 649–656, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Kassam G, Choi KS, Ghuman J, Kang HM, Fitzpatrick SL, Zackson T, Zackson S, Toba M, Shinomiya A, Waisman DM. The role of annexin II tetramer in the activation of plasminogen. J Biol Chem 273: 4790–4799, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Kubista H, Sacre S, Moss SE. Annexins and membrane fusion. In: Subcellular Biochemistry: Fusion of Biological Membranes and Related Problems, edited by Hilderson and Fuller. New York: Kluwer Academic/Plenum, 2000, p. 73–131. [DOI] [PubMed]
- 48.Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, Tsukita S. Ca2+-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr Biol 9: 1035–1038, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Kunzelmann-Marche C, Freyssinet JM, Martinez MC. Loss of plasma membrane phospholipid asymmetry requires raft integrity. Role of transient receptor potential channels and ERK pathway. J Biol Chem 277: 19876–19881, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Kusumi A, Koyama-Honda I, Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic 5: 213–230, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Laffafian I, Hallett M. Lipid-protein cargo transfer: a mode of direct cell-to-cell communication for lipids and their associated proteins. J Cell Physiol 210: 336–342, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Lambert D, O'Neill CA, Padfield PJ. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem J 387: 553–560, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambert D, O'Neill CA, Padfield PJ. Methyl-β-cyclodextrin increases permeability of Caco-2 cell monolayers by displacing specific claudins from cholesterol rich domains associated with tight junctions. Cell Physiol Biochem 20: 495–506, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Lambert O, Gerke V, Bader MF, Porte F, Brisson A. Structural analysis of junctions formed between lipid membranes and several annexins by cryo-electron microscopy. J Mol Biol 272: 42–55, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Lee DBN, Jamgotchian N, Allen SG, Kan FWK, Hale IL. Annexin A2 heterotetramer: role in tight junction assembly. Am J Physiol Renal Physiol 287: F481–F491, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Lee DBN, Jamgotchian N, Allen SG, Ward HJ. Tight junction and lipid rafts. Structural similarities and possible role of annexin A2 heterotetramer calcium binding. Proteins 2: 44–51, 2007. [Google Scholar]
- 57.Leroy A, de Bruyne GK, Oomen LC, Mareel MM. Alkylphospholipids reversibly open epithelial tight junctions. Anticancer Res 23: 27–32, 2003. [PubMed] [Google Scholar]
- 58.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Interferon-γ and tumor necrosis factor-α disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol 126: 67–80, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Li Q, Zhang Q, Zhang M, Wang C, Zhu Z, Li N, Li J. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS Lett 275: 411–420, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Liemann S, Huber R. Three-dimensional structure of annexins. Cell Mol Life Sci 53: 516–521, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol 8: 74–84, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Liu DZ, Morris-Natschke SL, Kucera LS, Ishaq KS, Thakker DR. Structure-activity relationships for enhancement of paracellular permeability by 2-alkoxy-3-alkylamidopropylphosphocholines across Caco-2 cell monolayers. J Pharm Sci 88: 1169–1174, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Liu L, Fisher AB, Zimmerman UJ. Lung annexin II promotes fusion of isolated lamellar bodies with liposomes. Biochim Biophys Acta 1259: 166–172, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Lynch RD, Francis SA, McCarthy KM, Casas E, Thiele C, Schneeberger EE. Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occludin. Exp Cell Res 313: 2597–2610, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrink SJ, Mark AE. Molecular view of hexagonal phase formation in phospholipid membranes. Biophys J 87: 3894–3900, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142: 117–127, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin TA, Das T, Mansel RE, Jiang WG. Enhanced tight junction function in human breast cancer cells by antioxidant, selenium and polyunsaturated lipid. J Cell Biochem 101: 155–166, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4: 225–236, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic 5: 231–240, 2004. [DOI] [PubMed] [Google Scholar]
- 70.McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. In press. [DOI] [PubMed]
- 71.McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Skare IB, Lynch RD, Schneeberger EE. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci 113: 3387–3398, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC Jr, McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403: 785–789, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Miller RG Do “lipidic particles” represent intermembrane attachment sites? Nature 287: 166–167, 1980. [DOI] [PubMed] [Google Scholar]
- 74.Mishra S, Joshi PG. Lipid raft heterogeneity: an enigma. J Neurochem 103 Suppl 1: 135–142, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 96: 511–516, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, Hunziker W. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet 73: 1293–1301, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller WA Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 24: 327–334, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Nakata T, Sobue K, Hirokawa N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J Cell Biol 110: 13–25, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nichols GE, Borgman CA, Young WW Jr. On tight junction structure: Forssman glycolipid does not flow between MDCK cells in an intact epithelial monolayer. Biochem Biophys Res Commun 138: 1163–1169, 1986. [DOI] [PubMed] [Google Scholar]
- 80.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci 113: 1771–1781, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 69: 1329–1336, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pinto da Silva P, Kachar B. On tight-junction structure. Cell 28: 441–450, 1982. [DOI] [PubMed] [Google Scholar]
- 83.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 22: 146–158, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Podbilewicz B, Leikina E, Sapir A, Valansi C, Suissa M, Shemer G, Chernomordik LV. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev Cell 11: 471–481, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137: 1393–1401, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci USA 100: 3971–3976, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Senda T, Okabe T, Matsuda M, Fujita H. Quick-freeze, deep-etch visualization of exocytosis in anterior pituitary secretory cells: localization and possible roles of actin and annexin II. Cell Tissue Res 277: 51–60, 1994. [DOI] [PubMed] [Google Scholar]
- 88.Senda T, Yamashita K, Okabe T, Sugimoto N, Matsuda M. Intergranular bridges in the anterior pituitary cell and their possible involvement in Ca2+-induced granule-granule fusion. Cell Tissue Res 292: 513–519, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry 27: 6197–6202, 1988. [DOI] [PubMed] [Google Scholar]
- 91.Staehelin LA Structure and function of intercellular junctions. Int Rev Cytol 39: 191–283, 1974. [DOI] [PubMed] [Google Scholar]
- 92.Stankewich MC, Francis SA, Vu QU, Schneeberger EE, Lynch RD. Alterations in cell cholesterol content modulate Ca2+-induced tight junction assembly by MDCK cells. Lipids 31: 817–828, 1996. [DOI] [PubMed] [Google Scholar]
- 93.Stevenson BR, Anderson JM, Bullivant S. The epithelial tight junction: structure, function and preliminary biochemical characterization. Mol Cell Biochem 83: 129–145, 1988. [DOI] [PubMed] [Google Scholar]
- 94.Stevenson BR, Goodenough DA. Zonulae occludentes in junctional complex-enriched fractions from mouse liver: preliminary morphological and biochemical characterization. J Cell Biol 98: 1209–1221, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swairjo MA, Concha NO, Kaetzel MA, Dedman JR, Seaton BA. Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat Struct Biol 2: 968–974, 1995. [DOI] [PubMed] [Google Scholar]
- 96.Tang VW Proteomic and bioinformatic analysis of epithelial tight junction reveals an unexpected cluster of synaptic molecules. Biol Direct 1: 37, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Top D, de Antueno R, Salsman J, Corcoran J, Mader J, Hoskin D, Touhami A, Jericho MH, Duncan R. Liposome reconstitution of a minimal protein-mediated membrane fusion machine. EMBO J 24: 2980–2988, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol 9: 268–273, 1999. [DOI] [PubMed] [Google Scholar]
- 99.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2: 285–293, 2001. [DOI] [PubMed] [Google Scholar]
- 100.Turin L, Behe P, Plonsky I, Dunina-Barkovskaya A. Hydrophobic ion transfer between membranes of adjacent hepatocytes: a possible probe of tight junction structure. Proc Natl Acad Sci USA 88: 9365–9369, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126: 741–754, 2006. [DOI] [PubMed] [Google Scholar]
- 102.van der Merwe PA, Barclay AN. Transient intercellular adhesion: the importance of weak protein-protein interactions. Trends Biochem Sci 19: 354–358, 1994. [DOI] [PubMed] [Google Scholar]
- 103.van Genderen IL, van Meer G, Slot JW, Geuze HJ, Voorhout WF. Subcellular localization of Forssman glycolipid in epithelial MDCK cells by immuno-electronmicroscopy after freeze-substitution. J Cell Biol 115: 1009–1019, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci 110: 1113–1121, 1997. [DOI] [PubMed] [Google Scholar]
- 105.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci 118: 1427–1436, 2005. [DOI] [PubMed] [Google Scholar]
- 106.van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature 322: 639–641, 1986. [DOI] [PubMed] [Google Scholar]
- 107.van Oss CJ Energetics of cell-cell and cell-biopolymer interactions. Cell Biophys 14: 1–16, 1989. [DOI] [PubMed] [Google Scholar]
- 108.Van Venetie R, Verkleij AJ. Analysis of the hexagonal II phase and its relations to lipidic particles and the lamellar phase. A freeze-fracture study. Biochim Biophys Acta 645: 262–269, 1981. [DOI] [PubMed] [Google Scholar]
- 109.Verkleij AJ, Momvers C, Leunissen-Bijvelt J, Ververgaert PH. Lipidic intramembranous particles. Nature 279: 162–163, 1979. [DOI] [PubMed] [Google Scholar]
- 110.Waisman DM Annexin II tetramer: structure and function. Mol Cell Biochem 149–150: 301–322, 1995. [DOI] [PubMed]
- 111.Wang P, Chintagari NR, Gou D, Su L, Liu L. Physical and functional interactions of SNAP-23 with annexin A2. Am J Respir Cell Mol Biol 37: 467–476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong JL, Koppel DE, Cowan AE, Wessel GM. Membrane hemifusion is a stable intermediate of exocytosis. Dev Cell 12: 653–659, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A. Angiotensin II controls occludin function and is required for blood-brain barrier maintenance: relevance to multiple sclerosis. J Neurosci 27: 9032–9042, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamada A, Irie K, Hirota T, Ooshio T, Fukuhara A, Takai Y. Involvement of the annexin II-S100A10 complex in the formation of E-cadherin-based adherens junctions in MDCK cells. J Biol Chem 280: 6016–6027, 2005. [DOI] [PubMed] [Google Scholar]
- 115.Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta 1778: 709–716, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zahraoui A, Louvard D, Galli T. Tight junction, a platform for trafficking and signaling protein complexes. J Cell Biol 151: F31–F36, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zampighi GA, Zampighi LM, Fain N, Lanzavecchia S, Simon SA, Wright EM. Conical electron tomography of a chemical synapse: vesicles docked to the active zone are hemi-fused. Biophys J 91: 2910–2918, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeuschner D, Stoorvogel W, Gerke V. Association of annexin 2 with recycling endosomes requires either calcium- or cholesterol-stabilized membrane domains. Eur J Cell Biol 80: 499–507, 2001. [DOI] [PubMed] [Google Scholar]