Abstract
Tumor necrosis factor-α (TNF-α) has been implicated in the pathogenesis of hypertension and renal injury. However, the direct effects of TNF-α on renal hemodynamic and excretory function are not yet clearly defined. We examined the renal responses to infusion of TNF-α (0.33 ng·g−1·min−1) in anesthetized mice. Renal blood flow (RBF) and glomerular filtration rate (GFR) were determined by PAH and inulin clearance. The urine was collected from a cannula inserted into the bladder. Following the 60-min control clearance period, TNF-α infusion was initiated and 15 min were given for stabilization followed by another 60-min clearance period. TNF-α alone (n = 7) caused decreases in RBF (7.9 ± 0.3 to 6.4 ± 0.3 ml·min−1·g−1) and GFR (1.04 ± 0.06 to 0.62 ± 0.08 ml·min−1·g−1) as well as increases in absolute (0.8 ± 0.3 to 1.4 ± 0.3 μmol·min−1·g−1) and fractional excretion of sodium (0.5 ± 0.2 to 1.5 ± 0.4%) without affecting arterial pressure. TNF-α also increased 8-isoprostane excretion (8.10 ± 1.09 to 11.13 ± 1.34 pg·min−1·g−1). Pretreatment with TNF-α blocker etanercept (5 mg/kg sc; 24 and 3 h before TNF-α infusion; n = 6) abolished these responses. However, TNF-α induced an increase in RBF and caused attenuation of the GFR reduction in mice pretreated with superoxide (O2−) scavenger tempol (2 μg·g−1·min−1; n = 6). Pretreatment with nitric oxide (NO) synthase inhibitor nitro-l-arginine methyl ester (0.1 μg·g−1·min−1; n = 6) resulted in further enhancement in vasoconstriction while natriuresis remained unaffected in response to TNF-α. These data suggest that TNF-α induces renal vasoconstriction and hypofiltration via enhancing the activity of O2− and thus reducing the activity of NO. The natriuretic response to TNF-α is related to its direct effects on tubular sodium reabsorption.
Keywords: superoxide, nitric oxide, renal hemodynamic, sodium excretion
tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine produced by leukocytes, monocytes, and macrophages. However, it can also be generated by endothelial cells and in the kidney by mesangial and tubular epithelial cells upon inflammation and tissue injury (3, 54). Dong et al. (12) showed that resident dendritic cells in the kidney are the predominant TNF-α-secreting cells in early renal ischemia-reperfusion injury. ANG II has also been reported to increase TNF-α production in monocytes (25), in renal epithelial cells (16), and in the mammalian heart (30). Increased production of TNF-α has been reported in ANG II-dependent hypertensive rats (17) as well as in Dahl salt-sensitive rats (13, 21) and is involved in the progression of renal injury in chronic ANG II-infused rats (14). Inhibition of angiotensin-converting enzyme (ACE) or AT1 receptors attenuates renal tubulointerstitial injury and reduces expression of cytokines and matrix proteins (7, 46). Moreover, ANG II-induced hypertension is considered a chronic inflammatory disorder characterized by growth of vascular smooth muscle cells, proliferation and infiltration of monocytes in the kidney (29), and increased production of proinflammatory cytokine TNF-α by T lymphocytes (23, 48). It has been demonstrated that T cells are activated to increase the production of TNF-α in ANG II-induced hypertension. Furthermore, blockade of TNF-α normalized blood pressure and vascular superoxide production in this model of hypertension (23).
In addition, it has been reported that TNF-α mediates renal injury in Dahl salt-sensitive hypertensive rats via activation of NADPH oxidase and reduced nitric oxide (NO) bioavailability (50). In aortic endothelial cells, TNF-α has been reported to potentiate protein tyrosine nitration through activation of both NADPH oxidase and the endothelial isoform of nitric oxide synthase (eNOS) (52). Furthermore, Li et al. (37) demonstrated that TNF-α mediates the mechanical trauma-induced myocardial apoptosis via oxidative and nitrosative stress by increasing NADPH oxidase and NOS expression. Interestingly, hypertension induced by chronic administration of a subpressor dose of ANG II has been reported to be associated with activation of NADPH oxidase (8) and upregulation of NOS (28, 31).
Collectively, the aforementioned data suggest that TNF-α plays an important role in ANG II-dependent and other forms of hypertension and that it might produce its effects by regulating the activity of NADPH oxidase and NOS and thus superoxide (O2−) and NO production. However, the role of TNF-α in renal hemodynamic and excretory function is not yet clearly defined. Thus the present study was designed to examine the renal responses to acute intravenous administration of human recombinant TNF-α in mice.
MATERIALS AND METHODS
Animal Preparation
All the experimental procedures described in this study were performed in accordance with the guidelines and practices established by the Tulane University Animal Care and Use Committee. C57BL6 mice (Jackson Laboratories, Bar Harbor, ME), weighing between 22 and 25 g, were housed in a temperature- and light-controlled room and allowed free access to a standard diet (Ralston-Purina, St. Louis, MO) and tap water. The animals were anesthetized with a combination of Inactin (thiobutabarbital sodium, 100 mg/kg ip, Sigma, St. Louis, MO) and ketamine (6 mg/kg ip, Vedco, St. Joseph, MO) to measure renal blood flow (RBF), glomerular filtration rate (GFR), and renal excretory function as described before (27). Supplemental doses of ketamine (6 mg/kg iv) were administrated as required. The mice were placed on a servo-controlled surgical table that maintained body temperature at 37°C, and a tracheostomy was performed. The animals were allowed to breathe air enriched with oxygen (O2) by placing the exterior end of the tracheal cannula inside a small plastic chamber into which humidified 95% O2-5% CO2 was continuously passed. The right carotid artery was cannulated with polyethylene tubing (PE-10) connected to a pressure transducer (AcqKnowledge data acquisition system, Biopac) for continuous recording of arterial pressure (MAP) and heart rate (HR). The right jugular vein was catheterized with a PE-10 tube for fluid infusion at a rate of 3 μl/min with the help of an infusion pump (CMA). During surgery, an isotonic saline solution containing 6% albumin (bovine serum, Calbiochem, La Jolla, CA) was infused. The bladder was catheterized with a PE-90 tube via a suprapubic incision for urine collection. After surgery, the infusion fluid was replaced with an isotonic saline solution containing 1% albumin, 7.5% inulin (Inutest, Laevosan, Linz/Donau, Austria), and 1.5% PAH (Merck Sharpe & Dohme, West Point, PA), which was also used as vehicle for TNF-α infusion solution.
Experimental Protocol
Two sets of experiments were conducted in mice: responses to a single-dose infusion of TNF-α and the dose-response relationship of TNF-α.
In the first set of experiments, responses to a single-dose (0.33 ng·g−1·min−1 iv) infusion of human recombinant TNF-α (Invitrogen-Biosource) were assessed. This dose of TNF-α was selected from the findings of previous studies with TNF-α (5, 41). We considered this dose of TNF-α as physiologically relevant because it was lower than the dose that was used to induce cytokine-mediated cardiac dysfunction (5). This dose did not alter systemic arterial pressure significantly and thus allowed us a reasonable assessment of the renal responses to TNF-α without any confounding arterial pressure-induced effects in the kidney. Responses to this single-dose infusion of TNF-α were assessed in control animals (n = 7) as well as in animals pretreated with etanercept (n = 6), tempol (n = 6), and nitro-l-arginine methyl ester (l-NAME; n = 6). Etanercept (Immunex), a TNF-α blocker, is a fusion protein which binds with circulating TNF-α and prevents it from acting on cellular TNF-α receptors (47). Etanercept was administered (5 mg/kg sc) in conscious mice 1 day before the experiment. A second dose was also given to the anesthetized mice 3 h before TNF-α infusion on the day of experiment (36). Mice pretreated with etanercept were considered to determine the specificity of the responses to TNF-α. To determine the role of O2− production in the responses to TNF-α, experiments were conducted in mice pretreated with tempol, a O2− scavenging agent. Tempol (Sigma) was given at a rate of 2 μg·g−1·min−1 starting 2 h before TNF-α infusion. Experiments in mice pretreated with NOS inhibitor l-NAME (Sigma) were conducted to determine the role of NO in the responses to TNF-α. l-NAME was given at a rate of 0.1 μg·g−1·min−1 starting 2 h before TNF-α infusion. Tempol/l-NAME infusions were continued until the end of experiments along with TNF-α infusion. A separate set of time control experiments (n = 7) was also conducted with a similar protocol with vehicle (saline) infusion without TNF-α.
The basic protocol with infusion of TNF-α in all the experiments in treated and untreated mice described earlier was as follows. After a 60-min equilibration period following completion of surgical procedures, two consecutive 30-min control urine collections (basal period) were made. An arterial blood sample (100 μl) was then taken for measurements of basal hematocrit and plasma PAH, inulin, and sodium/potassium concentrations. Then, an infusion of TNF-α was started at a rate of 0.33 ng·g−1·min−1 and continued until the end of the experiment. Fifteen minutes after the initiation of TNF-α infusion (stabilization period), another two 30-min urine clearance collections were made (treatment period). After the final collection period, another arterial blood sample (100 μl) was taken for measurements of hematocrit, plasma PAH, inulin, and sodium/potassium concentrations. At the end of the experiment, the animals were killed with a high dose of ketamine (60 mg/kg iv) and the kidneys were removed and weighed.
A second set of experiments was conducted in mice (n = 5) to determine the dose-response relationship of TNF-α which was infused at incremental doses of 0.1, 0.3, and 0.5 ng·g−1·min−1. These doses were considered to include the dose (0.33 ng·g−1·min−1) in earlier experiments at the mid-range level. The protocol in these experiments was as follows. After a 60-min equilibration period following completion of surgical procedures, two consecutive 30-min control urine collections (basal period) were made. An arterial blood sample (100 μl) was then taken for measurements of basal hematocrit and plasma PAH, inulin, and sodium/potassium concentrations. Then, an infusion of TNF-α was initiated at a rate of 0.1 ng·g−1·min−1 and after a 15-min of stabilization period, a 30-min urine collection was made. Another infusion of TNF-α was then started at a rate of 0.3 ng·g−1·min−1 and after a 15-min stabilization period, another 30-min urine collection was made. Subsequently, third infusion of TNF-α was then started at a rate of 0.5 ng·g−1·min−1 and after a 15-min stabilization period, a final 30-min urine clearance collection was done. At the end of this urine collection, another arterial blood sample (100 μl) was taken for measurements of hematocrit, plasma PAH, inulin, and sodium/potassium concentrations. The animals were then killed and kidneys were taken out and weighed.
TNF-α RNA Isolation and Real-Time RT-PCR in Renal Tissues
Total RNA was extracted from the preserved kidney by using TRIzol reagent provided by the manufacturer (Invitrogen) and was reverse transcribed by using oligo (dT) and reverse transcriptase. Expression levels of TNF-α mRNA were determined by using specific primers. GAPDH was used as a housekeeping gene. Real-time RT-PCR (qRT-PCR) was performed in 384-well PCR plates with the use of Bio-Rad PCR Master Mix (iTaq SYBR Green Supermix with ROX) and the ABI Prism 7900 sequence-detection system (Applied Biosystems). The primer sequences for real-time PCR for GAPDH were AGACAGCCGCATCTTCTTGT (forward) and CTTGCCGTGGGTAGAGTCAT (reverse) and for TNF-α were GTCGTAGCAAACCACCAAGC (forward) and TGTGGGTGAGGAGCACATAG (reverse) (41).
Calculations and Statistical Analysis
Urine volume (V) was measured gravimetrically. Blood and urine samples collected during systemic experiments were analyzed for inulin, PAH, and sodium/potassium concentrations as reported earlier (27). 8-Isoprostane (UIsoV) concentration in the urine was measured by enzyme immunoassay (Cayman Chemical) (33). Inulin and PAH concentrations were determined by spectrophotometry, and sodium/potassium concentrations were determined by flame photometry. The value for inulin clearance is considered as GFR, and the value for PAH clearance is considered as renal plasma flow. RBF is calculated from renal plasma flow and hematocrit values. The concentrations of sodium in urine and blood were used to calculate the urinary sodium excretion rate (UNaV) and fractional excretion of sodium (FENa), respectively. Renal vascular resistance (RVR) is calculated by dividing the value of mean systemic arterial pressure with the value of RBF. The mean values obtained during the first two control collection periods were considered as “basal values,” while the mean of the values collected during the two TNF-α infusion periods was taken as the “treatment value.” The differences in the values between the basal and the treatment periods are considered as the responses to TNF-α treatment. All values were normalized per gram of kidney weight. Results are expressed as means ± SE. Differences between basal and treatment values in the same set of experiments were analyzed by a paired Student's t-test. Comparison of the data among the different set of experiments was made by one-way ANOVA, followed by the Student-Newman-Keuls post hoc test for multiple comparisons. Differences were considered significant at P < 0.05.
RESULTS
First Set of Experiments
Responses to single-dose infusion of TNF-α on MAP and HR.
At the dose of 0.33 ng·g−1·min−1, TNF-α infusion per se was found to have no significant effect on MAP. The responses to TNF-α in different group of experiments were as follows: control group: 100 ± 3 to 99 ± 4 mmHg; etanercept treated-group: 96 ± 2 to 95 ± 2 mmHg; tempol-treated group: 80 ± 4 to 82 ± 3 mmHg; and l-NAME-treated group: 131 ± 3 to 129 ± 5 mmHg. A small decrease in MAP was observed in all the animals due to the first blood collection, which was the same (∼7 mmHg) in all the experimental groups. Once the MAP recordings reached a stabilized level after the first blood sample collection (usually taking 3–5 min), TNF-α infusion was initiated. There were significant increases in HR during TNF-α infusion, which was absent only in etanercept-treated animals. The HR responses to TNF-α in different groups of experiments were as follows: control group: 466 ± 6 to 541 ± 11 beats/min (P < 0.05); etanercept-treated group: 456 ± 8 to 481 ± 11 beats/min; tempol-treated group: 431 ± 29 to 484 ± 16 beats/min (P < 0.05); and l-NAME-treated group: 461 ± 9 to 497 ± 13 beats/min (P < 0.05). No significant differences were observed in plasma Na+ and K+ levels after TNF-α infusion in any group. The plasma Na+ levels after TNF-α infusion are as follows: control group: 150.4 ± 0.8 to 152.4 ± 1.3 meq/l; etanercept-treated group: 159.6 ± 1.8 to 161.9 ± 1.1 meq/l; tempol-treated group: 155.5 ± 1.2 to 153 ± 1.6 meq/l; and l-NAME-treated group: 157 ± 3.1 to 154 ± 1.9 meq/l. The plasma K+ levels after TNF-α infusion among different groups are as follows: control group: 4.3 ± 0.1 to 4.5 ± 0.3 meq/l; etanercept-treated group: 4.1 ± 0.2 to 4.2 ± 0.3 meq/l; tempol-treated group: 4.2 ± 0.2 to 4.2 ± 0.2 meq/l; and l-NAME-treated group: 4.6 ± 0.3 to 4.7 ± 0.3 meq/l.
Responses to single-dose infusion of TNF-α on renal hemodynamics.
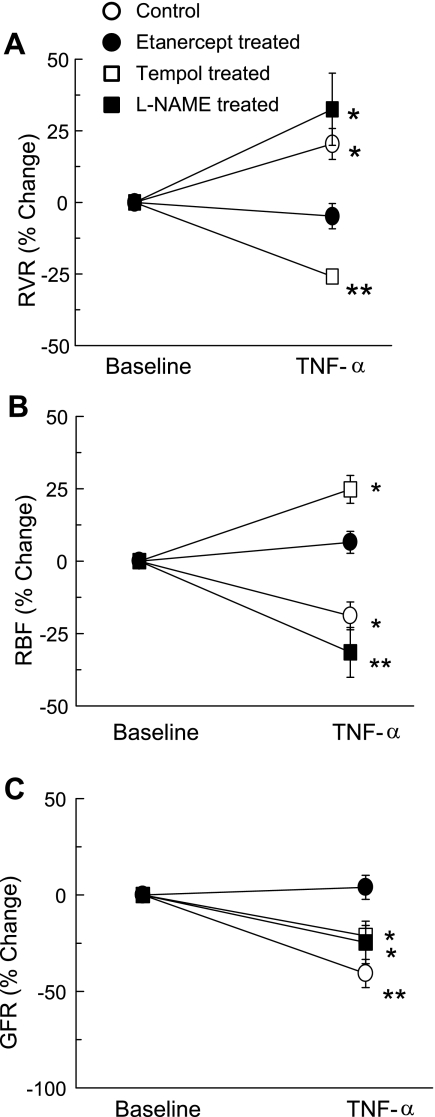
The absolute changes in RBF, RVR, and GFR in response to TNF-α infusion are given in the Table 1, and percent responses are illustrated in Fig. 1. Figure 1A illustrates the percent response of RVR to TNF-α treatment. TNF-α alone increases RVR by 20.4 ± 5.4% (P < 0.05) from the baseline value, which was not observed in etanercept-pretreated mice. In the animals treated with tempol, TNF-α decreased RVR by 25.9 ± 1.5% (P < 0.01) from the baseline value. In contrast, in l-NAME-treated animals, TNF-α infusion resulted in increases in RVR (36.1 ± 10.4%; P < 0.05) from basal values. TNF-α infusion alone resulted in 18.9 ± 4.8% (P < 0.05) decreases in RBF from the baseline value (Fig. 1B). This effect of TNF-α on RBF was found to be blocked in mice pretreated with the TNF-α blocker etanercept. However, in the tempol-treated animals, TNF-α markedly increased RBF (24.8 ± 4.8%; P < 0.05) from the baseline value. In contrast, in l-NAME-treated mice, TNF-α infusion resulted in decreases in RBF (31.5 ± 8.6%; P < 0.05 vs. baseline). The magnitude of this TNF-α-induced decrease in RBF during l-NAME treatment appears to be greater than that during TNF-α infusion alone, although found statistically not significant. Figure 1C illustrates the GFR responses to acute TNF-α infusion. TNF-α infusion in anesthetized mice caused 40.7 ± 7.3% (P < 0.01) decreases in GFR from the baseline value. The reduction in GFR by TNF-α was found to be blocked in the etanercept-pretreated mice. In addition, in the animals treated with tempol, TNF-α caused decreases (21.2 ± 5.4%; P < 0.05) in GFR from basal values. The magnitude of TNF-α-induced decreases in GFR during tempol treatment was smaller (P < 0.05) than that during TNF-α infusion alone. Furthermore, TNF-α infusion in l-NAME-treated animals also caused decreases in GFR (29.6 ± 8.7%; P < 0.05) from the baseline value.
Table 1.
Absolute responses to TNF-α administration (0.33 ng/g/min) on renal hemodynamics and excretory function in mice
| Animal Group |
Control (n = 7) |
Etanercept-Treated (n = 6)
|
Tempol-Treated (n = 6)
|
l-NAME-Treated (n = 6)
|
||||
|---|---|---|---|---|---|---|---|---|
| Basal | TNF-α | Basal | TNF- α | Basal | TNF- α | Basal | TNF- α | |
| RBF, ml·min−1·g−1 | 7.9±0.3 | 6.4±0.3* | 6.8±0.6 | 7.1±0.5 | 8.1±0.2 | 10.1±0.4* | 8.0±0.1 | 5.5±0.7† |
| RVR, mmHg·ml·min·g−1 | 13.9±0.7 | 16.6±0.9* | 14.6±1.5 | 13.8±0.9 | 11.4±0.4 | 8.4±0.3† | 17.8±0.3 | 24.1±2.0* |
| GFR, ml·min−1·g−1 | 1.04±0.07 | 0.62±0.09† | 1.04±0.07 | 1.09±0.07 | 1.22±0.06 | 0.95±0.02* | 1.44±0.14 | 0.98±0.09* |
| V, μl·min−1·g−1 | 9.3±1.4 | 15.0±2.0* | 5.1±0.2 | 5.2±0.5 | 4.6±0.5 | 9.3±1.6* | 8.2±1.3 | 15.6±3.1* |
| UNaV, μmol·min−1·g−1 | 0.75±0.22 | 1.37±0.23* | 0.69±0.09 | 0.85±0.07 | 0.42±0.07 | 1.12±0.26* | 1.31±0.30 | 2.75±0.59* |
| FENa, % | 0.53±0.23 | 1.45±0.40* | 0.28±0.05 | 0.38±0.05 | 0.18±0.05 | 0.81±0.23* | 0.51±0.16 | 1.78±0.33* |
| UKV, μmol·min−1·g−1 | 1.16±0.06 | 1.29±0.08 | 1.25±0.13 | 1.28±0.09 | 1.36±0.10 | 1.49±0.09 | 1.38±0.20 | 1.16±0.14 |
| UIsoV, pg·min−1·g−1 | 8.10±1.09 | 11.13±1.34† | 7.31±0.56 | 7.08±0.43 | 6.37±0.92 | 5.97±1.40 | 9.25±0.31 | 14.11±074† |
Values are means ± SE; n = no. of animals. l-NAME, nitro-l-arginine methyl ester; RBF, renal blood flow; RVR, renal vascular resistance; GFR, glomerular filtration rate; V, urine flow; UNaV, urinary sodium excretion rate; UKV, urinary potassium excretion rate; FENa, fractional excretion of sodium; UIsoV, urinary 8-isoprostane excretion rate.
P < 0.05,
P < 0.01 vs. corresponding basal value.
Fig. 1.
Percent responses to TNF-α treatment (0.33 ng·g−1·min−1) on renal vascular resistance (RVR; A), renal blood flow (RBF; B), and glomerular filtration rate (GFR; C) in mice. *P < 0.05, **P < 0.01 vs. baseline value.
Responses to single-dose infusion of TNF-α on renal excretory function.
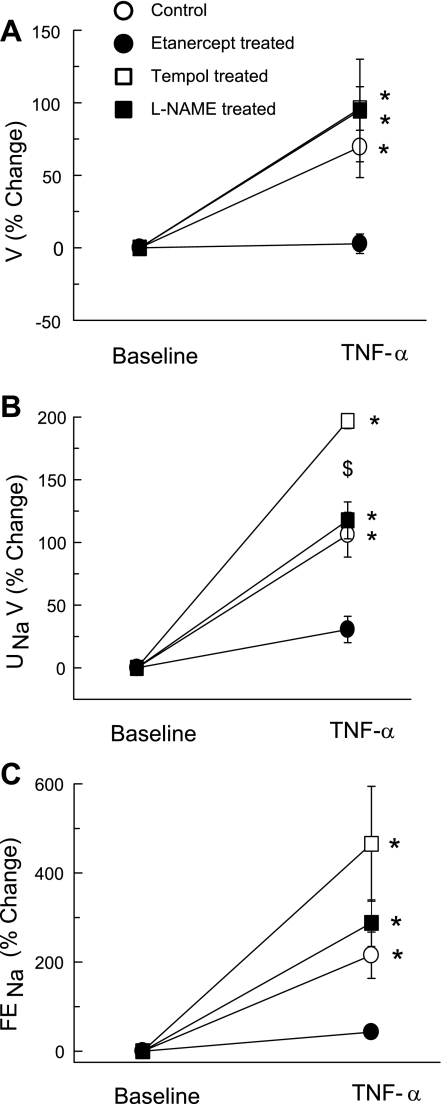
Figure 2 illustrate the effects of TNF-α infusion on renal excretory function. TNF-α infusion alone in mice caused marked increases in V (69.5 ± 21.1%; P < 0.05; Fig. 2A), UNaV (106.1 ± 17.8%; P < 0.01; Fig. 2B), and FENa (215.3 ± 51.9%; P < 0.05; Fig. 2C) from their respective baseline values. These effects of TNF-α were not observed when TNF-α was given to etanercept-pretreated mice. Moreover, during tempol or l-NAME treatment also, TNF-α caused increases in V (96.1 ± 15.0%; P < 0.01 and 94.7 ± 35.4%; P < 0.05) from their respective baseline values, which were similar to those during TNF-α treatment alone. However, during tempol treatment, TNF-α resulted in marked increases in UNaV (196.9 ± 6.2%; P < 0.01) and FENa (465.5 ± 128.8%; P < 0.05) from their respective baseline values. The degree of TNF-α-induced increases in UNaV and FENa during tempol treatment was greater (P < 0.05) than that during TNF-α infusion alone. Similarly, in l-NAME-treated animals, TNF-α caused similar increases in UNaV (117.6 ± 14.7%; P < 0.01) and FENa (287.7 ± 52.2%; P < 0.05) from their respective baseline values, as by TNF-α alone. The baseline UNaV value in l-NAME-treated animals was higher than that in vehicle-treated animals; however, this difference was only borderline significant statistically (1.31 ± 0.30 vs. 0.69 ± 0.17 μmol·min−1·g−1; P = 0.062; Table 1).
Fig. 2.
Percent responses to TNF-α treatment (0.33 ng·g−1·min−1) on urine flow (V; A), urinary sodium excretion rate (UNaV; B), fractional excretion of sodium (FENa; C) in mice. *P < 0.05, **P < 0.01 vs. baseline value. $P < 0.05 vs. values in control mice.
Urinary 8-isoprostane excretion rate responses to TNF-α infusion are summarized in Table 1. TNF-α infusion in mice caused 38.01 ± 2.86% increases in UIsoV (P < 0.01) from the baseline value. This response to TNF-α was not observed when TNF-α was given to etanercept-pretreated mice. Similarly, during tempol treatment, TNF-α-induced increases in UIsoV were absent. However, during l-NAME treatment, TNF-α caused marked increases in UIsoV (52.62 ± 6.71; P < 0.01) from the baseline value. Although the magnitude of these TNF-α-induced increases in UIsoV during l-NAME treatment appears to be higher than those during TNF-α infusion alone, it was not statistically significant.
In time control (vehicle treated) experiments (n = 7), no significant difference was observed between the basal and treatment period values, which were as follows: MAP, 89 ± 4 to 91 ± 4 mmHg; HR, 468 ± 11 to 499 ± 32 beats/min; RBF, 8.0 ± 0.9 to 7.7 ± 0.6 ml·min−1·g−1; RVR, 13.8 ± 1.3 to 13.6 ± 1.3 mmHg·ml·min·g−1; GFR, 1.00 ± 0.04 to 1.07 ± 0.06 ml·min−1·g−1; V, 8.4 ± 1.7 to 7.8 ± 1.6 μl·min−1·g−1; UNaV, 0.69 ± 0.17 to 0.65 ± 0.15 μmol·min−1·g−1; FENa, 0.53 ± 0.15 to 0.52 ± 0.17%; UKV, 1.55 ± 0.25 to 1.59 ± 0.06 μmol·min−1·g−1; UIsoV, 6.89 ± 1.04 to 6.96 ± 0.7 pg·min−1·g−1; and plasma Na+ and K+ concentration, 155.9 ± 1.1 to 155.2 ± 1.2 and 4.3 ± 0.2 to 4.4 ± 0.5 meq/l, respectively.
Second Set of Experiments
Responses to incremental doses of TNF-α on MAP, HR, renal hemodynamics and excretory function.
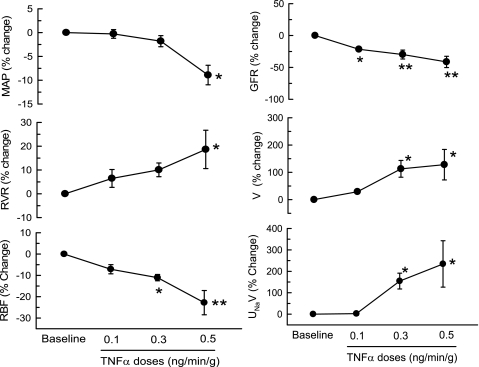
Table 2 summarizes the absolute responses to incremental doses of TNF-α. Figure 3 also illustrates some of these responses in percent changes. At 0.1 ng·g−1·min−1 infusion rate, TNF-α did not cause any significant change in MAP, RBF, UNaV, and V except an increase in HR (13 ± 3%; P < 0.01) and a decrease in GFR (21.5 ± 2.9%; P < 0.05) compared with basal values. At 0.3 ng·g−1·min−1, TNF-α resulted in a significant fall in RBF (11.1 ± 1.5%; P < 0.05) and GFR (29.7 ± 7%; P < 0.01) as well as significant increases in V (103 ± 25%; P < 0.05), UNaV (142 ± 31%; P < 0.05), and FENa (284 ± 37%; P < 0.05) without altering MAP (2 ± 1%) compared with baseline values. Similarly, at 0.5 ng·g−1·min−1 TNF-α also caused a significant fall in RBF (22.7 ± 5.7%; P < 0.01) and GFR (41.3 ± 8.9%; P < 0.01) and significant increases in V (120 ± 43%; P < 0.05), UNaV (204 ± 89%; P < 0.05), and FENa (518 ± 105%; P < 0.01) along with a significant fall in MAP (9 ± 2%; P < 0.01) compared with the baseline values. The plasma Na+ and K+ concentration measured before and after TNF-α infusions were not different and are as follows: 152.4 ± 2.3 to 153.4 ± 2.5 and 4.8 ± 0.5 to 4.9 ± 0.3 meq/l, respectively. The responses at the dose of 0.3 ng·g−1·min−1 were comparable with the responses to a single-dose (0.33 ng·g−1·min−1) infusion in the first set of experiments.
Table 2.
Absolute responses to different doses of TNF-α administration on mean arterial pressure, heart rate, renal hemodynamics, and excretory function in mice
| Basal |
TNF-α, ng·min−1·g−1 |
|||
|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | ||
| MAP, mmHg | 98±5 | 98±5 | 97±6 | 90±3† |
| HR, beats/min | 434±9 | 488±15† | 551±11† | 572±12† |
| RBF, ml·min−1·g−1 | 10.2±0.9 | 9.5±0.7 | 9.1±0.9* | 7.7±0.4† |
| GFR, ml·min−1·g−1 | 1.08±0.13 | 0.92±0.14* | 0.76±0.12† | 0.65±0.13† |
| V, μl·min−1·g−1 | 4.4±1.1 | 5.4±1.1 | 8.5±1.6* | 9.4±2.3* |
| UNaV, μmol·min−1·g−1 | 0.34±0.16 | 0.42±0.24 | 0.73±0.28* | 0.80±0.27* |
| FENa, % | 0.19±0.09 | 0.30±0.17 | 0.62±0.23* | 0.85±0.19† |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate.
P < 0.05,
P < 0.01 vs. corresponding basal value.
Fig. 3.
Percent responses to incremental doses (0.1, 0.3, 0.5 ng·g−1·min−1) of TNF-α administration on mean arterial pressure (MAP), renal vascular resistance (RVR), renal blood flow (RBF), glomerular filtration rate (GFR), urine flow (V) and urinary sodium excretion rate (UNaV) in mice. *P < 0.05, **P < 0.01 vs. baseline value.
Renal mRNA expression of TNF-α in response to a single-dose (0.33 ng·g−1·min−1) infusion of TNF-α.
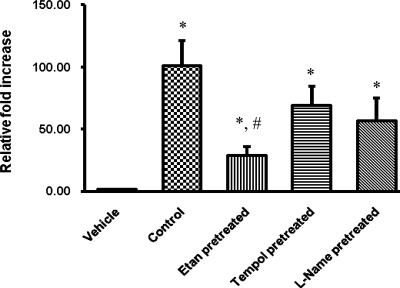
Results are illustrated in Fig. 4. TNF-α infusion induced an increase (P < 0.05) in the mRNA levels of TNF-α (101 ± 20-fold) in the kidney compared with control vehicle-treated animals. This increase in TNF-α expression in response to TNF-α infusion was significantly attenuated (P < 0.05) in mice pretreated with TNF-α blocker etanercept compared with the vehicle-treated animals. However, a significant increase in TNF-α expression was observed in response to TNF-α infusion in the animals treated with tempol or l-NAME (69 ± 15- and 57 ± 18-fold, respectively; P < 0.05) compared with vehicle-treated animals.
Fig. 4.
Renal mRNA expression of TNF-α in response to TNF-α treatment (0.33 ng·g−1·min−1) in mice. Values are means ± SE. Control, vehicle-treated mice. Etan, etanercept-treated mice. *P < 0.05 vs. vehicle-treated mice. #P < 0.05 vs. control value.
DISCUSSION
The present study demonstrated that acute administration of the proinflammatory cytokine TNF-α in mice resulted in decreases in RBF and in GFR but caused increases in urine flow and sodium excretion without altering arterial pressure. These renal responses to TNF-α were abolished in mice pretreated with the TNF-α blocker etanercept, confirming the specificity of these direct effects of TNF-α in the kidney. To our knowledge, this is the first study in which the direct effects of this cytokine on renal hemodynamics and excretory function were assessed. As TNF-α is released in the plasma during inflammatory conditions, it can exert its effects in different organ systems, including the kidney. The estimated renal plasma concentration of TNF-α achieved during the infusion period in the present study was ∼30 pmol, which was within the range of plasma concentrations of this cytokine reached during inflammatory conditions, as reported in many experimental studies (4, 23, 40). Thus the results obtained in the present study provide useful information regarding a possible physiological and pathophysiological role of this cytokine in the regulation of renal hemodynamics and excretory functions, particularly in the conditions that are associated with inflammatory responses. It should be noted here that the effect of exogenous TNF-α might be different in anesthetized and conscious animals. Indeed, various anesthetics have been shown to reduce the release of proinflammatory cytokines, including TNF-α, and reduce the lethality of lipopolysaccharide, which may result from the decrease in the activities of nuclear factor-κB and activator protein 1 and the reduction of the expression of p38 mitogen-activated protein kinase (19, 53). Thus it may be possible that exogenous administration of TNF-α can cause accentuated renal responses in conscious animals than those in anesthetized animals, and the present results may be interpreted accordingly. It could be argued that a centrally mediated action of TNF-α (1) can induce soluble mediators especially prostaglandin E2 within the cells of the blood-brain barrier that readily penetrate into the brain to initiate neural mechanisms (55) to alter sympathetic nerve activity. However, in the present study we found no change in blood pressure by TNF-α infusion, suggesting that the dose used in this study did not alter the sympathetic activity to a degree that could influence significantly renal hemodynamics and renal function. As TNF-α was observed to induce dose-dependent increases in HR along with no change (in low dose) or a decrease (in high dose) in arterial pressure, it can reasonably be assumed that this cytokine also decreased stroke volume. Previous studies also demonstrated an increase in HR in response to acute administration of TNF-α (20, 43). Although chronic administration of TNF-α for 5 days in rats did not affect HR or blood pressure, it caused a decrease in fractional shortening, indicating a systolic dysfunction in TNF-α-treated animals (41). In the present study, although HR increases, the presumed decreases in stroke volume/cardiac output may also indicate such systolic dysfunction in response to acute infusion of TNF-α. Although the plasma volume in these acute, anesthetized mice preparations was not measured in the present study, there were no significant changes in plasma Na+ and K+ concentrations following TNF-α infusion.
It could also be argued that l-NAME or tempol treatment might alter the effective plasma concentration of TNF-α in the kidney. This possibility seems unlikely as it was observed in the present study that basal levels of RBF were not much different between l-NAME- or tempol-treated mice, indicating that the kidneys in both conditions received more or less the same amount of TNF-α from its infusion. Moreover, TNF-α administration also induces similar levels of renal tissue TNF-α mRNA expression in l-NAME- and tempol-treated mice, indicating that the effective concentrations of TNF-α in the kidney in those two conditions might not be much different. We observed that TNF-α markedly increases the mRNA levels of TNF-α in the kidney. Moreover, the TNF-α-induced increase in TNF-α mRNA levels was found significantly attenuated in etanercept-pretreated mice; however, it remained unaffected in tempol- or l-NAME-pretreated mice, suggesting that TNF-α can induce its own mRNA message independently of NO and O2−, at least in the present experimental setting. This increase in the renal TNF-α mRNA expression due to exogenous TNF-α in this short period is interesting but consistent with many previous studies which demonstrated the significant increases in TNF-α mRNA expression in a time period as short as 30 min in response to different stimuli like ANG II or coronary artery occlusion (18, 30, 49). Moreover, it is a well-known property of the proinflammatory cytokines to amplify their own secretion in a feed-forward manner (11). By a well-organized and comprehensive study, Taishi et al. (49) very recently reported that TNF-α treatment in rat hypothalamic culture significantly increased IL-1β (56-fold), TNF-α (43-fold), and IL-6 (5-fold) mRNA levels at 30 min. The same treatment with TNF-α of cortical culture increased mRNA levels of these genes further [IL-1β (>30-fold), TNF-α (>50-fold), and IL-6 (30-fold)] at 30 min. These data suggest that TNF-α can increase its own as well as other cytokine expression over a time period as short as 30 min. As the present study was designed to determine the effect of circulating TNF-α on renal hemodynamic and function, we limit our assessment of the tissue mRNA levels only in the kidney; however, it should be noted here that such changes in tissue TNF-α mRNA level were also observed in the hypothalamus during coronary artery occlusion (18) or in myocardium during ANG II administration (30). The finding that etanercept did not completely block the tissue TNF-α mRNA induction by circulating TNF-α could be due to the fact that etanercept is a recombinant fusion protein which is a soluble TNF-α receptor. It does not bind to trans-membrane TNF-α as effectively as would be done by anti-TNF-α antibodies such as infliximab, adalimumab, etc. (47), indicating its reduced efficacy in intracellular targets, which could be the reason for this incomplete blockade of tissue TNF-α mRNA induction. The dose of etanercept used in the present study, although effective in preventing TNF-α-induced changes in renal hemodynamics and excretory function, may not be sufficient enough to prevent tissue TNF-α mRNA induction effectively. It should be noted here that the assessment of mRNA activity could not necessarily reflect protein activity; thus the mRNA data in this may be interpreted accordingly. However, it appears that exogenous TNF-α can stimulate the renal mRNA expression of TNF-α equally in the condition NOS or O2− inhibition.
The vasoconstrictor effect of TNF-α was not seen when this cytokine was administered in mice pretreated with tempol (an O2− scavenger). It was observed that TNF-α infusion in tempol-treated animals resulted rather in an increase in RBF and also an attenuation of reductions in GFR. These findings suggest that an enhanced O2− activity plays a role in mediating renal vasoconstrictor and hypofiltration responses to this cytokine. Indeed, there was an increased rate of UIsoV (a marker for enhanced O2− activity) in response to TNF-α administration in these mice that was ameliorated in etanercept as well as in tempol-treated mice. Previous studies (10, 39) also demonstrated that an increase in O2− activity during inhibition of superoxide dismutase enzyme exerted a renal vasoconstrictor response. TNF-α was shown to induce O2− as well as NO production (37, 52). Thus it is conceivable that the renal vasodilatory response to TNF-α observed in tempol-treated mice resulted from an enhancement of NO bioavailability due to an increase in the production of NO as well as to a reduction in O2− level, as the latter was scavenged by tempol. It has been reported that l-NAME administration resulted in an increase in O2− activity (32, 38). Thus the TNF-α-induced enhancement of O2− activity would be even greater in the condition of NOS inhibition than that in an intact condition. Indeed, TNF-α induced more increases in UIsoV during l-NAME treatment than TNF-α alone in the present study. The greater reduction in RBF by TNF-α in l-NAME-treated mice compared with untreated mice indicates that a concomitant increase in NO production helps to offset the action of enhanced O2− activity induced by TNF-α. It has also been suggested that in vascular smooth muscle cells, O2− could exert its direct vasoconstrictor action, possibly by enhancing intracellular calcium accumulation (9). Collectively, these results indicate that the effects of TNF-α on RBF and GFR are mediated by both an enhancement in O2− activity as well as a reduction in NO activity.
8-Isoprostane is generally believed to be formed endogenously due to oxidation of membrane phospholipids by a potent oxidant, peroxynitrite, that is produced during an in vivo chemical reaction between NO and O2− (26, 45). However, it has also been demonstrated that UIsoV increased when endogenous NO production was inhibited by nitro-l-arginine administration. This increase in UIsoV was ameliorated completely by the O2− scavenger tempol, indicating that such increased formation of 8-isoprostane results from direct peroxidation of lipids by O2− (38, 44). In the present study, we also demonstrated that TNF-α-induced increases in UIsoV were more pronounced in the l-NAME-pretreated (NOS-inhibited) animals, supporting the notion that O2− can directly interact with lipids to produce 8- isoprostane.
The involvement of both O2− and NO production in the actions of TNF-α has been demonstrated in many experimental settings (22, 28, 36, 37, 50). In aortic endothelial cells, TNF-α has been reported to potentiate the protein tyrosine nitration and the production of O2− and NO through activation of NADPH oxidase and NOS, respectively (52). In cardiomyocytes, TNF-α has been reported to mediate nonlethal trauma-induced apoptosis by overproduction of NO and O2− via increased expression of inducible NOS and NADPH oxidase (37). Moreover, recently it has also been shown that ANG II increases the expression of gp91phox (a subunit of NADPH oxidase) and eNOS in the myocardium of wild-type mice, which were absent in TNF-α knockout mice. However, when TNF-α knockout mice were treated with human recombinant TNF-α, ANG II caused increases in blood pressure as well as upregulation of gp91phox and eNOS (22, 48). Preliminary data from our laboratory also demonstrated that ANG II-induced increases in urinary nitrate/nitrite and isoprostane excretion rate were significantly lower in TNF-α knockout mice compared with control animals (34).
It was noted that TNF-α induced reduction in GFR was partially attenuated in l-NAME-treated animals but the reduction in RBF responses to TNF-α was further enhanced in these animals. These findings indicate that in the condition of NOS inhibition, TNF-α induced a similar influence on both preglomerular and postglomerular resistance segments that helped to maintain GFR although there was a marked decrease in RBF, as also suggested earlier (27, 42). It is known that the magnitude of GFR depends on the filtration forces that depend on the balance between pre- and postglomerular vascular resistances (42). In conditions in which an equipotent balance is maintained, GFR may remain the same or change in an opposite direction to the changes in RBF. Such a situation is noted during administration of many agents such as ANG II (a vasoconstrictor) or acetylcholine (a vasodilator) (42). In tempol- treated animals, an increase in NO bioavailability was further enhanced during TNF-α administration, resulting in an increase in RBF but GFR remained decreased, indicating a preferentially greater reduction in efferent vascular resistance in such a condition.
The exact cause of the natriuretic response to TNF-α despite its vasoconstrictor and hypofiltration effects in the present study is not yet clear. However, it seems that this effect of TNF-α was due to its direct influence on tubular sodium reabsorption as fractional excretion of sodium increased during TNF-α infusion. It was reported in previous in vitro studies that TNF-α exerts direct inhibitory action on Na+-K+-ATPase, Na+-K+-2Cl− cotransporter, and renal epithelial sodium channel (ENaC) activity in the renal tubule. Kreydiyyeh and Markossian (35) showed that TNF-α reduces the activity and expression of renal Na+-K+-ATPase and the Na+-K+-2Cl− cotransporter in the kidney cortex and medulla. In another in vitro study, TNF-α was shown to inhibit ENaC activity which is mediated by ceramide and the PKC-dependent pathway (2). Thus it is conceivable that the natriuretic effect of TNF-α in the present study could be due to its direct inhibitory action on tubular sodium transporters. Further experiments are needed to examine this mechanism more comprehensively. As Na+-K+-ATPase and the Na+-K+-2Cl− cotransporter play an important role in the Na+ reabsorption in the proximal tubules and in the thick ascending limb of the loop of Henle (15, 24), it is possible that inhibition of these transporters by TNF-α increases distal delivery and generates signals from the macula densa cells to afferent arterioles to elicit vasoconstriction by activation of the tubuloglomerular feedback (TGF) mechanism (6, 42). Such activation of the TGF mechanism may have partially influenced the RBF and GFR responses to TNF-α administration in the present study. However, further stringent in vivo and in vitro experiments using a direct functional and molecular approach will be required to examine this possibility.
TNF-α is produced by activated neutrophils and monocytes (51); however, it can also be generated by vascular endothelial cells and by mesangial and renal tubular epithelial cells in the kidney, particularly upon inflammation and tissue injury (3, 16, 54). More specifically, ANG II has been reported to increase TNF-α production in monocytes (25), in the thick ascending limb of the loop of Henle in the kidney (16), and in the mammalian heart (30). Increased production of TNF-α has been reported in many forms of experimental hypertension such as ANG II-dependent hypertension (23), Dahl salt-sensitive hypertension (21), DOCA-salt hypertension (13), and l-NAME-induced hypertension (4). Furthermore, Guzic et al. (23) showed that blockade of TNF-α normalized the blood pressure in ANG II-induced hypertension. Recently, it has also been shown that chronic ANG II administration also failed to cause a hypertensive response in TNF-α knockout mice (48) as well as in T lymphocyte-deleted mice (23). Collectively, all these studies suggest that TNF-α plays a key role in the pathogenesis of hypertension and cardiovascular disease, and targeting this proinflammatory cytokine may be a valuable strategy for lowering the incidence of hypertension and subsequent cardiovascular complications. Indeed, recently we have reported that chronic infusion of ANG II did not cause hypertension in TNF-α knockout mice (48). However, when a chronic replacement therapy was made with recombinant TNF-α, ANG II caused a similar hypertensive response, as observed in wild-type mice (48). Further investigation determining the chronic effects of TNF-α on renal hemodynamics and excretory function and involvement of different isoforms of NOS and NADPH oxidase in these responses would provide a better insight into the physiological and pathophysiological roles of TNF-α in the kidney.
In conclusion, present data demonstrate that TNF-α exerts a vasoconstrictor action but causes diuresis and natriuresis in the kidney. While a direct inhibitory action of TNF-α on epithelial sodium transport may cause natriuresis, our findings in the present study suggest that both an enhancement in O2− production, and thus a reduction in NO bioavailability due to its interaction with O2−, mediate the renal vasoconstrictor action of TNF-α.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-66432.
Acknowledgments
We gratefully acknowledge the technical support provided by Alexander Castillo.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Banks WA, Moinuddin A, Morley JE. Regional transport of TNF-alpha across the blood-brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol Aging 22: 671–676, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bao HF, Zhang ZR, Liang YY, Ma JJ, Eaton DC, Ma HP. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-α through protein kinase C. Am J Physiol Renal Physiol 293: F1178–F1186, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Baud L, Oudinet JP, Bens M, Noe L, Peraldi MN, Rondeau E, Etienne J, Ardaillou R. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int 35: 1111–1118, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Bourraindeloup M, Adamy C, Candiani G, Cailleret M, Bourin MC, Badoual T, Su JB, Adubeiro S, Roudot-Thoraval F, Dubois-Rande JL, Hittinger L, Pecker F. N-acetylcysteine treatment normalizes serum tumor necrosis factor-alpha level and hinders the progression of cardiac injury in hypertensive rats. Circulation 110: 2003–2009, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt B, Kribbs SB, Clubb FJ Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97: 1382–1391, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Braam B, Mitchell KD, Koomans HA, Navar LG. Relevance of the tubuloglomerular feedback mechanism in pathophysiology. J Am Soc Nephrol 4: 1257–1274, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Cooper ME, Wu LL, Cox AJ, Jandeleit-Dahm K, Kelly DJ, Gilbert RE. Blockade of the renin-angiotensin and endothelin systems on progressive renal injury. Hypertension 36: 561–568, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborti T, Gosh SK, Michael JR, Batabyl SK, Chakraborti S. Targets of oxidative stress in cardiovascular system. Mol Cell Biochem 187: 1–10, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Chen YF, Li PL, Zou AP. Oxidative stress enhances the production and actions of adenosine in the kidney. Am J Physiol Regul Integr Comp Physiol 281: R1808–R1816, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Chrousos GP The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann NY Acad Sci 917: 38–67, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension 47: 557–562, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Ferreri NR, Escalante BA, Zhao Y, An SJ, McGiff JC. Angiotensin II induces TNF production by the thick ascending limb: functional implications. Am J Physiol Renal Physiol 274: F148–F155, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Ferreri NR, Zhao Y, Takizawa H, McGiff JC. Tumor necrosis factor-alpha-angiotensin interactions and regulation of blood pressure. J Hypertens 15: 1481–1484, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 286: H2264–H2271, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes JM, Talamini MA, Fulton WB, Hanly EJ, Aurora AR, De Maio A. General anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin Vaccine Immunol 13: 281–288, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardiner SM, Kemp PA, March JE, Woolley J, Bennett T. The influence of antibodies to TNF-alpha and IL-1beta on haemodynamic responses to the cytokines, and to lipopolysaccharide, in conscious rats. Br J Pharmacol 125: 1543–1550, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD Jr. Renal NF-κB activation and TNF-α upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 291: R1817–R1824, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Guggillam A, Hartfield J, Haque M, Francis J. Ang II-induced oxidative stress in the heart is mediated through cytokines (Abstract). FASEB J 388: 16A, 2005. [Google Scholar]
- 23.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas M, Forbush B 3rd. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Hahn AW, Jonas U, Bühler FR, Resink TJ. Activation of human peripheral monocytes by angiotensin II. FEBS Lett 347: 178–180, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B, Zhao K, Whiteman M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: a personal view of recent controversies. Free Radic Res 31: 651–69, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Haque MZ, Majid DS. Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension 43: 335–40, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Hartfield JN, Haque M, Majid DSA, Francis J. Angiotensin II induced upregulation of myocardial eNOS m RNA is mediated through TNF-alpha (Abstract). FASEB J 20: 722A, 2005. [Google Scholar]
- 29.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Kalra D, Sivasubramanian N, Mann DL. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation 105: 2198–2205, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Kase H, Hashikabe Y, Uchida K, Nakanishi N, Hattori Y. Supplementation with tetrahydrobiopterin prevents the cardiovascular effects of angiotensin II-induced oxidative and nitrosative stress. J Hypertens 23: 1375–1382, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension 47: 568–572, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Kopkan L, Castillo A, Francis J, Majid DS. Renal responses to angiotensin II are attenuated in knockout mice lacking the gene for tumor necrosis factor-a (TNF-α). FASEB J 21: 595.20, 2007. [Google Scholar]
- 35.Kreydiyyeh SI, Markossian S. Tumor necrosis factor alpha down-regulates the Na+-K+ ATPase and the Na+K+2Cl− cotransporter in the kidney cortex and medulla. Cytokine 33: 138–144, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Jiao X, Tao L, Liu H, Cao Y, Lopez BL, Christopher TA, Ma XL. Tumor necrosis factor-α in mechanic trauma plasma mediates cardiomyocyte apoptosis. Am J Physiol Heart Circ Physiol 293: H1847–H1852, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Tao L, Jiao X, Liu H, Cao Y, Lopez B, Luan RH, Christopher T, Ma XL. TNF-alpha-initiated oxidative/nitrative stress mediates cardiomyocyte apoptosis in traumatic animals. Apoptosis 12: 1795–1802, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Malleo G, Mazzon E, Genovese T, Di Paola R, Muià C, Centorrino T, Siriwardena AK, Cuzzocrea S. Etanercept attenuates the development of cerulein-induced acute pancreatitis in mice: a comparison with TNF-alpha genetic deletion. Shock 27: 542–551, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-α-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol 293: H2726–H2737, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi K, Saigusa T. Sympathetic nervous responses during cytokine-induced fever in conscious rabbits. Pflügers Arch 433: 691–698, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Praticò D, Lawson JA, Rokach J, FitzGerald GA. The isoprostanes in biology and medicine. Trends Endocrinol Metab 12: 243–247, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Romero JC, Reckelhoff JF. State-of-the-Art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 34: 943–949, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Ortega M, Esteban V, Rupérez M, Sánchez-López E, Rodríguez-Vita J, Carvajal G, Egido J. Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens 15: 159–166, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 301: 418–426, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 51: 1345–51, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taishi P, Churchill L, De A, Obal F Jr, Krueger JM. Cytokine mRNA induction by interleukin-1beta or tumor necrosis factor alpha in vitro and in vivo. Brain Res 1226: 89–98, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J Hypertens 23: 165–174, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Vassalli P The pathophysiology of TNF-alpha. Annu Rev Immunol 10: 411–452, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Yang B, Rizzo V. TNF-α potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol 292: H954–H962, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Yang FL, Li CH, Hsu BG, Tsai NM, Lin SZ, Harn HJ, Chen HI, Liao KW, Lee RP. The reduction of tumor necrosis factor-alpha release and tissue damage by pentobarbital in the experimental endotoxemia model. Shock 28: 309–316, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Yard BA, Daha MR, Kooymans-Couthino M, Bruijn JA, Paape ME, Schrama E, van Es LA, van der Woude FJ. IL-1 alpha stimulated TNF α production by cultured human proximal tubular epithelial cells. Kidney Int 42: 383–389, 1992. [DOI] [PubMed] [Google Scholar]
- 55.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-α in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284: R916–R927, 2003. [DOI] [PubMed] [Google Scholar]