Abstract
Acute kidney injury induced by renal ischemia-reperfusion (I/R) compromises microvascular density and predisposes to chronic kidney disease (CKD) and sodium-dependent hypertension. VEGF-121 was administered to rats fed a standard (0.4%) sodium diet at various times following recovery from I/R injury for up to 35 days. VEGF-121 had no effect on the initial loss of renal function, as indicated by serum creatinine levels measured 24 h after injury. Serum creatinine levels declined thereafter, indicative of renal repair. Rats were then switched to an elevated (4.0%) sodium diet for an additional 28 days to induce CKD. The 4.0% sodium diet enhanced renal hypertrophy, interstitial volume, albuminuria, and cardiac hypertrophy relative to postischemic animals maintained on the 0.4% sodium diet. Administration of VEGF-121 from day 0 to 14, day 0 to 35, or day 3 to 35 after I/R suppressed the effects of sodium diet on CKD development, while delayed administration of VEGF-121 from day 21 to 35 had no effect. Endothelial nitric oxide synthase protein levels were upregulated in postischemic animals, and this effect was significantly increased by the 4.0% sodium diet but was not influenced by prior treatment with VEGF. Conversely, microvascular density was preserved in postischemic animals treated with VEGF-121 relative to vehicle-treated postischemic animals. These data suggest that early, but not delayed, treatment with VEGF-121 can preserve vascular structure after ischemia and influence chronic renal function in response to elevated sodium intake.
Keywords: ischemia, interstitial fibrosis, peritubular capillaries, salt sensitivity
renal ischemia-reperfusion (I/R) results in acute kidney injury (AKI), characterized by substantial damage to the renal tubular system and reduced glomerular filtration. Although regeneration of the renal tubular system contributes to the recovery from AKI following I/R, overall recovery is not complete, and a predisposition to secondary kidney disease remains (2, 3). Interestingly, although renal blood flow typically returns to sham control levels, microvascular density in the kidney is significantly compromised and is associated with exacerbated hypoxia, potentially contributing to the progressive development of interstitial fibrosis (4, 5).
In a recent report from our laboratory, it was also demonstrated that I/R injury results in significant increases in blood pressure in rats fed an elevated-sodium diet after resolution of the initial insult (26). In addition, development of secondary chronic kidney disease (CKD) was dramatically augmented in after I/R in rats fed an elevated-sodium diet. The nature of the salt sensitivity in animals after I/R may relate, in part, to the loss of renal microvascular structure, which has been reported in several models of interstitial disease associated with hypertension (11–13, 16, 17, 21).
The reduction of capillary density after I/R suggests that endogenous repair responses in the postischemic kidney are insufficient to maintain vascular structure. Although a number of potential factors with negative trophic effects are elevated after I/R injury, inhibition of VEGF expression has also been reported during the 1st wk of the normal recovery phase (6). The loss of endogenous VEGF during a potentially critical window of the early recovery response suggests that supplementation with exogenous VEGF may preserve microvascular density after I/R injury. Therefore, the following study evaluated the effects of VEGF-121 supplementation during recovery from renal I/R, the potential of VEGF-121 to maintain renal vascular structure, and the ability of VEGF-121 to preserve chronic renal function after the initial resolution of the insult.
METHODS
Animals.
Animal care before and during the experimental procedures was conducted in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols had received prior approval by the Institutional Animal Care and Use Committees at Indiana University.
Male Sprague-Dawley rats (∼250 g body wt) were housed in pairs in standard shoe-box cages and exposed to a 12:12-h light-dark cycle. Animals were acclimated to a laboratory diet (AIN 76A, Dyets, Bethlehem, PA) with a defined 0.4% sodium content. Some of the animals were subsequently fed the same diet with a defined 4.0% sodium content. Food and water were available ad libitum.
Acute renal failure was induced as follows. Rats were anesthetized with ketamine (100 mg/kg ip) and pentobarbital sodium (25 mg/kg ip) and placed on a heated surgical table. After a midline incision, the blood supply to the kidneys was interrupted by application of microvascular clamps on the renal pedicles of both kidneys (26). After 40 min, the clamps were released, and reperfusion was visualized. Another group of rats was subjected to sham surgery: the kidneys were exposed but were not touched.
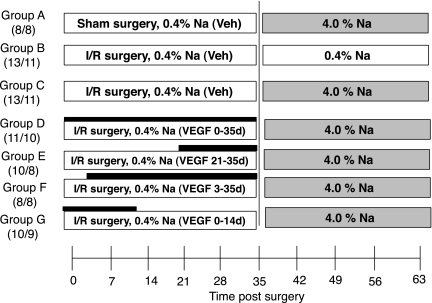
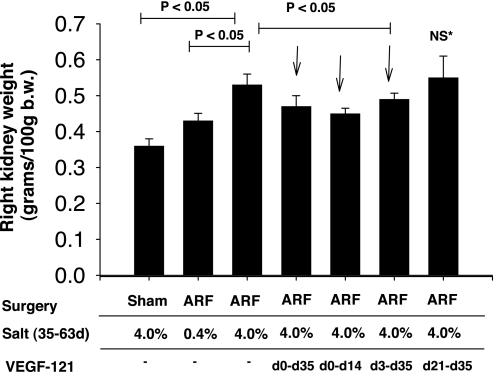
The study design, which is outlined in Fig. 1 , is based on the paradigm established in our previous report characterizing the predisposition to salt-sensitive hypertension after recovery from I/R (26). All animals were allowed to recover for 35 days (i.e., 5 wk after I/R or sham surgery), during which they were fed a 0.4% sodium diet and then allowed to recover for an additional 28 days (total 63 days after surgery), during which they were fed a 0.4% sodium diet or an elevated (4.0%) sodium diet. VEGF-121 was the generous gift of Scios (Mountain View, CA). Four groups of animals were injected with VEGF-121 (100 μg·kg−1·day−1 sc, in 2 daily injections). The low rate of attrition indicated by the number of animals that completed the protocol is consistent with this injury model and was not noticeably different between groups.
Fig. 1.
Schema outlining experimental groups described in Figs. 2, 4–8, 10, and 11. All animals were initially acclimated to a standard sodium (0.4% Na) diet for 1–2 wk before sham surgery or 40 min of bilateral renal ischemia [ischemia-reperfusion (I/R)]. During the first 5 wk (0–35 days) of recovery, animals were fed 0.4% sodium diet; during recovery weeks 5–9 (35–63 days), animals were fed 0.4% Na diet or an elevated-sodium (4.0% Na) diet. Groups A, B, and C were treated with saline vehicle (Veh) twice daily following surgery. Groups D, E, F, and G were treated with VEGF-121 (solid horizontal bar): 2 groups received VEGF-121 immediately after reperfusion by subcutaneous injection and twice daily thereafter for up to 14 or 35 days after I/R with a total dose of 100 μg·kg−1·day−1, and 2 groups received the same dose of VEGF-121 beginning on day 3 or day 21. Number of animals in each group is shown in parentheses: the first number is the number of animals that began the protocol, and the second number is the number of animals that completed the protocol.
In addition to the animals indicated in Fig. 1, a second group of animals was studied to determine the effect of VEGF-121 administration on vascular density; these animals were treated as described above, except they were allowed to recover for 5 wk, during which they were fed the 0.4% Na diet only. Similarly, a third group of animals was treated identically but was allowed to recover for 3 days for determination of the potential effect of VEGF-121 on early tissue repair responses.
Measurement of renal function.
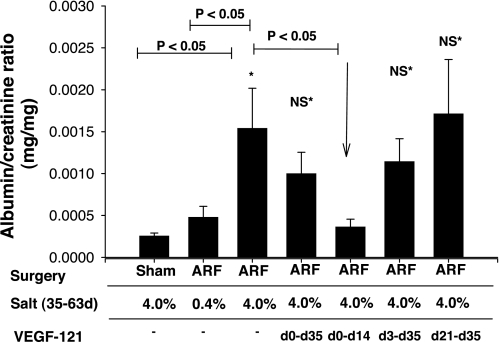
For measurement of serum creatinine, tail blood samples (<0.5 ml) were collected into heparinized tubes, and plasma was obtained by centrifugation. Serum creatinine values were determined using the Creatinine II analyzer (Beckman Coulter). Urine was obtained from 24-h collections at 1, 5, 6, 7, 8, and 9 wk after injury. Volume from metabolic cages was determined gravimetrically. Urine albumin content was measured using the Albumin Blue 580 fluorescence method (Fluka), as previously described (5). The pattern of albumin excretion between groups was similar at all time points after exposure to the 4.0% sodium diet at 5 wk (not shown); therefore, for clarity, the data in Fig. 4 are from the final collection point.
Fig. 4.
Effect of bilateral I/R injury (ARF), VEGF-121, and dietary salt on development of albuminuria. Urinary albumin and creatinine levels were obtained from 24-h collections. Data are shown at day 63, just before animals were killed. Values are means ± SE. *P < 0.05 vs. sham. VEGF-121-treated groups were compared with ARF 4.0% group. P values were determined by ANOVA and Student-Newman-Keuls post hoc test.
Assessment of interstitial cellularity.
At 63 days after I/R, rats were anesthetized with ketamine HCl (60 mg/kg), xylazine (6 mg/kg), and acepromazine maleate (0.9 mg/kg). The kidneys were quickly removed and cut longitudinally. Some kidney pieces were fixed by immersion in 10% formalin and processed in paraffin for histological analysis. Other pieces were fixed in 2% paraformaldehyde and processed on a cryostat, and desiccated pieces were stored at −20°C for immunofluorescence analysis (see below). Other pieces were snap frozen in liquid nitrogen, stored at −80°C, and subsequently used for Western blot analysis (see below).
For morphometric measurements, paraffin-embedded sections were stained with picrosirius red (31). The extent of interstitial expansion was determined on photomicrographs of these sections, as previously described (27). A minimum of five representative photomicrographs were obtained using standard light microscopy (Nikon Spot camera system) from each zone of the kidney of each animal. Computer-aided image analysis (Image J) was used to apply an arbitrary array of points over the photomicrographs (704 points per microscopic field), and points that lay over cellular and fibrotic structures of the interstitium were counted. Data are presented as the average number of points per microscopic field. Interstitial expansion was also characterized by immunohistochemical detection of the macrophage marker ED-1 (Serotech) and double immunohistochemical detection of α-smooth muscle actin (Zymed) and S100A4 (Dako); these procedures are described in detail elsewhere (23, 26).
Localization of renal microvessels was carried out by staining with antibodies against platelet endothelial adhesion molecule (PECAM)/CD31 (SEW31, a generous gift of Dr. Peter Newman, Blood Center of Southeast Wisconsin) and cablin, a marker of the basal lamina of vascular cells (8) (a generous gift of Dr. Robert Bacallao, Indiana University). Immunofluorescence analysis was carried out after nonspecific sites were blocked with 2% BSA and 0.2% gelatin (Sigma) in 0.1 M phosphate-buffered saline, incubated overnight at 4°C in primary antibodies (1:1,000 dilution of serum), and detected using anti-rabbit Cy3 (Molecular Probes).
Microvessel density was analyzed using a Nikon microscope equipped with mercury epifluorescence, and images were obtained using a Nikon Spot camera and Spot Basic software (Nikon). Microvessel density was determined using techniques analogous to those utilized in our previous studies (5). A minimum of five random images from the cortex and outer medulla of each kidney were analyzed using Image J software; images were overlaid with a 12 × 12 grid, and vessel density was determined from the number of positively stained vessels that intersected the overlaid gridlines (5). Data were normalized and expressed as mean percent change relative to sham-operated control animals.
For Western blot analysis, frozen kidney samples were homogenized in 50 mM Tris·HCl (pH 8.0) containing 10% DMSO and 2 mM PMSF using a Teflon-and-glass homogenizer. The homogenate was cleared of large tissue fragments by centrifugation for 5 min at 3,000 g. Protein content was determined using a Bio-Rad protein assay as follows: 100 μg of total protein were loaded onto 10% precast polyacrylamide gels (Bio-Rad), and proteins were separated at 100 V for 90 min and transferred onto nitrocellulose sheets (Bio-Rad) at 20 V for 12 h at 4°C. After they were blocked, the membranes were incubated overnight with primary antibody against rat endothelial nitric oxide synthase (eNOS, 0.2 μg/ml; Sigma). Blots were then incubated with anti-rabbit peroxidase-conjugated secondary antibody and developed using chemiluminescent substrate (Supersignal, Pierce). Chemiluminescent signals were visualized and quantified using a FujiFilm LAS1000 image acquisition system and analysis software. For quantification, signals for bands of interest were subtracted from local background; data for each individual sample are expressed relative to the mean signal derived from the sham-operated control group.
RESULTS
To assess the effects of exogenous VEGF-121 during recovery from I/R on the development of CKD, we subjected rats acclimated to a 0.4% sodium diet to renal I/R and continued the 0.4% sodium diet for 35 days. Rats were treated with VEGF-121 at various times following the initiation of reperfusion, beginning immediately on day 0 or on day 3 or 21 (Fig. 1). On day 35, we switched some rats to the 4.0% sodium diet to induce CKD and evaluated the effects of prior VEGF-121 treatment on the development of sodium-sensitive kidney disease following recovery from I/R.
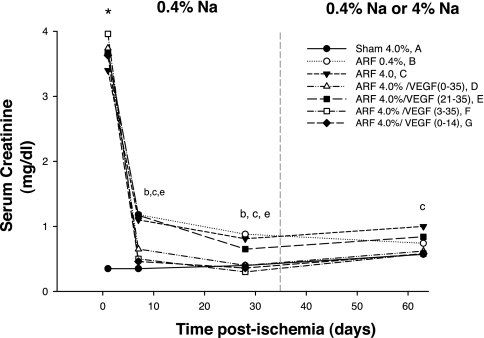
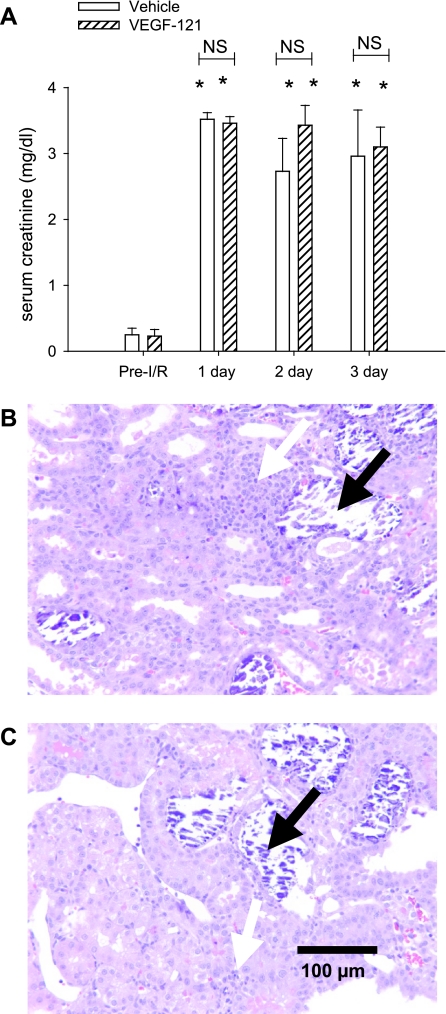
The increase in serum creatinine was similar in all groups of animals (range 3.4–3.9 mg/dl) 24 h after I/R injury, and the early injury response of the two groups of animals that were treated with VEGF-121 immediately after reperfusion (groups D and G) was not different from the response of any other group subjected to I/R injury (Fig. 2). All groups of animals subjected to I/R injury demonstrated evidence of functional recovery by days 7 and 28, as indicated by resolution of serum creatinine values; however, in contrast to some of our earlier studies (4, 26, 27), serum creatinine levels did not return completely to sham-operated control levels. Animals treated with vehicle through day 7 (groups B, C, and E) had the least efficient recovery, whereas those that were treated with VEGF-121 beginning on day 0 or day 3 (groups D, F, and G) showed more efficient recovery, with creatinine levels not statistically different from sham-operated control levels. An additional group of animals was used to examine the effects of VEGF-121 on early injury and repair processes in more detail for up to 3 days after I/R. Serum creatinine levels were not different between vehicle- and VEGF-121-treated groups at 1, 2, or 3 days after I/R. When kidney tissue morphology was evaluated at 3 days after I/R, vehicle- and VEGF-121-treated groups manifested a similar and substantial degree of tubular damage within the outer medullary region, with evidence of necrotic cell casts and inflammation (Fig. 3, B and C). These data indicate that VEGF-121 does not alter the early course of renal injury or tubular repair following I/R.
Fig. 2.
Effect of bilateral I/R injury [acute renal failure (ARF)], VEGF-121, and dietary sodium (0.4% and 4.0%) on serum creatinine. Dashed line at 35 days indicates switch of some groups to 4.0% sodium diet. Statistically significant difference (by ANOVA and Student-Newman-Keuls post hoc test) is as follows: *P < 0.05, all ARF groups vs. sham; bP < 0.05, all ARF groups vs. sham at 7 days; cP < 0.05, all ARF groups vs. sham at 28 days; eP < 0.05, all ARF groups vs. sham at 63 days.
Fig. 3.
Effect of VEGF-121 on renal function and morphology in early postischemic period. A: serum creatinine before and 1, 2, and 3 days after I/R in animals treated with vehicle or VEGF-121. Values are not significantly (NS) different at any time point. *P < 0.05 vs. pre-I/R (by Student's t-test). B and C: photomicrographs through renal outer medulla of postischemic vehicle- and VEGF-121-treated rats, respectively, at 3 days of recovery. Kidneys manifest a significant degree of tubular damage and dilatation in both groups of animals that is not distinguishable at this time point. Black arrows, necrotic cell casts; white arrow, inflammation.
Exposure of post-I/R rats to the 4.0% sodium diet beginning on day 35 resulted in substantial evidence of secondary CKD. Albumin excretion was not affected by I/R injury alone if animals were maintained on the 0.4% sodium diet for the duration of the recovery period (Fig. 4) ; however, when post-I/R animals were exposed to the 4.0% sodium diet for 4 wk (from day 35 to 63), albumin excretion was significantly increased (Fig. 4). The salt-induced albuminuria was significantly attenuated by exposure to VEGF-121 (from day 0 to 14); animals exposed to VEGF from day 0 to 35 or from day 3 to 35 tended to have lower albumin excretion rates, but these values were not statistically significant. Renal hypertrophy was also significantly enhanced in rats exposed to the 4.0% sodium diet relative to post-I/R animals maintained on the 0.4% sodium diet. Rats treated with VEGF-121 in the early phase of recovery (from day 0 to 14, day 0 to 35, and day 3 to 35) did not manifest the increase in renal hypertrophy, whereas rats treated with VEGF-121 beginning on day 21–35 manifested hypertrophy comparable to that of vehicle-treated animals (Fig. 5).
Fig. 5.
Effect of bilateral I/R injury (ARF), VEGF-121, and dietary salt on renal hypertrophy. Data were normalized for body weight and are expressed as means ± SE. *P < 0.05 vs. sham. VEGF-treated groups were compared with ARF 4.0% group. P values were determined by ANOVA and Student-Newman-Keuls post hoc test.
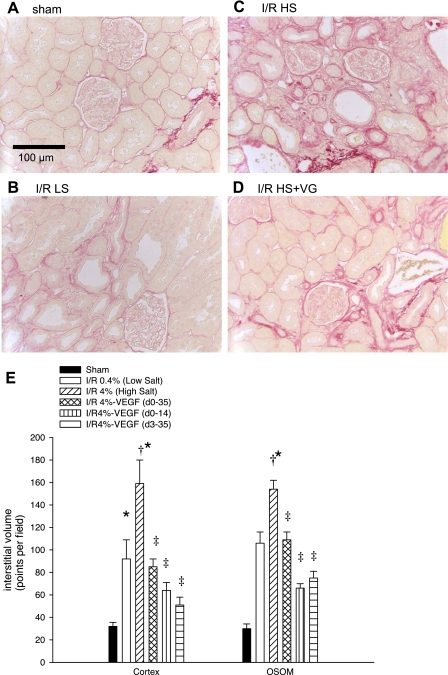
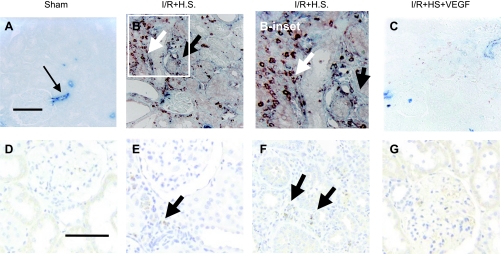
Picrosirius red staining was utilized to visualize interstitial scarring in postischemic tissues, which is exacerbated by elevated salt intake (Fig. 6, A–D). Interstitial volume scores derived from morphometric analysis demonstrate interstitial expansion by I/R injury that is significantly enhanced with increased sodium intake (Fig. 6E). However, VEGF-121 significantly attenuated salt-induced interstitial volume expansion (Fig. 6). Similar to our previous studies, the interstitial expansion was characterized, in part, by a dramatic increase in deposition of S100A4-positive fibroblasts (Fig. 7, A vs. B), α-smooth muscle actin-positive myofibroblasts (Fig. 7, A vs. B), and ED-1-labeled macrophages (Fig. 7, D vs. E and F). All these parameters resolved in kidneys of rats treated with VEGF-121 (Fig. 7, C and G).
Fig. 6.
Effect of bilateral I/R injury, VEGF-121, and dietary salt on renal structure. Renal cortex of rat kidneys were stained with picrosirius red. A–D: photomicrographs from sham-operated control, post-I/R + 0.4% Na diet, post-I/R + 4.0% Na diet, and post-I/R + 4.0% Na diet + VEGF-121 (VG) from day 0 to 35. Scale is as shown in A. E: quantitative assessment of tubulointerstitial volume based on a point-counting technique for renal cortex and outer stripe of outer medulla (OSOM). Values are means ± SE. Statistical significance (1-way ANOVA and Student-Newman-Keuls post hoc test) is as follows: *P < 0.05 vs. sham. †P < 0.05 vs. I/R 0.4%. ‡P < 0.05 vs. I/R 0.4% and I/R 4.0%.
Fig. 7.
Effect of bilateral I/R injury, VEGF-121, and dietary salt on renal expression of smooth muscle actin, S100A4, and ED-1. A: representative section through renal cortex of rat kidney following sham surgery stained with α-smooth muscle actin (blue) and S100A4 (red) as an index of myofibroblasts and fibroblasts, respectively. Arrow indicates arteriolar staining. B and B-inset: section from a post-I/R rat exposed to high salt (HS); note discrete S100A4 staining and light smooth muscle actin staining (black arrow) in interstitial area. White arrow indicates colocalized S100A4-smooth muscle actin staining in interstitium. C: section from post-I/R rat treated with VEGF from day 0 to 14 and then exposed to high salt. Note relative absence of blue and red staining. D–G: ED-1 staining (brown) of sections from sham animal (D), post-I/R animal exposed to high salt [cortex (E) and outer medulla (F)], and post-I/R animal treated with VEGF from day 0 to 14 (G). Black arrows indicate ED-1-positive cells in interstitial space, predominantly in cortex and outer medulla of post-I/R animals exposed to high salt; few such cells were observed in VEGF-treated animals. Images represent ≥4 animals per group. Scale bars (100 μm) in A and D represent scale for A–C and D–G, respectively.
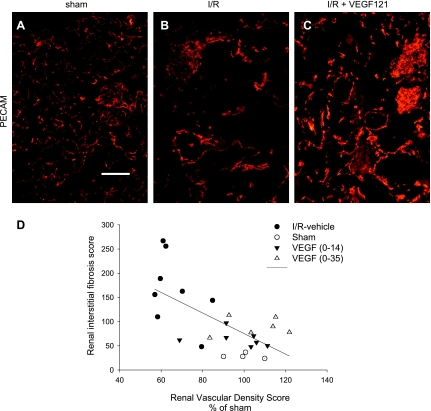
We sought to evaluate the potential relationship between interstitial fibrosis and renal microvascular density in post-I/R rats treated with vehicle or VEGF-121. Recovery from I/R in combination with increased sodium intake resulted in a substantial reduction in PECAM-labeled microvessels relative to sham-operated control animals treated identically with elevated sodium intake (Fig. 8, A vs. B). This reduction was attenuated by the administration of VEGF-121 from day 0 to 14 (Fig. 8C) and day 0 to 35 after I/R (not shown). The relationship between microvessel density and interstitial fibrosis in sham-operated, post-I/R, and VEGF-treated animals is shown in Fig. 8D; the data suggest a relationship between the reduction in microvessel density and the development of interstitial fibrosis following I/R and elevated sodium intake that is affected by VEGF-121 (Fig. 8D).
Fig. 8.
Effect of bilateral I/R injury, VEGF-121, and dietary salt on renal blood vessel density and its relationship to interstitial fibrosis. A–C: renal microvessels visualized by immunofluorescence detection of platelet endothelial cell adhesion molecule (PECAM)/CD31 in kidneys from sham-operated animal, post-I/R animal treated with vehicle and exposed to high salt from day 35 to 63, and post-I/R animal exposed to high salt from day 35 to 63 and treated with VEGF-121 from day 0 to 14. D: microvessel density quantified from individual animals in A–C (as well as the group treated with VEGF from day 0 to 35). Individual vascular density values were normalized to mean of sham group. Linear regression ANOVA demonstrated a significant (P < 0.005) correlation between vascular density and resultant fibrosis (R2 = 0.43).
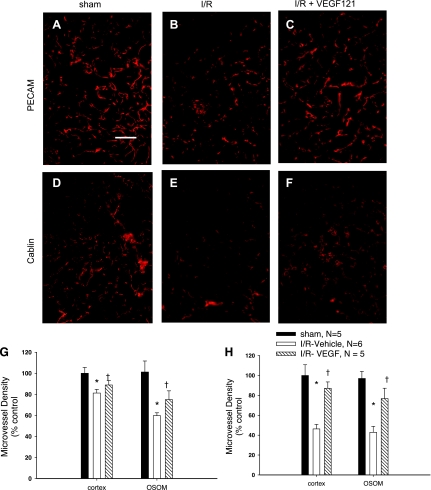
To determine whether the effects of VEGF-121 on vessel density could be observed before the development of overt interstitial fibrosis, which is exacerbated by a high-salt diet, vascular density was evaluated in rat kidney after 5 wk of recovery from I/R in animals maintained on the 0.4% sodium diet. Immunofluorescence histochemistry for PECAM/CD31 or another blood vessel marker, cablin, was used to visualize blood vessels in the kidney (Fig. 9). Morphometric quantification of the cortex and outer medulla revealed significant reductions in relative vascular density by PECAM and cablin histochemistry. The blood vessel loss was significantly attenuated in the cortex and outer medulla by treatment with VEGF-121. Thus VEGF-121 protection of blood vessel density during the renal repair response may defend against the predisposition to develop fibrosis in response to elevated salt intake.
Fig. 9.
Effect of bilateral I/R and VEGF-121 on microvascular density. A–F: renal microvessels visualized by immunofluorescence detection of PECAM/CD31 and cablin in kidneys from sham-operated animals, post-I/R animals at 5 wk, and post-I/R animals treated with VEGF-121. G and H: microvessel density for PECAM and cablin, respectively. Values are means ± SE. Statistical significance (by Student's t-test) is as follows: *P < 0.05 vs. sham; †P < 0.05 vs. I/R-vehicle.
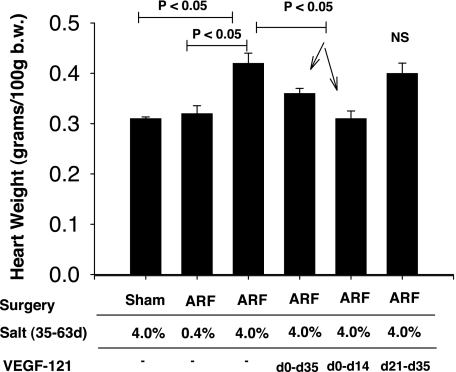
Exposure of rats to elevated salt after renal I/R, as described here, results in a significant increase in blood pressure (26). Although blood pressure was not measured in the present study, the combination of renal I/R and the 4.0% sodium diet resulted in significant cardiac hypertrophy, which was blocked by early (from day 0 to 14 and day 0 to 35), but not late (from day 21 to 35), exposure to VEGF (Fig. 10).
Fig. 10.
Effect of bilateral I/R injury, VEGF-121, and dietary salt on cardiac hypertrophy. Values are means ± SE. Values for ARF 4.0% group are significantly different from those for sham 4.0% groups (ANOVA and Student-Newman-Keuls post hoc test). VEGF-treated groups were compared with ARF 4.0% group. Heart weights were not recorded for VEGF-treated group from day 3 to 35.
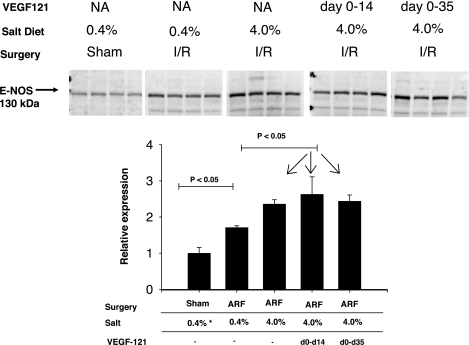
We sought to determine whether VEGF-121 might alter additional factors, with the potential to influence the pathogenesis of kidney disease and, potentially, hypertension in the setting of I/R injury. We evaluated eNOS protein expression, since CKD and hypertension are associated with reductions in renal eNOS expression, which may be attenuated by VEGF-121 treatment (20). However, eNOS protein expression was not inhibited after recovery from I/R injury but, rather, was enhanced by I/R injury (Fig. 11). Moreover, a significant further enhancement of eNOS protein expression was observed in kidneys of post-I/R rats with elevated sodium intake, and prior treatment of rats with VEGF-121 had no detectable effect on eNOS protein levels (Fig. 11).
Fig. 11.
Effect of bilateral I/R injury, VEGF-121, and dietary salt on endothelial nitric oxide synthase (eNOS) protein expression. Top: Western blots from whole kidney extracts of rats after sham or I/R injury and treatment with vehicle (NA) or VEGF-121. Bottom: densitometric quantification of data from Western blots. Values (means ± SE) are expressed relative to values obtained in sham-operated control rats. P values were determined by ANOVA and Student-Newman-Keuls post hoc test.
DISCUSSION
Renal I/R results in reversible and nonreversible injuries to the kidney (2). The recovery from AKI following I/R is due to the resolution of vasoconstriction and inflammation and an elaborate tubular regenerative response (2). The remodeling of the proximal tubule in the early postischemic period consists of multiple cellular processes characterized by dedifferentiation of surviving proximal tubule cells to a more simplified phenotype, which proliferate, migrate, and redifferentiate to reestablish a proximal tubule similar to that of control animals (9). Regeneration is likely the result of a well-orchestrated repair process that is influenced by the local production of mitogenic growth factors and the activation of developmental signaling pathways (1, 9, 10, 22, 24).
Although this repair response is impressive, certain elements of renal structure and function are not restored after injury. Data from our laboratory have shown a consistent reduction in peritubular capillary density after the initial resolution of I/R when measured 4–40 wk after injury (5). However, the mechanism and timing of vascular dropout are not clear. There is evidence of compromised endothelial and smooth muscle structure and function within a few hours of I/R injury (29, 30, 32). These observations suggest that capillary injury begins early following the renal insult. Because of the apparent lack of vascular repair, it appears that that the local milieu that is thought to effectively facilitate tubular repair is not sufficient to maintain the structural integrity of the renal microvasculature.
VEGF is an important growth factor involved in neoangiogenesis and vascular trophism. VEGF is produced in the proximal tubule of the kidney and has been suggested to provide tonic trophic support of the renal microvasculature (14). This concept is supported by the observation that VEGF expression in proximal tubules is attenuated in disease models associated with reductions of peritubular capillary density and, further, by the observation of Kang et al. (13, 15) that supplementation with VEGF-121 attenuated the loss of peritubular capillary density and improved long-term renal function after 5/6 nephrectomy or administration of cyclosporin.
Recently published data from our laboratory suggest that VEGF expression is also compromised in the early postischemic kidney (6). However, in contrast to other progressive disease models in which VEGF expression is chronically reduced, VEGF expression was transiently reduced for only up to 1 wk after injury and then restored to values of sham-operated controls by 5 wk of injury. These observations suggest that reductions in VEGF may compromise vascular stability during the early remodeling phase and, also, that restoration of VEGF is not sufficient to drive vascular repair at later times following recovery. It has been speculated that this may be due to other negative trophic factors, such as transforming growth factor-β or angiostatin (7, 27). In addition, the lack of repair may be due to poor responsiveness of the renal endothelium during the later phase of recovery.
It has been hypothesized that the loss of peritubular capillaries compromises long-term function by exacerbating hypoxia and promoting interstitial fibrosis (2, 3). Interestingly, moderate tubulointerstitial damage and capillary dropout are associated with salt-sensitive hypertension in models of subtle renal injury (12). Indeed, postischemic animals manifest salt-induced hypertension and exacerbated secondary kidney disease. However, it is unclear whether blood vessel loss directly contributes to salt sensitivity. The present study was designed 1) to investigate the potential of VEGF-121 to preserve peritubular capillaries over the course of CKD induced by elevated salt intake following recovery from I/R injury and 2) to determine the efficacy of VEGF-121 at different times during the early recovery response. These experiments were done to glean information about the biological responsiveness of the system at different times following I/R, which may impact potential windows of therapeutic intervention.
In the present study, we utilized recombinant human VEGF-121 to investigate VEGF activity in renal vascularization and long-term function following I/R injury. VEGF-121 occurs naturally via differential mRNA splicing of exons 6 and 7 of the VEGF gene, resulting in a molecule that can engage VEGF receptors but lacks the heparin-binding domain (25). Therefore, it is useful for systemic injections, and we observed no apparent side effects of prolonged treatment with VEGF-121.
VEGF-121 treatment was initiated immediately following reperfusion injury (day 0) in two of the four VEGF-121 treatment groups; VEGF-121 administration did not affect the severity of the loss of function immediately following the initiating insult. All groups showed evidence of recovery through the first 5 wk following I/R, although a persistent elevation in serum creatinine was observed at 4 wk after injury in the vehicle-treated group and the group in which VEGF-121 treatment was delayed. This result is inconsistent with our previous finding that serum creatinine values are similar to controls at these late recovery time points. Under the conditions of the present study, early VEGF-121 treatment may have resulted in a slightly enhanced restoration of renal perfusion, as indicated by the more efficient resolution of serum creatinine in those groups 7 days after I/R (Fig. 2). However, we were unable to detect any differences in creatinine levels or tubular regeneration up 3 days after I/R.
Importantly, administration of VEGF-121 early in the recovery phase attenuated the development of CKD in animals subsequently exposed to the elevated-salt diet. Even treatment only from day 0 to 14 effectively inhibited albuminuria, hypertrophy, and interstitial scarring, which were promoted by elevated salt intake from day 35 to 63. In addition, although blood pressure was not directly measured in the present study, VEGF-121-treated animals did not manifest cardiac hypertrophy. In contrast, 14 days of treatment beginning on day 21 did not affect progression of CKD in response to subsequent exposure to elevated salt intake. This suggests that there is a critical window of time in the early injury/recovery phase that is influenced by VEGF and imparts chronic salt sensitivity in this model. Interestingly, Long et al. (18) recently showed that administration of VEGF-121 had no substantial effect on the salt-dependent phase of hypertension following an initiating injury induced by transient ANG II infusion. Such observations may be consistent with a protective role of VEGF during an injury process but a relative lack of response after establishment of injury (18).
It is widely believed that maintenance of normal blood pressure in response to elevated sodium intake is dependent on alterations in renal (particularly medullary) hemodynamics that promote natriuresis. The natriuretic responses may be promoted, in part, by eNOS activity, since eNOS protein expression in the kidney is enhanced following acclimation to a high-salt diet in Sprague-Dawley rats (19). The salt sensitivity of postischemic animals would be consistent with the impaired eNOS activity/expression in this model. Interestingly, eNOS expression is impaired in a model of renal microvascular injury and preserved by VEGF-121 treatment (28). In contrast, eNOS protein expression is not diminished but, rather, is enhanced by I/R injury and is further enhanced by elevated salt, whereas treatment with VEGF-121 did not affect eNOS expression. The present experiment did not allow us to determine whether eNOS activity was preserved in postischemic animals or was influenced by VEGF-121. Therefore, little functional significance should be construed from alterations of eNOS protein levels alone. In the present study, we attempted to measure urinary nitrite/nitrate levels of postischemic animals. However, no clear pattern of activity could be determined with confidence, in part, because of excessively high variance (not shown). It is clear that more careful analysis of eNOS/nitric oxide activity using more precise methodologies is warranted to determine whether this pathway is compromised after I/R, promoting chronic kidney dysfunction.
The present study supports the idea that preservation of vascular structure following I/R may preserve renal function during challenge with an elevated salt intake. Indeed, VEGF-121 treatment attenuated the loss of renal blood vessels induced by I/R. However, we do not yet understand the mechanism of vascular preservation by VEGF-121 perhaps, in part, because of our lack of understanding of the nature of vascular dropout in this setting. No reports have provided a detailed characterization of endothelial cell death or apoptosis following I/R. The lack of effect of VEGF treatment following delayed administration might be explained if VEGF-121 has little effect on proliferation but potently inhibits cell death. Clearly, studies geared toward understanding the effects of the early events of I/R injury on endothelial cell dropout represent critical future investigations of the pathogenesis of AKI.
Regardless of the mechanism, we hypothesize that vascular preservation represents an important component of the preservation of long-term function following I/R injury. We suggest that vascular rarefaction not only promotes renal hypoxia, but it also limits the vasodilatory capacity of the renal medulla to impair sodium excretion. This hypothesis should be addressed in future studies by evaluation of local hemodynamic and sodium excretory responses. We further suggest that early alterations of endothelial stability, due to an absent or inadequate vascular repair response, are important in the development of CKD following AKI. Finally, our findings further support the idea that future therapies that target vascular stability may be warranted to address long-term complications following AKI.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-63114.
Acknowledgments
The authors thank Bhadrani Chelladurai and Kristen Bachman for technical assistance and Dr. Vincent Gattone for assistance with picrosirius red staining.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bacallao R, Fine LG. Molecular events in the organization of renal tubular epithelium: from nephrogenesis to regeneration. Am J Physiol Renal Fluid Electrolyte Physiol 257: F913–F924, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Basile DP Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–13, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Basile DP, Donohoe DL, Roethe K, Mattson DL. Chronic renal hypoxia following ischemia/reperfusion injury: effects of l-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Basile DP, Fredrich K, Weihrauch DW, Hattan N, Chilian WM. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol 286: F893–F902, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Charron AJ, Xu W, Bacallao RL, Wandinger-Ness A. Cablin: a novel protein of the capillary basal lamina. Am J Physiol Heart Circ Physiol 277: H1985–H1996, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Devarajan P Update on the mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1517, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Hammerman MR Growth factors and apoptosis in acute renal injury. Curr Opin Nephrol Hypertens 7: 419–424, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RJ, Gordon KL, Suga S, Duijvestijn AM, Griffin K, Bidani A. Renal injury and salt-sensitive hypertension after exposure to catecholamines. Hypertension 34: 151–159, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int 52: 1169–1179, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model. II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson R. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Kang DH, Kim YG, Andoh TF, Gordon KL, Suga S, Mazzali M, Jefferson JA, Hughes J, Bennett W, Schreiner GF, Johnson RJ. Post-cyclosporine-mediated hypertension and nephropathy: amelioration by vascular endothelial growth factor. Am J Physiol Renal Physiol 280: F727–F736, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kim YG, Suga SI, Kang DH, Jefferson JA, Mazzali M, Gordon KL, Matsui K, Breiteneder-Geleff S, Shankland SJ, Hughes J, Kerjaschki D, Schreiner GF, Johnson RJ. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int 58: 2390–2399, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension 33: 1013–1019, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Long D, Mu W, Price K, Roncal C, Schreiner GF, Woolf AS, Johnson R. Vascular endothelial growth factor administration does not improve microvascular disease in the salt-dependent phase of post-angiotensin II hypertension. Am J Physiol Renal Physiol 291: F1248–F1254, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension 27: 688–692, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Nangaku M, Alpers CE, Pippin J, Shankland SJ, Adler S, Kurokawa K, Couser WG, Johnson RJ. A new model of renal microvascular endothelial injury. Kidney Int 52: 182–194, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Nangaku M, Alpers CE, Pippin J, Shankland SJ, Kurokawa K, Adler S, Johnson RJ, Couser WG. Renal microvascular injury induced by antibody to glomerular endothelial cells is mediated by C5b-9. Kidney Int 52: 1570–1578, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Nony P, Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther 304: 905–912, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Pechman K, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Safirstein RL Cell cycle events during renal injury. Ren Fail 21: 427–431, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya M Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct 26: 25–35, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Spurgeon KS, Donohoe DL, Basile DP. Transforming growth factor-β in acute renal failure: receptor expression, effects on cell proliferation, cellularity and vascularization after recovery from injury. Am J Physiol Renal Physiol 288: F568–F577, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Suga S, Kim Y, Joly A, Puchacz E, Kang DH, Jefferson JA, Abraham JA, Hughes J, Johnson R, Schreiner GF. Vascular endothelial growth factor (VEGF-121) protects rats from renal infarction in thrombotic microangiopathy. Kidney Int 60: 1297–1308, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 285: F191–F198, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Torres V, Wang X, Qian Q, Somlo S, Harris PC, Gattone V. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol 282: F1150–F1155, 2002. [DOI] [PubMed] [Google Scholar]