Abstract
We tested the anti-inflammatory agent methyl-2-acetamidoacrylate (M2AA), an ethyl pyruvate analog, in a cecal ligation-and-puncture (CLP) model of sepsis in CD-1 mice. M2AA administration at the time of CLP improved survival, renal function, kidney histology, liver injury, and splenocyte apoptosis, and lowered cytokine levels (TNF-α, IL-6, IFN-γ, and IL-10). When M2AA treatment was delayed 6 h (but not 12 h), M2AA still significantly reduced kidney dysfunction, liver injury, splenocyte apoptosis, and cytokine levels. NF-κB, a M2AA target, was transiently activated in spleen, peaking at 6 h; kidney and liver NF-κB increased steadily with a plateau at 12–24 h. M2AA reduced NF-κB activation in spleen at 6 h and in kidney and liver at 24 h. Splenectomy diminished the ability of M2AA to reduce cytokines, especially IL-6, but M2AA still decreased kidney and liver dysfunction, suggesting that splenic NF-κB is not central to M2AA action. In contrast, beneficial effects of chloroquine on cytokines and organ damage were neutralized by splenectomy, demonstrating a spleen-specific chloroquine target. Because M2AA and chloroquine act differently, we tested this combination. Survival at 96 h was highest with combination therapy (57%) vs. chloroquine (38%), M2AA (47.6%), or vehicle (5%). The benefit of combination therapy over chloroquine or M2AA alone did not reach statistical significance, indicating potential mechanistic overlap. We conclude that the transient target(s) for M2AA responsible for the narrow 6-h therapeutic window is not splenic NF-κB. Identifying this new target and downstream signaling pathways could lengthen the therapeutic window and improve combination therapy with chloroquine.
Keywords: cecal ligation-puncture, spleen, apoptosis, nuclear factor-κB, chloroquine
the mortality rate of sepsis and septic shock remain unacceptably high and have not changed in several decades (9). Approximately 50% of septic patients develop acute kidney injury (AKI) (28, 34). The mortality rate of septic patients with AKI remains as high as 70%, and the development of AKI in septic patients predicts a poor outcome (28). Ethyl pyruvate (EP) is a derivative of an endogenous antioxidant pyruvate (35). EP can scavenge reactive oxygen species and can downregulate proinflammatory cytokines both in vitro and in vivo. EP inhibits high-mobility group box 1 (HMGB1) and decreases the activation of p38 mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) (35, 45, 47, 51). EP also preserves mucosal histology and permeability after mesenteric ischemia-reperfusion injury (40) and survival and intestinal damage after hemorrhagic shock (42), alcoholic hepatitis (52), and coronary ischemia and reperfusion (50). Previously, our group (2, 33) reported that EP can decrease sepsis-induced AKI and multiple organ failure in a clinically relevant sepsis model of cecal ligation and puncture (CLP), in which mice were treated with fluids and antibiotics. Because of its chemical instability in aqueous solution, EP needs to be prepared immediately before injection in a calcium ion-containing solution (Ringers EP solution) to increase its stability (8) and used shortly after dilution, which limits its usefulness as a therapeutic agent. EP analogs, including methyl-2-acetamidoacrylate (M2AA), also have anti-inflammatory properties and inhibit LPS-induced nitric oxide, TNF-α, NF-κB, and amelioration of the increase of epithelial permeability (38), but with more stable chemical properties.
In the present study, we have evaluated the effect of M2AA, a more potent and more stable analog of EP, on sepsis-induced AKI and multiple organ failure. Our group (54) recently reported that chloroquine (CQ) improves survival of CLP-induced sepsis and reduces renal injury; others have shown that CQ improves survival following hemorrhagic shock (7) and protects mice challenged with oligodeoxynucleotides containing CpG motif (CpG-ODN) and LPS (16). In the present study we have examined the effect of M2AA and CQ and compared the involvement of the spleen in their mechanisms of action by using splenectomy. We hypothesized that a combination therapy with two effective drugs, which have different mechanisms of action, might have a synergistic benefit. Therefore, we also examined a combination treatment with M2AA and CQ.
METHODS
Animals.
Animal care followed the National Institutes of Health (NIH) criteria for the care and use of laboratory animals in research, under a protocol approved by the National Institute of Diabetes and Digestive and Kidney Diseases animal care and use committee. Male CD-1 mice (7–9 wk; Charles River Laboratories, Wilmington, MA) had free access to water and chow before and after surgery.
Polymicrobial CLP sepsis-induced AKI.
CLP was performed as previously described with some modifications (5, 33). Our group (5) has previously reported, as have others (23), that the length of the ligated cecum determines the severity of sepsis and, ultimately, survival, kidney dysfunction (33), and liver damage (3–5). In this study, we ligated the cecum at 12 mm to obtain a model of moderately severe sepsis. The cecum was punctured twice with a 21-gauge needle, gently squeezed to express a small amount of fecal material, and then returned to the central abdominal cavity. In sham-operated animals, the cecum was isolated but neither ligated nor punctured. The abdominal incision was closed in two layers with 6-0 nylon sutures. After surgery, 30 ml/kg body wt of prewarmed normal saline was given intraperitoneally (53). All animals received antibiotic and fluid therapy subcutaneously (imipenem/cilastatin, 14 mg/kg in 1 ml of normal saline at 6 h and 7 mg/kg in 1 ml of normal saline at 18 h after surgery). Twenty-four hours after surgery, blood was collected by cardiac puncture for measurement of serum markers of organ injury and cytokine responses. Kidneys, liver, and spleen were fixed in 10% neutral buffered formalin.
Splenectomy.
Splenectomy or sham splenectomy was performed immediately before CLP surgery. An incision was made in the left flank, and the spleen was isolated. The splenic pedicles were ligated using 4-0 silk sutures, and the spleen was removed. In the sham splenectomy group, a flank incision was made, the spleen was isolated, but the pedicles were not ligated and the spleen was not removed. The incision was closed using 6-0 nylon sutures.
Treatment with M2AA and CQ.
M2AA (Sigma-Aldrich, St. Louis, MO) at the dose of 8 mg/kg dissolved in normal saline (0.3 ml) or an equal volume of normal saline was injected intraperitoneally at 0, 6, or 12 h after CLP surgery. CQ (50 mg/kg; Sigma-Aldrich) dissolved in 0.3 ml saline or an equal volume of saline was administered by oral gavage 3 h before CLP surgery. In the splenectomy and combination therapy treatment, the control CLP group received vehicle 3 h before and 0 h after CLP, the CQ group received vehicle at 0 h, and the M2AA group received vehicle 3 h before CLP.
Survival study.
Survival after surgery was assessed every 6 h within the first 48 h and then every 8 h for 4 days. Antibiotic injection (14 mg/kg imipenem/cilastatin) and fluid resuscitation (1 ml of normal saline) were started 6 h after CLP by subcutaneous injection and then repeated with less antibiotic (7 mg/kg imipenem/cilastatin in 1 ml of normal saline) every 12 h for 4 days. Morbidly ill animals were euthanized.
Blood chemistries and cytokine measurements.
Blood urea nitrogen (BUN), aspartate transaminase (AST), alanine transaminase (ALT), amylase, creatine kinase (CK), and lactate dehydrogenase (LDH) were measured using an autoanalyzer (Hitachi 917; Boehringer Mannheim, Indianapolis, IN). Serum creatinine was measured by HPLC (57). TNF-α, IL-6, IL-10, and IFN-γ were measured by ELISA (R&D Systems, Minneapolis, MN).
Bacterial abundance in blood and peritoneal fluid.
Peritoneal fluid or blood was analyzed for the presence of bacteria by dilution plating onto blood agar (Remel, Lenexa, KS) and colony counting after 24-h incubation at 37°C. Bacterial counts were expressed on a log10 scale.
Quantification of NF-κB activation.
The activity of NF-κB p65 was measured with a TransAM NF-κB family transcription factor assay kit (Active Motif, Carlsbad, CA) as previously described (5), using 100 μg of whole cell extracts from kidney, liver, and spleen tissue. Values of each sample were normalized to a positive control: Raji nuclear extract (Active Motif).
Histology.
The 10% formalin-fixed, paraffin-embedded kidney sections were stained with periodic acid-Schiff (PAS) reagent (Sigma-Aldrich). Histological changes in the cortex and in the outer stripe of the outer medulla (OSOM) were assessed by quantitative measurements of tissue damage by a blinded observer. Tubular damage was defined as tubular epithelial swelling, loss of brush border, vacuolar degeneration, necrotic tubules, cast formation, and desquamation. The degree of kidney damage was estimated at ×200 magnification, using five randomly selected fields for each animal, by the following criteria: 0, normal; 1, area of damage <25% of tubules; 2, damage involving 25–50% of tubules; 3, damage involving 50–75% of tubules; and 4, 75–100% of the area being affected.
Immunohistochemical analysis of active caspase-3 in spleen.
Immunohistochemical staining of 4-μm paraffin sections was performed as previously described with anti-active caspase-3 antibody (Cell Signaling Technology, Beverly, MA) (4). Active caspase-3 staining, a marker of apoptosis, was examined in five randomly chosen ×200 fields of white pulp and expressed as positive cells per high-power field.
Statistical analysis.
Differences between the groups were examined for statistical significance by performing Student's t-test or analysis of variance (ANOVA) with an appropriate correction. Comparisons between survival curves were performed using a log-rank test (SigmaStat 3.1; Systat Software, Point Richmond, CA). A P value <0.05 was accepted as statistically significant.
RESULTS
M2AA improved survival and kidney dysfunction after polymicrobial sepsis.
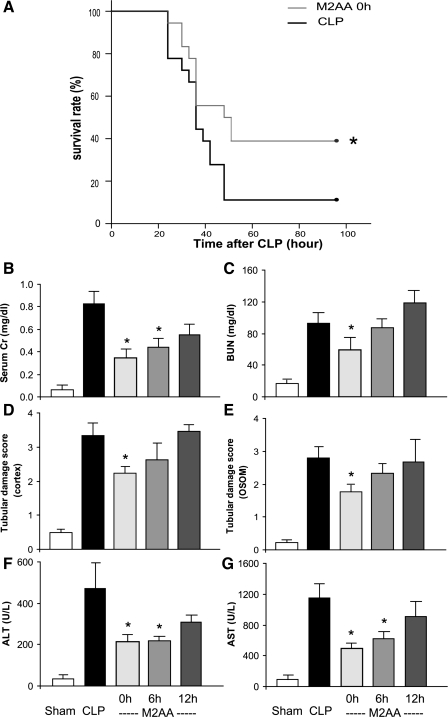
We determined whether M2AA altered sepsis-induced mortality, renal dysfunction, and liver injury in young outbred CD-1 mice treated with fluid and antibiotics. Intraperitoneal M2AA (8 mg/kg) given immediately after CLP surgery altered sepsis-induced mortality and organ injury in CLP. Survival at 96 h after CLP was 39% in M2AA-treated mice and 11% in CLP with vehicle-treated mice. (P < 0.05; Fig. 1A). M2AA treatment immediately after CLP also significantly reduced, by 24 h after surgery, kidney dysfunction evaluated as serum creatinine and BUN levels (Fig. 1, B and C), renal tubular injury evaluated as histology damage score in the cortex (Fig. 1D and Supplemental Fig. 1) and OSOM (Fig. 1E and Supplemental Fig. 1), and liver injury evaluated as increases in ALT and AST (Fig. 1, F and G). (Supplemental data for this article is available online at the American Journal of Physiology-Renal Physiology website.)
Fig. 1.
Methyl-2-acetamidoacrylate (M2AA) improves survival, kidney dysfunction, and liver injury after polymicrobial sepsis. A: survival analysis of control cecal ligation and puncture (CLP; solid line, n = 18) or M2AA at 0 h after CLP (shaded line, n = 18). *P < 0.05 vs. control. B–G: kidney function evaluated as serum creatinine (Cr) and blood urea nitrogen (BUN) levels (B and C), kidney tubular histology damage score in the cortex (D) and outer stripe of the outer medulla (OSOM; E), and liver function evaluated as increases in alanine transaminase (ALT; F) and aspartate transaminase (AST; G) at 24 h after surgery in sham group (n = 8) or groups treated with vehicle plus CLP (n = 8–11), M2AA 0 h after CLP (n = 9–11), M2AA 6 h after CLP (n = 9–11), or M2AA 12 h after CLP (n = 7–10). *P < 0.05 vs. vehicle plus CLP.
To explore the clinical potential of M2AA, we tested its window of therapeutic opportunity. M2AA (8 mg/kg) was given at 6 h after CLP surgery, when clinical symptoms first appear, or 12 h after CLP. M2AA administered at 6 h after CLP significantly attenuated kidney and liver dysfunction; however, M2AA was not effective when given at 12 h after CLP (Fig. 1, B–G). We also tested additional doses (4, 8, and 40 mg/kg), all of which improved kidney function; in contrast, 80 and 400 mg/kg M2AA did not improve kidney function (Supplemental Fig. 2), which may be related to toxicity at those doses. At 80 mg/kg M2AA, four of six mice died within 18 h, and at 400 mg/kg M2AA, five of six mice died within 6 h. Therefore, to maximize the efficacy and yet minimize the risk of toxicity, we used 8 mg/kg M2AA in all subsequent experiments.
M2AA reduced splenocyte apoptosis in polymicrobial sepsis.
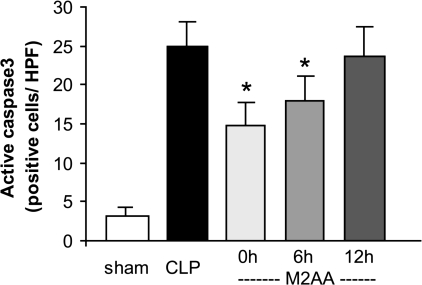
The spleen is injured in sepsis; splenocyte apoptosis is associated with the severity of sepsis outcome and can contribute to immune depression (17, 19–22). EP reduces apoptosis both in vitro and in vivo (6, 44, 48). Therefore, we administered M2AA at 0, 6, or 12 h after CLP and evaluated the effect of M2AA on splenocyte apoptosis by active caspase-3 immunohistochemistry at 24 h after CLP. The apoptotic cells were found mostly in the white pulp of spleen but not in kidney or liver, as previously reported (4). M2AA administered immediately or 6 h after CLP, but not at 12-h delayed treatment, significantly reduced splenocyte apoptosis (Fig. 2 and Supplemental Fig. 3). Although spleen injury and liver/kidney injury occur by apparently different mechanisms (apoptosis associated vs. apoptosis independent, respectively), the therapeutic window for damage to both organs is similar (Figs. 1, B–G, and 2).
Fig. 2.
M2AA attenuated splenocyte apoptosis in CLP sepsis: effect of M2AA in splenocyte apoptosis at 24 h after CLP. Splenocyte apoptosis was detected by immunohistochemistry of active caspase-3 in the spleen at 24 h after surgery in sham group (n = 4) or groups treated with vehicle plus CLP (n = 4), M2AA 0 h after CLP (n = 5), M2AA 6 h after CLP (n = 5), or M2AA 12 h after CLP (n = 4). *P < 0.05 vs. vehicle plus CLP.
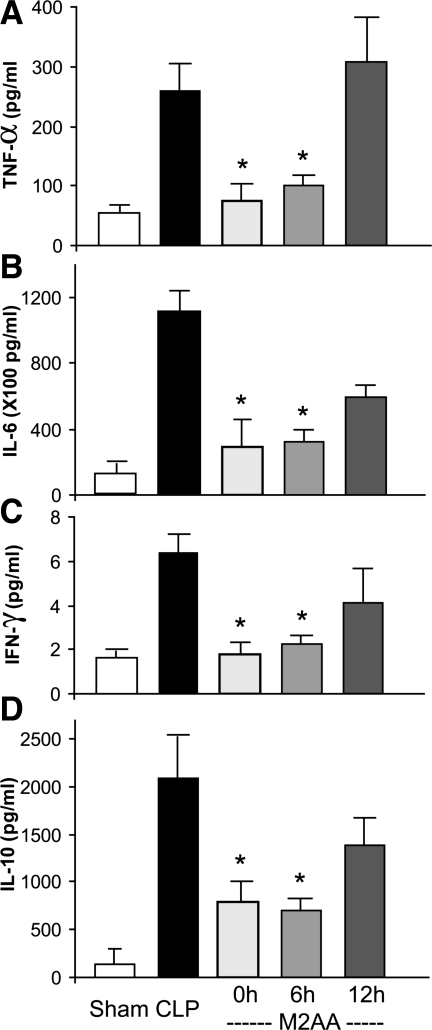
M2AA improved cytokine response in polymicrobial sepsis.
Sepsis increases pro- and anti-inflammatory cytokines; the balance between pro- and anti-inflammatory cytokines is associated with severity of sepsis (10, 36, 41, 46, 49). Our group previously reported increases in proinflammatory cytokines TNF-α and IL-6 (4, 15, 54, 55) and an anti-inflammatory cytokine IL-10 in our CLP model (3, 5, 54). Therefore, we determined the therapeutic window for M2AA treatment to reduce CLP-induced increases in the proinflammatory cytokines TNF-α, IL-6, and IFN-γ (Fig. 3, A–C) and the anti-inflammatory cytokine IL-10 (Fig. 3D). M2AA administered at the time of CLP or 6 h after CLP inhibited both pro- and anti-inflammatory cytokines, suggesting a balanced inhibition of inflammatory pathways. However, when administered 12 h after CLP, M2AA did not significantly inhibit the cytokine response (Fig. 3), in parallel with the therapeutic window for kidney, liver, and spleen damage (Figs. 1 and 2).
Fig. 3.
M2AA improves cytokine response. Serum proinflammatory cytokines (TNF-α, A; IL-6, B; and IFN-γ, C) and an anti-inflammatory cytokine (IL-10; D) at 24 h after surgery in sham group (n = 5) or groups treated with vehicle plus CLP (n = 8–9), M2AA 0 h after CLP (n = 9–10), M2AA 6 h after CLP (n = 11), or M2AA 12 h after CLP (n = 8–10). *P < 0.05 vs. vehicle plus CLP.
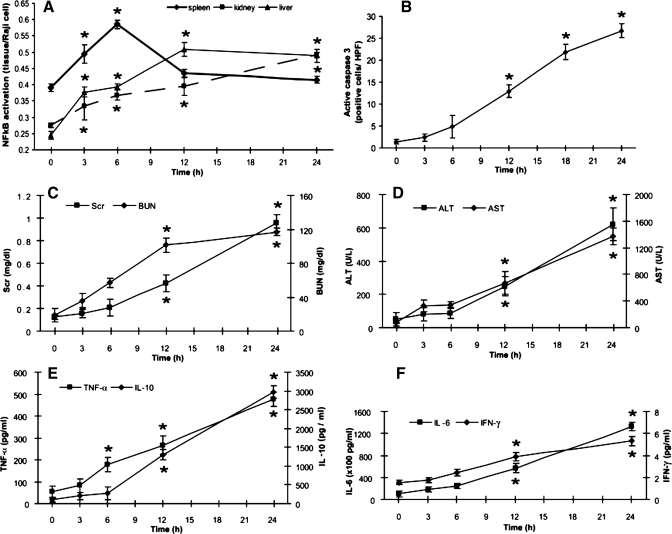
Time course of NF-κB activation, splenocyte apoptosis, kidney dysfunction, liver injury, and cytokine response in CLP sepsis.
M2AA had wide-ranging effects, preventing and preempting (at 6 h but not at 12 h) kidney, liver, and splenic injury. We wanted to find an early, transient, and locally activated signaling pathway inhibited by M2AA that might explain both the 6-h therapeutic window of M2AA (Figs. 1, B–G, and 3) and the localization of apoptosis to the spleen (Supplemental Fig. 3). NF-κB plays a critical role in both inflammation and apoptosis and in organ injury. Moreover, EP and its analogs, including M2AA, can directly inhibit NF-κB activation in vitro (38). Therefore, we determined the time course of NF-κB activation, active caspase-3-mediated apoptosis, and organ injury in spleen, liver, and kidney examined responses of inflammatory cytokines. NF-κB levels were significantly elevated in the spleen as early as 3 h, reached a maximum value at 6 h, and then decreased (Fig. 4A). In contrast, NF-κB activation in kidney and liver increased gradually and reached a maximum at 24 h. We found evidence for apoptosis in the spleen (Supplemental Fig. 3) but not in liver or kidney (data not shown). Splenocyte apoptosis was significantly increased after 12 h and maximal at 24 h (Fig. 4B and Supplemental Fig. 4). In contrast, kidney and liver dysfunction were not significant until 12 h (Fig. 4, C and D), although in both cases, organ injury followed NF-κB activation. In our model, TNF-α was increased at 6 h, although the other cytokines (IL-6, IFN-γ, and IL-10) increased at 12 h (Fig. 4, E and F). Both the cytokine response and organ dysfunction were maximal at 24 h. Interestingly, early NF-κB activation preceded organ damage/dysfunction in the spleen, kidney, and liver. These data suggest that 1) the sepsis response occurred very early (3 h after surgery) and that all of the parameters had already reached a high level at 12 h, which might explain why delayed treatment with M2AA at 12 h was ineffective; and 2) the decrease in NF-κB activation in spleen, kidney, and liver might decrease the damage to each organ in sepsis.
Fig. 4.
Time course of NF-κB activation, splenocyte apoptosis, kidney dysfunction, liver injury, and cytokine responses after CLP. Animals were killed at indicated times for measurement of each parameter. A: activated NF-κB p65 in spleen, kidney, and liver were measured at indicated time points and normalized by Raji nuclear extract. B: time course of splenocyte apoptosis detected by immunohistochemistry of active caspase-3 in the spleen. C–F: kidney function as measured by serum Cr and BUN (C), liver function as measured by ALT and AST (D), and cytokine levels (TNF-α and IL-10, E; Il-6 and IFN-γ, F) (n = 4–6 per group). *P < 0.05 vs. 0 h.
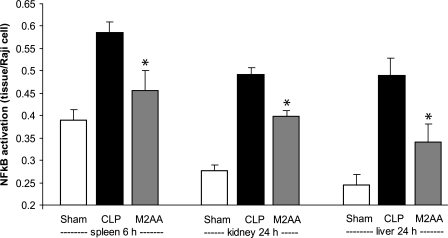
M2AA reduced NF-κB activation in spleen, kidney, and liver.
NF-κB activation preceded splenocyte apoptosis, appearance of inflammatory cytokines, and organ injury; the activation at 6 h in the spleen was particularly striking (Fig. 4A). Most of the organ injury was maximal at 24 h. Therefore, we evaluated the effect of M2AA on NF-κB activation in spleen at 6 h (because of its early activation) and in kidney and liver at 24 h. We found that M2AA reduced NF-κB activation in the spleen at 6 h and also in liver and kidney at 24 h and after CLP surgery (Fig. 5 and Supplemental Fig. 5).
Fig. 5.
M2AA reduced NF-κB activation in spleen, kidney, and liver. We evaluated the effect of M2AA on NF-κB p65 activation in spleen at 6 h or in kidney and liver at 24 h. NF-κB activation (normalized by Raji cell nuclear extract) in sham group (n = 4–6) or groups treated with vehicle plus CLP (n = 4) or M2AA (n = 4). *P < 0.05 vs. control CLP.
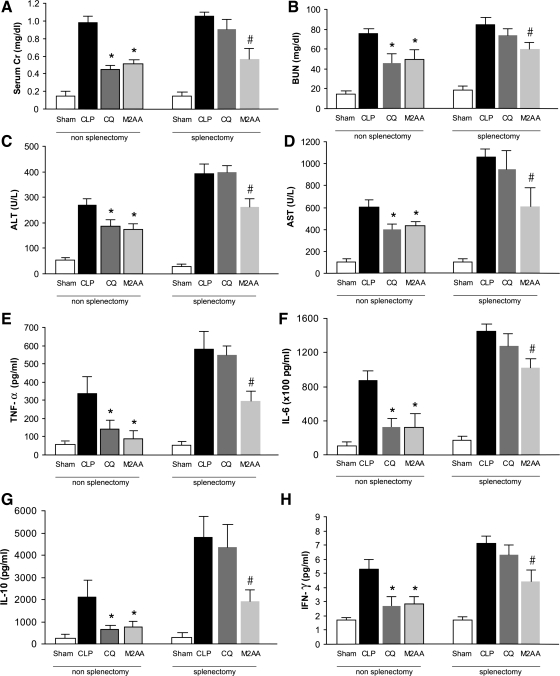
Target of CQ, but not M2AA, was localized to the spleen.
Our group (54) recently showed that CQ reduces the mortality and organ injury from sepsis, in part, via inhibition of TLR9 receptors, which are maximally expressed in the spleen. In the current study, we found that M2AA inhibited mortality and organ injury, in part, by inhibition of NF-κB activation. Since NF-κB is activated early in the spleen, we hypothesized that an early target of M2AA could also be in the spleen. Hence, both CQ and M2AA could act primarily in the spleen, albeit not necessarily acting on the same drug targets. Therefore, we determined whether the action of either CQ or M2AA could be prevented in splenectomized mice. Splenectomy or sham surgery was performed at the same time as CLP surgery to minimize compensation by other peripheral lymphoid organs. CQ or vehicle was administered orally 3 h before CLP and M2AA, or vehicle was administered intraperitoneally immediately after CLP. Interestingly, M2AA still reduced sepsis-induced kidney dysfunction, liver injury in splenectomized mice, and, to a lesser extent, TNF-α and IL-10 levels (Fig. 6). However, after splenectomy, M2AA had very little residual effect on sepsis-induced IFN-γ and, especially, IL-6 levels (Fig. 6). Because M2AA inhibited organ damage without inhibiting all cytokines, a generalized inhibition of cytokines cannot account for the protective effect of M2AA on organ damage. Removal of the spleen in the absence of M2AA or CQ did not decrease CLP-induced organ damage or cytokine response, confirming a nonessential role for the spleen in the pathogenesis of sepsis (see discussion). In contrast, CQ could no longer inhibit sepsis-induced cytokine induction or kidney or liver dysfunction in splenectomized mice, revealing that chloroquine is entirely dependent on the spleen for its action. Although both M2AA and CQ have drug targets in the spleen, the cumulative impact of M2AA on sepsis outcomes depends on drug targets in other organs such as NF-κB activation in kidney and liver.
Fig. 6.
Comparison of M2AA and chloroquine (CQ) to improve sepsis-induced organ injury and cytokine levels in splenectomized mice. Splenectomy was performed at the same time as CLP surgery. Kidney dysfunction (A and B), liver injury (C and D), and cytokine levels (E–H) were determined at 24 h after surgery in mice subjected to sham surgery (n = 4–6) or treatment with vehicle plus CLP (n = 7–8), CQ (50 mg/kg) 3 h before CLP (n = 7–8), or M2AA 0 h after CLP (n = 6–8). *P < 0.05 vs. vehicle plus CLP in nonsplenectomy group. #P < 0.05 vs. vehicle plus CLP in splenectomy group.
As further evidence that CQ and M2AA act via different mechanisms, we found that M2AA did not significantly alter the bacterial counts in blood or the peritoneal cavity at 24 h after CLP (Supplemental Fig. 6). In contrast, our group (54) previously showed that CQ reduced blood bacterial counts without affecting peritoneal fluid bacterial counts.
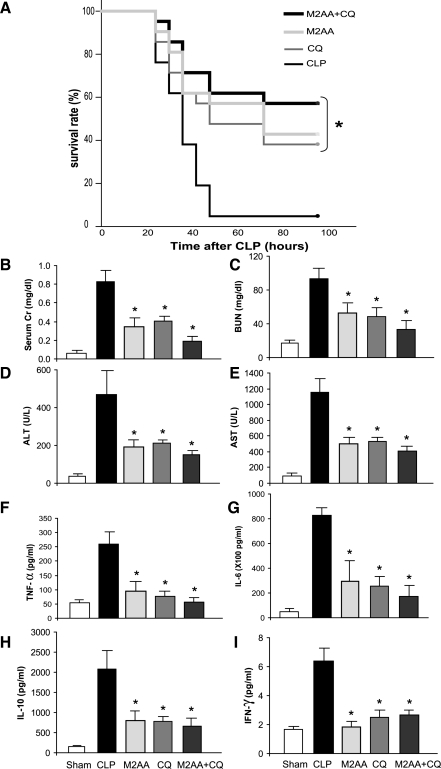
Effect of combination therapy of M2AA and CQ on survival and organ injury in CLP sepsis model.
The divergent responses of M2AA and CQ to polymicrobial sepsis in splenectomized mice, as well as their different effects on blood bacterial counts (54), suggested that these two agents might have different drug targets and pathways of action. Therefore, we examined whether a combination of both drugs might be synergistic. The survival rate at 96 h of control, CQ treatment, M2AA treatment, and combined treatment was 5, 38, 47.6, and 57%, respectively (n = 21 per group) (Fig. 7A). Both single-treatment groups improved survival compared with the vehicle-treated group (P < 0.05), and the combination therapy group showed the highest survival rate, although the differences between combination therapy and single-treatment groups were not statistically significant because of the small number of animals relative to the incremental benefit of combination treatment. The same tendency of improvement was observed in organ injury and some of the cytokine levels (Fig. 7, B–E).
Fig. 7.
Effect of M2AA and CQ combination therapy on survival, kidney dysfunction, liver injury, and cytokine levels in CLP sepsis model. A: survival curves from mice given vehicle (CLP; n = 21), M2AA 0 h after CLP (n = 21), CQ 3 h before CLP (n = 18), or M2AA + CQ treatment (n = 21). Kidney (B and C) and liver function (D and E) and cytokine responses (F–I) at 24 h after surgery are shown in sham group (n = 6) and in groups treated with vehicle plus CLP (n = 8–11), M2AA (n = 8–11), CQ (n = 7–11), or combination therapy (n = 7–11). *P < 0.05 vs. vehicle plus CLP.
DISCUSSION
We evaluated the effects of the EP analog M2AA on sepsis-induced mortality and organ injury in an outbred CD-1 strain mouse model of polymicrobial sepsis; the outbred strain is more similar to the genetically heterogeneous human population than typically used inbred strains (5). In this clinically relevant model of polymicrobial sepsis, we found that 1) M2AA improved mortality and reduced kidney dysfunction, liver injury, splenic apoptosis, and cytokine levels, even when treatment was delayed for 6 h; 2) NF-κB from spleen, but not kidney or liver, was activated by 6 h after CLP and then decreased; 3) M2AA reduced spleen NF-κB activation at 6 h CLP, yet also decreased splenocyte apoptosis at 24 h CLP; 4) after splenectomy, M2AA retained its effectiveness on sepsis, as indicated by almost all parameters measured, revealing that the spleen is not essential for M2AA action; 5) in contrast, CQ was no longer effective in splenectomized mice, as indicated by all parameters measured, identifying a spleen-specific target for CQ; and 6) despite apparently divergent mechanisms, combination M2AA and CQ therapy showed modest additivity.
M2AA improves survival, kidney dysfunction, liver injury, splenic apoptosis, and cytokine levels in polymicrobial sepsis.
Previously, our group (2, 33) reported the effectiveness of EP in a mouse model of polymicrobial sepsis. Translation from preclinical to clinical studies has been difficult for EP because of its chemical lability. M2AA, an EP analog, shares similar biological and mechanistic properties with EP; it is anti-inflammatory and is known to inhibit NF-κB and IL-1 receptor-associated kinase (25). Indeed, M2AA had a spectrum of action similar to that of EP in our mouse CLP model treated with fluids and antibiotics; both agents improved survival, reduced kidney and liver injury, and reduced both pro- and anti-inflammatory activities. M2AA had a shorter therapeutic window, since EP was active when treatment was delayed at 6 or 12 h, whereas M2AA was only active at 6, but not 12 h, after CLP. This shorter therapeutic window could be a consequence of several factors, including failure of M2AA to act on sufficient targets to reverse advanced organ damage, different pharmacodynamics of M2AA and EP, and/or additional beneficial effects of EP not found with M2AA.
Sepsis causes apoptosis in the thymus, spleen, gastrointestinal tract, and lung (13, 19, 20). Splenocyte apoptosis in sepsis may contribute to the impaired immune response and accelerate the severity of sepsis (21, 22, 43). Moreover, injection of apoptotic spleen cells into the CLP sepsis model accentuates the severity of sepsis (17). EP inhibits apoptosis both in vitro and in vivo (6, 44, 48). In the present study we found evidence for CLP-induced apoptosis in the spleen but not in kidney and liver; furthermore, M2AA administered at 0 or 6 h (but not 12 h) after CLP attenuated splenocyte apoptosis. It is not known whether M2AA inhibits splenic apoptosis by reducing sepsis severity or acting directly on splenocytes to suppress an apoptotic pathway.
Relationship of NF-κB activation, splenocyte apoptosis, and cytokine response in CLP sepsis, and reversal by M2AA.
We sought to find a pathway that was activated early, transiently, and inhibited by M2AA, since such a pathway might explain downstream responses, and the 6-h therapeutic window of M2AA. NF-κB is a transcription factor activated in immune and endothelial cells and in cells undergoing rapid differentiation. NF-κB is responsible for a broad spectrum of responses including inflammatory/immunoregulatory functions, apoptosis, adhesion molecule production, and cell cycle regulation (1, 32). NF-κB is known to be activated in many organs in sepsis models and has potent effects on downstream signaling pathways and tissue injury. EP directly inhibits p65 NF-κB and thereby attenuates inflammatory responses in vivo (11, 38, 45). Other EP analogs, including M2AA, also inhibit NF-κB activation (38).
Thus we evaluated the time course of NF-κB activation in the spleen, kidney, and liver, organs with widely different pathological responses to sepsis, and compared findings with downstream events such as TNF-α and organ damage. NF-κB activation occurred early (3 h in the spleen) and was followed by increases in serum TNF-α at 6 h. Other cytokines (IL-6, IFN-γ, and IL-10), splenocyte apoptosis, kidney dysfunction, and liver injury were detectable starting at 12 h after CLP. This is representative of a pleiotropic cytokine response during sepsis, where NF-κB mediates the activation of many cytokines, and in turn, several cytokines can activate NF-κB (32). Interestingly, although NF-κB is activated in many organs after sepsis, the pattern of NF-κB activation in spleen was quite different from that in kidney or liver. Splenic NF-κB activation spiked early (maximum at 6 h), whereas liver and kidney NF-κB slowly increased over 24 h. Apoptosis was also limited to the spleen and not detected in kidney or liver (data not shown). The finding that the surge of spleen NF-κB activation precedes splenocyte apoptosis suggests a possible link between NF-κB activation and splenocyte apoptosis in polymicrobial sepsis, although NF-κB is typically antiapoptotic (1, 31, 32) with few examples of proapoptotic activity (26, 39). The combined effects of M2AA on NF-κB and apoptosis may be an improvement over strategies to specifically inhibit splenic apoptosis either genetically or pharmacologically (18).
It is interesting that the slow increase in kidney and liver NF-κB activation occurs without triggering apoptosis or local inflammation (data not shown). Indeed, NF-κB activation is usually associated with local TNF-α production and subsequent triggering of an inflammatory response. The role of this parenchymal (i.e., kidney and liver cell) NF-κB activation is unknown but could be responsible for resolving inflammation (29, 30).
M2AA treatment in splenectomized mice and combination therapy with CQ.
CQ has been shown to improve survival of CLP-induced sepsis following hemorrhagic shock (7) or in CpG-ODN- and LPS-challenged mice (16). We recently showed that CQ reduced the severity of sepsis, including a reduction in sepsis-induced AKI, a reduction of splenocyte apoptosis, and an increase in systemic bacterial clearance. Most of the benefit from CQ might be attributed to inhibition of TLR9, one of several targets for CQ, and TLR9 resides primarily in spleen (54). Similarly, M2AA reduces the severity of sepsis, and this benefit is also associated with a decrease in early splenic NF-κB activation and a decrease in splenocyte apoptosis, consistent with a spleen-centered mechanism for M2AA. To address whether the spleen could be the common locus of these two anti-inflammatory drugs or even the site of a potentially unifying mechanism, we directly tested whether CQ and M2AA were still effective in splenectomized mice. The splenectomy or sham surgery was performed at the time of CLP surgery to minimize compensation from another lymphoid organ. The interpretation of the experiment is somewhat obscured, because splenectomy alone increased the severity of CLP-induced sepsis, including more severe clinical symptoms (data not shown), higher liver injury (ALT and AST), and higher cytokine levels (TNF-α, IL-6, IFN-γ, and IL-10), consistent with increased mortality (27). The spleen therefore contributes a net inhibitory effect on the inflammatory response during sepsis. However, this is not uniform, since serum IL-10 was increased over twofold with splenectomy/CLP, whereas serum creatinine was not significantly affected by splenectomy.
Factoring in this basal effect of splenectomy resetting the severity of sepsis, it is clear that CQ, as shown by every parameter we measured, is unable to protect against sepsis-induced kidney and liver dysfunction and increases in cytokines when the spleen is removed. The protective effect of CQ is dependent on one or more targets in the spleen. Although the beneficial effects of CQ are widespread, they evidently originate from the spleen. This is plausible, because TLR9 is found primarily in B cells and dendritic cells in spleen (12), but the source of CpG DNA or an alternative TLR9 ligand during the course of sepsis has not been elucidated.
In contrast to CQ, the relationship between M2AA and the spleen is less clear. Despite an early, transient activation of splenic NF-κB that coincided with the narrow window of therapeutic opportunity, much of the benefit of M2AA remained, perhaps because extrasplenic NF-κB is either more important than splenic NF-κB or becomes more prominent upon removal of the spleen. Even after splenectomy, M2AA significantly decreased all parameters, but the magnitude of the remaining benefit varied, ranging from IL-10 and TNF-α having the most intact M2AA response to IL-6 and IFN-γ having a minimal residual M2AA response.
Therefore, extrasplenic NF-κB activates most of the cytokines, whereas splenic NF-κB activation may be involved in a subset of the cytokine response, but this distinction is revealed only after M2AA treatment: IL-10, TNF-α, IL-6, and IFN-γ are increased comparably when the spleen was removed in the absence of M2AA. This adaptive, context-specific complexity is a common theme for the immune system, including NF-κB regulation (14), Toll-like receptors (37), and the cytokine network (56). For example, splenectomy worsened an otherwise beneficial effect of nicotine in a mouse CLP model (24).
Without completely understanding the mechanisms of CQ and M2AA, we nevertheless can conclude that these two agents have distinct mechanisms of action, where CQ acts, at least initially, on the spleen and M2AA is not dependent on the spleen. Further supporting this distinction, M2AA had no effect on bacterial load (Supplemental Fig. 6), whereas CQ significantly decreased bacterial load (54). These differences provided a rationale to test M2AA and CQ as a combination therapy. The individual treatments (M2AA or CQ) and combined treatment (M2AA and CQ) showed significant improvement in survival, kidney and liver injury, and cytokine levels compared with control. Although we did not detect a statistically significant synergistic effect from combination therapy (Fig. 7), the combination therapy group had the highest survival rate and lowest serum creatinine level. The failure to observe a statistically significant effect could be caused by insufficient numbers of animals (low power), particularly in the survival experiment; hence, it is difficult to draw any firm conclusions. However, in a different sepsis model, we have observed synergy between CQ and soluble FLT-1 using roughly the same number of animals. Thus the strategy of using drugs with nonoverlapping mechanisms is still conceptually valid, but the marginal effect of M2AA and CQ must be understood before further development of this particular combination. We speculate that there may be greater overlap between the two agents; perhaps one acts downstream of another, and their divergence at the level of the spleen may have little overall significance if other compensatory or parallel mechanisms blur the distinction between M2AA and CQ. There also may be a limit to using combinations of anti-inflammatory agents, where the capacity to inhibit the inflammatory response becomes saturated. The remaining organ dysfunction that is resistant to M2AA and CQ may be mediated by downstream signaling components that are irreversibly activated. Alternatively, the remaining organ dysfunction may be caused by parallel mediators. In either case, identification of these targets, their time course of action, specific inhibitors, and windows of therapeutic opportunity should lead to better combination therapies with M2AA and/or CQ.
Conclusion.
We have demonstrated that M2AA, a potent EP analog, improved survival and organ injury in a clinically relevant mouse CLP model. Delayed treatment starting at 6 h, but not 12 h, also improved sepsis outcomes. The mechanisms of its protection may include reduction of NF-κB activation in different organs, leading to the decrease in splenocyte apoptosis and cytokine surge. M2AA still showed almost all of its protective effects in splenectomized animals but did not increase bacterial clearance, demonstrating that inhibition of splenic NF-κB activation was not essential for the actions of M2AA. Since these latter two findings were not seen with CQ, the apparently different mechanisms of action provided a rationale for combination therapy. Finally, we found only a modest benefit of M2AA and CQ combination therapy; however, we speculate that combination therapy may be more effective when using drugs with vastly different mechanisms of action.
GRANTS
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Supplementary Material
Acknowledgments
Present address of H. Yasuda: First Department of Internal Medicine, Hamamatsu University School of Medicine, Hamamatsu 431-3192, Japan.
Present address of K. Doi: Department of Nephrology and Endocrinology, Tokyo University Hospital, Tokyo 13-8655, Japan.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Campbell KJ, Perkins ND. Regulation of NF-kappaB function. Biochem Soc Symp: 165–180, 2006. [DOI] [PubMed]
- 2.Dear JW, Kobayashi H, Jo SK, Holly MK, Hu X, Yuen PST, Brechbiel MW, Star RA. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int 67: 2159–2167, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Dear JW, Leelahavanichkul A, Aponte A, Hu X, Constant SL, Hewitt SM, Yuen PST, Star RA. Liver proteomics for therapeutic drug discovery: inhibition of the cyclophilin receptor CD147 attenuates sepsis-induced acute renal failure. Crit Care Med 35: 2319–2328, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PST, Hewitt SM, Sher A, Star RA. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int 69: 832–836, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doi K, Hu X, Yuen PST, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TEN, Frokiaer J, Nielsen S, Star RA. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int 73: 1266–1274, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epperly M, Jin S, Nie S, Cao S, Zhang X, Franicola D, Wang H, Fink MP, Greenberger JS. Ethyl pyruvate, a potentially effective mitigator of damage after total-body irradiation. Radiat Res 168: 552–559, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Ertel W, Morrison MH, Ayala A, Chaudry IH. Chloroquine attenuates hemorrhagic shock-induced immunosuppression and decreases susceptibility to sepsis. Arch Surg 127: 70–75; discussion 75–76, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Fink MP Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: potential benefits of resuscitation with Ringer's ethyl pyruvate solution. Curr Opin Clin Nutr Metab Care 5: 167–174, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time. Crit Care Med 26: 2078–2086, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181: 176–180, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther 312: 1097–1105, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 7: 247–253, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev 210: 171–186, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Holly MK, Dear JW, Hu X, Schechter AN, Gladwin MT, Hewitt SM, Yuen PST, Star RA. Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int 70: 496–506, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Z, Jiang Z, Liangxi W, Guofu D, Ping L, Yongling L, Wendong P, Minghai W. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int Immunopharmacol 4: 223–234, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci USA 100: 6724–6729, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6: 813–822, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med 25: 1298–1307, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27: 1230–1251, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis 35: 585–592, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA 96: 14541–14546, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock 24, Suppl 1: 52–57, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson AS, Johansson-Haque K, Okret S, Palmblad J. Ethyl pyruvate modulates acute inflammatory reactions in human endothelial cells in relation to the NF-kappaB pathway. Br J Pharmacol 154: 1318–1326, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi AR, Chung CS, Song GY, Lomas J, Priester RA, Ayala A. NF-kappaB activation has tissue-specific effects on immune cell apoptosis during polymicrobial sepsis. Shock 18: 380–386, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kang SC, Choudhry MA, Matsutani T, Schwacha MG, Rue LW, Bland KI, Chaudry IH. Splenectomy differentially influences immune responses in various tissue compartments of the body. Cytokine 28: 101–108, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Klenzak J, Himmelfarb J. Sepsis and the kidney. Crit Care Clin 21: 211–222, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434: 1138–1143, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med 7: 1291–1297, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y, Zhou Y, Shen P. NF-kappaB and its regulation on the immune system. Cell Mol Immunol 1: 343–350, 2004. [PubMed] [Google Scholar]
- 32.Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Miyaji T, Hu X, Yuen PST, Muramatsu Y, Iyer S, Hewitt SM, Star RA. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int 64: 1620–1631, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273: 117–123, 1995. [PubMed] [Google Scholar]
- 35.Reade MC, Fink MP. Bench-to-bedside review: amelioration of acute renal impairment using ethyl pyruvate. Crit Care 9: 556–560, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med 9: 517–524, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131: 1124–1136, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Sappington PL, Cruz RJ Jr, Harada T, Yang R, Han Y, Englert JA, Ajami AA, Killeen ME, Delude RL, Fink MP. The ethyl pyruvate analogues, diethyl oxaloproprionate, 2-acetamidoacrylate, and methyl-2-acetamidoacrylate, exhibit anti-inflammatory properties in vivo and/or in vitro. Biochem Pharmacol 70: 1579–1592, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: a question of life or death. J Biochem Mol Biol 35: 28–40, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med 29: 1513–1518, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med 21: 160–172, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Tawadrous ZS, Delude RL, Fink MP. Resuscitation from hemorrhagic shock with Ringer's ethyl pyruvate solution improves survival and ameliorates intestinal mucosal hyperpermeability in rats. Shock 17: 473–477, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol 171: 909–914, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Tsung A, Kaizu T, Nakao A, Shao L, Bucher B, Fink MP, Murase N, Geller DA. Ethyl pyruvate ameliorates liver ischemia-reperfusion injury by decreasing hepatic necrosis and apoptosis. Transplantation 79: 196–204, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA 99: 12351–12356, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to ‘immunoparalysis.' Chem Immunol 74: 162–177, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Wang LZ, Sun WC, Zhu XZ. Ethyl pyruvate protects PC12 cells from dopamine-induced apoptosis. Eur J Pharmacol 508: 57–68, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Wang TS, Deng JC. Molecular and cellular aspects of sepsis-induced immunosuppression. J Mol Med 86: 495–506, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Woo YJ, Taylor MD, Cohen JE, Jayasankar V, Bish LT, Burdick J, Pirolli TJ, Berry MF, Hsu V, Grand T. Ethyl pyruvate preserves cardiac function and attenuates oxidative injury after prolonged myocardial ischemia. J Thorac Cardiovasc Surg 127: 1262–1269, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Yang R, Gallo DJ, Baust JJ, Uchiyama T, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 283: G212–G221, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Yang R, Uchiyama T, Alber SM, Han X, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit Care Med 32: 1453–1459, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Yang S, Chung CS, Ayala A, Chaudry IH, Wang P. Differential alterations in cardiovascular responses during the progression of polymicrobial sepsis in the mouse. Shock 17: 55–60, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, Klinman DM, Yuen PST, Star RA. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1050–F1058, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasuda H, Yuen PST, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 69: 1535–1542, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7: 454–465, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Yuen PST, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol 286: F1116–F1119, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.