Abstract
The retinoic acids all-trans retinoic acid (AT-RA) and 9-cis retinoic acid (9C-RA) and the retinoic acid receptors RAR and RXR significantly induce transcriptional activity from a 200-bp PKD1 proximal promoter in transfected mammalian cells. This PKD1 promoter region contains Ets, p53, and GC box motifs, but lacks a canonical RAR/RXR motif. Mutagenesis of the Ets sites did not affect RA induction. In contrast, GC box mutations completely blocked stimulation by AT-RA and by RXRβ or RARβ. Mithramycin A, which prevents Sp1 binding, significantly reduced basal promoter activity and suppressed upregulation by AT-RA and RXR. The 200-bp proximal promoter could not be induced by AT-RA in Drosophila SL2 cells, which lack Sp1, but could be activated in these cells transfected with exogenous Sp1. Small interfering RNA knockdown of Sp1 in mammalian cells completely blocked RXRβ upregulation of the promoter. These data indicate that induction of the PKD1 promoter by retinoic acid is mediated through Sp1 elements. RT-PCR showed that AT-RA treatment of HEK293T cells increased the levels of endogenous PKD1 RNA, and chromatin immunoprecipitation showed the presence of both RXR and Sp1 at the PKD1 proximal promoter. These results suggest that retinoids and their receptors may play a role in PKD1 gene regulation.
Keywords: all-trans retinoic acid, 9-cis retinoic acid, RAR, RXR
autosomal dominant polycystic kidney disease (ADPKD) is a human genetic disorder with a frequency of 1 in 200–1,000 individuals and accounts for ∼10% of end-stage renal disease (2, 6, 22, 29, 47, 53, 58, 65). Approximately 85% of all ADPKD cases are due to mutations in the PKD1 gene, whose gene product is a large, membrane-spanning protein, polycystin-1. The remaining cases are due to mutations in the PKD2 gene. All ADPKD patients develop bilateral cystic kidneys. They can also have a variety of other extrarenal manifestations, including liver cysts, coronary artery disease, cardiac hypertrophy, hypertension, and cerebral aneurysms (17, 18, 20, 26, 48, 61).
While a two-hit loss-of-function mechanism has been hypothesized as the genetic cause of cyst initiation (5, 51, 52), there are conflicting observations as to whether cysts always result from abnormally decreased expression of the PKD genes and whether loss of both alleles is always required. On the one hand, Pkd1(+/−) mice develop few if any cysts in the kidney, whereas Pkd1(−/−) mice usually die in utero with massively cystic kidneys and cardiovascular abnormalities (4, 32, 38, 39, 44). On the other hand, there is evidence that haploinsufficiency at the Pkd1 locus can cause disease (36); there is immunocytochemical evidence for overexpression of polycystin-1 in cystic kidneys (46), and transgenic mice overexpressing a functional human PKD1 gene develop renal cysts (49, 60). Thus it is possible that ADPKD may be caused either by abnormally decreased (1- or 2-hits) or abnormally increased expression of the PKD1 gene.
Polycystin-1 appears to be developmentally regulated, with high expression during early embryogenesis in many tissues and organs, which decreases postnatally and is maintained at low levels in adults (9). Furthermore, the levels of PKD1 gene expression and polycystin-1 protein appear to govern in vitro cystogenesis (45). Thus any abnormality in the tight regulation of polycystin-1 protein levels may have important implications for normal physiological function and for cyst formation. Taken together, these observations signify the need to understand the transcriptional mechanisms regulating polycystin-1 levels. Earlier, our laboratory showed that the human PKD1 gene can be upregulated by β-catenin via a TCF/LEF consensus motif present within the PKD1 promoter (54). A number of other studies have also implicated β-catenin in the pathogenesis of PKD (28, 31, 62). We have also shown that the PKD1 proximal promoter contains two Ets binding elements, which are able to respond to the Ets factors Ets-1 and Fli-1 (50). It has also been recently demonstrated that p53 is a transcriptional repressor of PKD1, acting at the proximal promoter region (63). To further understand the mechanisms regulating PKD1 transcription, we have now looked for additional elements that could potentially regulate the expression of the PKD1 gene.
The retinoids, including all-trans retinoic acid (AT-RA) and 9-cis retinoic acid (9C-RA), are biologically active derivatives of vitamin A (13, 41). They have profound effects on the regulation of cell growth, differentiation, and apoptosis during embryonic development and within epithelial tissues in later life (8, 40, 42). The retinoids exert their effects through isoforms of RAR and RXR, the retinoic acid receptors, which are members of the nuclear hormone receptor superfamily. The RAR/RXR isoforms are ligand-dependent transcription factors that bind to DNA sequences, called RAR-elements (RAREs) or RXR-elements (RXREs), present in the promoter regions of target genes, including the RAR and RXR genes themselves. Upon ligand binding, the RARs/RXRs upregulate transcription of target genes through a variety of coactivators, such as steroid receptor coactivator-1 and p300/CBP [cAMP-response element binding protein (CREB)-binding protein] (10, 21, 24, 27, 66). In genes without canonical RA response elements, the RARs/RXRs have been shown to bind to and modulate activity of the transcription factor Sp1, resulting in an Sp1-mediated response to RA signaling (56, 59). Sp1 binds to a hexanucleotide sequence GGGCGG, called the “GC box” motif, or to variations of this sequence in which one or two nucleotides are substituted. Sp1 then activates transcription of GC box-containing genes by recruiting RNA polymerase II (19, 35). Disruption of one RXR isoform, RXRα, has been shown to cause conotruncal defects in mice, which are almost identical to the cardiac phenotype of Pkd1 null mice, suggesting that retinoids and Pkd1 may interact to play an important role in normal cardiac development (44).
In the present study, we have found that AT-RA, 9C-RA, and the RA receptors RAR and RXR activate the PKD1 promoter through a 200-bp proximal promoter region. We determined that there is an absence of canonical RAR or RXR elements in the 200-bp PKD1 proximal promoter, but that promoter activity is mediated through the ubiquitous transcription factor Sp1 and its binding sites in the proximal PKD1 promoter.
MATERIALS AND METHODS
Plasmid constructs.
A 3.3-kb human PKD1 promoter fragment (−3,346 to +33) subcloned in the promoterless luciferase reporter vector pGL2-Basic (Promega) was originally generated in our laboratory (54). Promoter deletion constructs (1.3 kb, 2.0 kb, and 200 bp) cloned in pGL2-Basic and/or pGL3-Basic were described previously (50, 54). The mammalian expression construct for Sp1 was from Dr. Yu-Chung Yang (Dept. of Pharmacology and Cancer Center, School of Medicine, Case Western Reserve University, Cleveland, OH). The constructs for RAR and RXR were from Dr. Ronald M. Evans, (Howard Hughes Medical Institute, the Salk Institute). The Sp1 pPac clone, for expression in Drosophila SL2 cells, was from Dr. Yu-Chung Yang.
Mutagenesis.
Two potential Ets binding sites (Ets-A and Ets-B) (50) and four of the six Sp1 sites (Sp1-A through Sp1-F) in the 200-bp proximal promoter region were mutated using the QuikChange site-directed mutagenesis kit (Stratagene) and the following double-stranded primers: Ets-A, 5′-GTG GGG CGG AGC Tct CGG AGG CCC CGC C-3′; Ets-B, 5′-GGA GGG TGA AGC CTC gtG ATG CCA GTC CCT CAT CG-3′; Sp1-A, 5′-CGC GTG Gtt CGG AGC TTC CG-3′; Sp1-B, 5′-GAG GCC aaG CCC TGC TGC CG-3′; Sp1-C, 5′-G CGA AGG Gtt CGG AGC CTG-3′; and Sp1-F, 5′-CC GTC taC CCC GCG CCG CGC-3′. The lowercase nucleotides represent the mutations. The 200-bp mutant fragments were subcloned into pGL2 and/or pGL3 and verified by sequencing.
Cells, transfections, and reporter assays.
HEK293T cells were cultured in DMEM with 4.5 g/l glucose, supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C (growth medium). HCT116 and COS-1 cells were maintained in DMEM/F12 (CellGro) containing 10% fetal bovine serum at 37°C. Drosophila SL2 cells were grown in Schneider's Drosophila medium with l-glutamine (GIBCO RL) at 26°C. Following overnight culturing in six-well plates, the cells were transfected using either the calcium phosphate method (HEK293T and HCT116 cells) or with Lipofectamine (GIBCO) (COS-1 cells) (43). A β-galactosidase expression plasmid (50 ng) was included to monitor transfection efficiency. After 6 h, the DNA-containing medium was removed, and the cells were washed with serum-free growth medium and incubated overnight in 1.5 ml/well of serum-free DMEM. The next morning, 0.5 ml of serum-free DMEM containing ethanol or the indicated amounts of RA (AT-RA or 9C-RA) was added to each well, and after 6 h the cells were harvested. In some experiments, cells were incubated with ethanol or AT-RA from the start of the transfection and were cultured posttransfection in DMEM containing 2% serum to which was added ethanol or AT-RA for an additional 40 h. Mithramycin A (MthA; 1 μM) was added following transfection, and the cells were harvested 40 h later. For reporter assays, cells were washed with ice-cold PBS and harvested in ice-cold 0.2 ml lysis buffer (0.5 M HEPES, 5% Triton X-100), lysed by vortexing (15 s, 2×) and cleared by centrifugation at 13,000 rpm for 5 min at 6°C. Luciferase assays were carried out using Luminol (Promega) as a substrate. β-Galactosidase assays were carried out in 1× reporter lysis buffer (Promega) using o-nitrophenyl-β-d-galactopyranoside (Sigma) as a substrate. Protein concentrations were determined with the Bio-Rad protein assay kit. The measured luciferase activity in each sample was normalized to either β-galactosidase activity or total protein. These normalized luciferase activities (RLU) were plotted using Microsoft Excel as the average ± SD of triplicate samples from typical experiments. Experiments were repeated at least two times. Statistical significance was determined using Student's t-test.
Transfection with small interfering RNA (siRNA) was carried out as follows. HEK293T cells were grown overnight in medium containing 10% serum in six-well plates (1.7 ml/well). The next day, the cells were transfected using the Lipofectamine 2000 (Invitrogen) method according to the manufacturer's instructions. Vector DNA or RXRβ expression plasmid (0.4 μg DNA) and the PKD1 promoter-luciferase reporter with or without siRNA duplex (80 pmol) against human Sp1 (SC-29487, Santa Cruz Biotechnology) were mixed with DMEM (total volume 150 μl/well) without serum and antibiotics (solution 1). Lipofectamine 2000 (6 μl/well) was diluted in DMEM (150 μl/well) without serum and antibiotics (solution 2). Solutions 1 and 2 were mixed and incubated for 25 min at room temperature. The mixed solution was added to the cells and the samples were incubated overnight at 37°C. Following incubation, the medium was replaced with fresh DMEM containing 5% serum and antibiotics, and the cells were harvested at 40 h.
SDS-PAGE and western blotting.
Expression of Sp1 in AT-RA-treated HEK293T cells was confirmed by 10% SDS-PAGE, followed by immunoblotting using rabbit anti-Sp1 antibody (sc-59, 1:10,000, Santa Cruz Biotechnology) and alkaline phosphate-conjugated secondary antibody (1: 8,000, Sigma). The protein bands were detected using CDP-Star (Amersham). The blots were reprobed with rabbit anti-actin antibody (A7434, Sigma).
Chromatin immunoprecipitation.
HEK293T cells were cross-linked for 10 min in 1% formaldehyde in PBS at room temperature. Cross-linking was stopped by adding glycine to a final concentration of 125 mM. Cells were washed with ice-cold PBS, harvested by centrifugation for 2 min at 5,000 rpm, resuspended in 0.5 ml of chromatin immunoprecipitation (ChIP) lysis buffer (10 mM Tris·HCl, pH 8.0, containing 10 mM NaCl, 0.5% NP-40, 1 mM EDTA, and protease inhibitor cocktail), and incubated on ice for 15 min. The crude nuclear extract collected by centrifugation at 2,500 rpm for 5 min was washed once with 0.2 ml of PBS, resuspended in 500 μl of 1× IP buffer (0.5% Triton X-100, 0.5% NP-40, 0.1% SDS in PBS, and protease inhibitor cocktail), and fragmented by sonication: 4 pulses for 15 s with 30-s cooling periods on ice, using a 2-mm probe at 60% output (Sonics model VCX 130, maximum 130 W). The fragmented chromatin was incubated with 5 μl of rabbit preimmune sera (R9133, Sigma), rabbit anti-RXR (D-20, Santa Cruz Biotechnology), or rabbit anti-Sp1 (PEP2, Santa Cruz Biotechnology). Following washing 3× with wash buffer (50 mM Tris·HCl, pH 8.0, containing 0.15 M NaCl, 0.5% Triton X-100, 0.5% NP-40, 0.1% SDS, 1 mM EDTA), the cross-links were reversed with elution buffer (1% SDS, 0.1 M NaHCO3, 0.4 M NaCl) for 3 h at 67°C. The DNA in the supernatant was purified by using a High Pure PCR Product Purification Kit (Boehringer Mannheim) and amplified for 35 cycles of 94°C for 45 s, 62°C for 45 s, 72°C for 45 s, with a final extension of 7 min at 72°C using the forward primer 5′-CTG CTG CCG ACC CTG TGG AG-3′ and the reverse primer 5′-GCG GCG GCG CGG GGC GGA CGG-3′ (135-bp product). The products were confirmed by sequencing.
Endogenous PKD1 expression.
HEK293T cells were treated with ethanol or AT-RA in DMEM containing 2% serum for 40 h. Total RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's protocol, and the samples were treated with DNase I (Ambion). RT-PCR was carried out with 1 μg RNA in a total volume of 25 μl using random primers and Superscript reverse transcriptase (Invitrogen) as described previously (54). Primers specific for PKD1 were forward, 5′-CGC CGC TTC ACT AGC TTC GAC-3′ and reverse, 5′-ACG CTC CAG AGG GAG TCC AC-3′, giving a 260-bp product; primers specific for the ribosomal protein L7 were forward, 5′-GCT TCG AAA GGC AAG GAG GAA GC-3′ and reverse, 5′-TCC TCC ATG CAG ATG ATG C-3′, giving a 440-bp product. Amplified PCR fragments were electrophoresed on 2% agarose gels containing ethidium bromide, and the bands were analyzed by National Institutes of Health Image software. Quantified band intensity was normalized to values for ribosomal protein L7 mRNA and plotted as relative units. The data were plotted with Microsoft Excel as the average ± SD of three independent experiments.
RESULTS
The PKD1 promoter is activated by retinoic acids and their receptors.
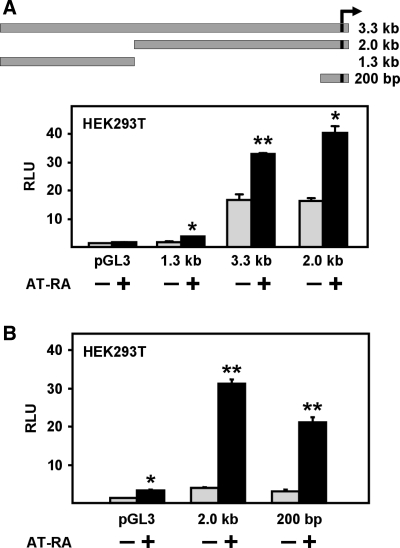
The 3.3-kb PKD1 promoter and its two proximal fragments, 2.0 kb and 200 bp, were found to have high basal promoter activity in transfected HEK293T cells and to be significantly induced by AT-RA treatment (Fig. 1, A and B). In contrast, the distal 1.3-kb fragment was found to have lower basal activity and to be less responsive to AT-RA. Our previous studies showed that the 200-bp proximal fragment has high promoter activity, suggesting that it contains functionally important transcriptional elements (54). Indeed, it has been recently shown that the 200-bp proximal fragment has functionally responsive Ets and p53 sites (50, 63). The relatively strong response to AT-RA of the 200-bp proximal promoter fragment suggests that the primary retinoic acid response is mediated by elements within the first 200 bp. Deletion analysis (data not shown) of the 2.0-kb promoter confirmed that the 200-bp proximal fragment is responsible for AT-RA induction. This AT-RA induction varied from 3-fold to up to >10-fold, depending on the experiment.
Fig. 1.
The PKD1 promoter is activated by all-trans retinoic acid (AT-RA). A and B: HEK293T cells were transfected with 1.0 μg of pGL3 or one of the PKD1 promoter-luciferase reporter constructs. The transfected cells were incubated with either ethanol (−) or 5 μM AT-RA (+) for 6 h. Luciferase activities were normalized to β-galactosidase activities. The average relative light unit (RLU) value obtained for the ethanol-treated (−), pGL3-transfected cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. All of the constructs were inducible to some degree. While in B, pGL3 is seen to respond to RA treatment, this was usually not the case (see A and Figs. 2 and 3). *P < 0.05. **P < 0.01.
The 200-bp PKD1 promoter fragment responded to both 9C-RA and AT-RA in a dose-dependent fashion (Fig. 2). This PKD1 promoter fragment also responded to AT-RA in monkey kidney COS-1 cells and human colon carcinoma HCT116 cells (Fig. 3), indicating that the response is not specific to HEK293T cells. It was also possible to stimulate the 200-bp promoter-reporter ∼2.5–6-fold by transfection of retinoic acid receptors alone, with RARβ showing the highest activity (Fig. 4). The only exception was RARγ, which elicited no increase over basal promoter activity. These data suggest that sequences in the 200-bp PKD1 proximal promoter can respond to vitamin A metabolites and their nuclear receptors.
Fig. 2.
The 200-bp proximal PKD1 promoter is stimulated by retinoic acid in a dose-dependent manner. HEK293T cells were transfected with 0.5 μg of the 200-bp PKD1 promoter-luciferase reporter construct in pGL3. The transfected cells were incubated with either ethanol (−) or the indicated concentrations of 9-cis retinoic acid (9C-RA) or AT-RA for 6 h. Luciferase activities were normalized to β-galactosidase activities. The average RLU value obtained for the ethanol-treated (−) cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. The 200-bp construct was induced by both 9C-RA and AT-RA (*P < 0.05. **P < 0.01. ***P < 0.001).
Fig. 3.
The proximal PKD1 promoter is activated by retinoic acid in COS-1 and HCT116 cells. COS-1 cells were transfected using Lipofectamine, with 1.0 μg of the 200-bp PKD1 promoter-luciferase reporter construct in pGL3. The cells were incubated for 2 h in serum-free/antibiotic-free growth medium containing DNA-Lipofectamine and then were placed in growth medium containing 2% serum (no antibiotics), with ethanol, or with 5 μM AT-RA, and were harvested after an additional 38 h. HCT116 cells were transfected using the calcium phosphate method and were treated with ethanol or with 5 μM AT-RA. Luciferase values were normalized to β-galactosidase activities. The average RLU value obtained for the ethanol-treated cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. The 200-bp construct was induced in both COS-1 and HCT116 cells (**P < 0.01).
Fig. 4.
Retinoic acid receptors can induce the 200-bp proximal PKD1 promoter. HEK293T cells were transfected with 0.2 μg of pGL3 or the 200-bp promoter-luciferase reporter construct together with 0.2 μg of pcDNA3 and retinoic acid receptors RXRβ, RARα, RARβ, or RARγ. The transfected cells were then incubated with growth medium containing 2% serum for 40 h. Luciferase values were normalized to protein concentration. The average RLU value obtained for the control (pGL3+pcDNA3) transfected cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. The 200-bp construct was induced by RXRβ, RARα, and RARβ, but not RARγ relative to the 200-bp+pcDNA3 control (**P < 0.01). RXRβ also seemed to induce pGL3 (*P < 0.05); however, this is likely to be nonspecific.
The 200-bp PKD1 proximal promoter lacks a canonical RAR/RXR response element.
Sequence analysis revealed that the 200-bp proximal promoter region does not have an RAR/RXR response element (Fig. 5). We recently showed that this proximal promoter sequence does have two consensus Ets elements (EREs), Ets-A and Ets-B, and there are two consensus p53 elements (Fig. 5) (50, 63). As there are reports that Ets factors can mediate a retinoic acid response and can modulate transcription of RA target genes (30), we thought that the EREs might be involved in RA-mediated transcriptional upregulation of the PKD1 promoter. To test this idea, site-directed mutagenesis was used to selectively disrupt the core EREs at each Ets site. Mutations were introduced either at Ets-A or Ets-B, and at both Ets-A and Ets-B (Fig. 5). The effects of these mutations were then determined in HEK293T cells following RA treatment. None of these ERE mutants, however, was able to suppress the transcriptional stimulation by AT-RA (data not shown).
Fig. 5.
Sequence of the 200-bp proximal promoter region of the human PKD1 gene. Two consensus Ets binding sites (Ets-A and Ets-B), two p53 motifs (p53), and six potential GC boxes (Sp1-A to Sp1-F) upstream of the transcription start site (bold arrow) are shown. The core sequence corresponding to each element is underlined (Ets and Sp1) or overlined (p53). Mutations were generated by replacing 2 base pairs at each site. The locations of the forward primer (F-primer) and the reverse primer (R-primer) for chromatin immunoprecipitation (ChIP)-PCR analysis are indicated by the long arrows.
Sp1 elements mediate the RA response.
The 200-bp sequence was also found to contain six canonical “GC box” (GGGCGG) motifs (Sp1-A to Sp1-F). Sp1 binds the canonical GC box motif with high affinity. The PKD1 promoter lacks TATA and CAAT boxes, and thus its basal transcription, like numerous other TATA-less promoters, may depend on Sp1. In genes without canonical RA response elements, the RARs/RXRs, have been shown to modulate the activity of Sp1, resulting in an Sp1-mediated response to RA signaling (56, 59). Thus we thought that RA might activate the PKD1 promoter through one or more of these Sp1 sites. Mutations were introduced into Sp1-A, Sp1-B, Sp1-C, and Sp1-F (Fig. 5). A double mutant at Sp1-B and Sp1-C (Sp1-BC) and a triple mutant at Sp1-B, Sp1-C, and Sp1-F (Sp1-BCF) were also generated. As shown in Fig. 6, mutations at either Sp1-A or Sp1-B alone did not cause a decrease in basal or RA-induced promoter activity. While the Sp1-C single mutation seemed to have little or no effect on basal or induced promoter activity, the Sp1-BC double mutation had significantly decreased basal activity and a reduced AT-RA responsiveness compared with wild-type (Fig. 6, bottom). The Sp1-F single mutation also had significantly decreased basal activity and a reduced AT-RA responsiveness. All of the single or double mutants could also be induced by cotransfected RXRβ (data not shown). In contrast to the above results, the triple mutant (Sp1-BCF), showed an almost complete loss of both basal promoter activity and induced promoter activity in response to either AT-RA or 9C-RA (Fig. 7A) or to the nuclear receptors RXRβ or RARβ (Fig. 7B). This result suggested that the RA response is mediated primarily through a combination of Sp1 sites that includes but is not restricted to Sp1-F. As mutations at site Sp1-B alone had no effect on basal and AT-RA- or RXRβ-stimulated activities (Fig. 6 and data not shown), and mutations at sites Sp1-C or Sp1-F alone caused some reductions in AT-RA induction, it is likely that a combination of Sp1-C and Sp1-F mediate the RA response in concert. Repeated attempts to introduce mutations into Sp1-D/Sp1-E failed, making it impossible to test these sites; however, the fact that the triple mutant Sp1-BCF was almost completely inactive makes it unlikely that Sp1-D/Sp1-E has a role in RA responsiveness.
Fig. 6.
Effect of GC box mutations on the response to AT-RA. Single and double mutations of GC box motifs in the 200-bp PKD1 promoter fragment were generated by substituting 2 base pairs within each core consensus site as shown in Fig. 5. HEK293T cells were transfected with 1.0 μg of wild-type (WT) 200-bp promoter-luciferase reporter or the mutant constructs (Sp1-A, Sp1-B, Sp1-C, Sp-BC, or Sp1-F) in pGL3. The transfected cells were incubated with either ethanol (−) or 5 μM AT-RA (+) for 6 h. Luciferase values were normalized to β-galactosidase activities. The average RLU value obtained for the ethanol (−)-treated, WT-transfected cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. Top: Sp1-A, Sp1-B, and Sp1-C mutations were all inducible (*P < 0.05, **P < 0.01) but had little effect on basal activity. Bottom: Sp1-BC and Sp1-F mutations were inducible (*P < 0.05) and had decreased basal and induced activities (#P < 0.05, ##P < 0.01) compared with the WT basal and induced control activities.
Fig. 7.
Sp1-BCF mutant is not inducible by retinoic acids or their receptors. The triple mutant, Sp1-BCF, was created by introducing mutations at the Sp1-B, Sp1-C, and Sp1-F sites in the 200-bp fragment. A: HEK293T cells were transfected with 1.0 μg of WT 200-bp promoter-luciferase reporter or the triple mutant construct in pGL3. The transfected cells were incubated with either ethanol (−), 5 μM AT-RA, or 10 μM 9C-RA for 6 h. Luciferase values were normalized to β-galactosidase activities. B: HEK293T cells were transfected with 0.2 μg of WT 200-bp promoter-luciferase reporter or the triple mutant construct in pGL3 together with 0.2 μg pcDNA3 (−), RXRβ, or RARβ. After transfection, the cells were incubated in growth medium containing 2% serum for 40 h. Luciferase values were normalized to protein concentration. The average RLU value obtained for the control (−) WT-transfected cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. The Sp1-BCF mutation significantly decreased basal (##P < 0.01) and induced (##P < 0.01, ###P < 0.001) activities compared with the WT basal and induced control activities.
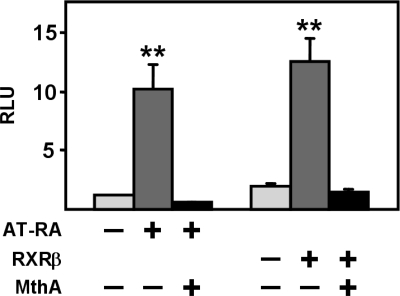
MthA completely blocks RA and RXR responses.
To test whether any of the GC boxes present in the 200-bp promoter sequence are induced by endogenous Sp1, we treated promoter-transfected HEK293T cells with MthA, a GC box inhibitor that specifically blocks interaction between Sp1 and the GC motif by masking GC-rich sequences (7, 23). As shown in Fig. 8, MthA treatment reduced the basal activity of the 200-bp PKD1 promoter by ∼70%, suggesting that one or more of the GC boxes is functional and that Sp1 is involved in basal transcription. As expected, MthA treatment had virtually no effect on the already very low basal activity of the Sp1-BCF mutant.
Fig. 8.
Effect of mithramycin A (MthA) on basal PKD1 promoter activity. HEK293T cells were transfected with 1.0 μg of WT or Sp1-BCF mutant 200-bp constructs. The transfected cells were incubated with growth media containing 2% serum and were treated with water (−) or 1 μM MthA for 36 h. Harvesting and assays were performed as in Fig. 1. Luciferase activities were normalized to β-galactosidase activities. The average RLU value obtained for the untreated WT-transfected cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. MthA significantly decreased basal activity of the WT 200-bp promoter (##P < 0.01) but not as completely as the Sp1-BCF triple mutation (###P < 0.001) compared with the respective WT controls.
To further establish a link between RA-mediated activation and Sp1, cells transfected with the wild-type 200-bp PKD1 promoter were treated with AT-RA in the absence or presence of MthA. As shown in Fig. 9 (left), the 10-fold activation of the promoter by AT-RA was completely suppressed by MthA. Induction of promoter activity by cotransfected RXRβ was also fully abolished by MthA (Fig. 9, right). These data further support the idea that Sp1 mediates the stimulation of the 200-bp PKD1 promoter by retinoid treatment.
Fig. 9.
Effect of MthA on induced PKD1 promoter activity. HEK293T cells were transfected with 0.5 μg of WT 200-bp PKD1 promoter construct only (left) or together with 0.4 μg of pcDNA3 (−) or RXRβ (+) (right). In promoter only-transfected cells (left), 50 ng of a β-galactosidase construct was included for a transfection control. The transfected cells were incubated with growth medium containing 2% serum plus ethanol (−) or 5 μM AT-RA (+), or water (−) or 1 μM MthA for 40 h. Luciferase activities were normalized to β-galactosidase activities (left) or protein concentrations (right). The average RLU value obtained for the untreated cells (far left) was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. AT-RA- or RXRβ-induced promoter activity (**P < 0.01) was completely blocked by MthA (below their respective basal promoter activities).
Exogenous Sp1 activates the 200-bp PKD1 promoter.
As Drosophila SL2 cells lack Sp1, but contain RAR/RXR homologs, we exploited this unique property of these cells to test the response of the 200-bp PKD1 promoter to exogenous Sp1. As shown in Fig. 10, the activity of the 200-bp promoter was significantly increased in the absence or presence of AT-RA after the cells were supplied with exogenous Sp1. In contrast, the promoter was not induced at all by AT-RA in the absence of Sp1. Taken together, these data support the idea that Sp1 can activate this promoter and thus can mediate the retinoid response.
Fig. 10.
Effect of exogenous Sp1 on basal and induced PKD1 promoter activity in Drosophila SL2 cells. Cells were grown in Schneider's Drosophila medium with l-glutamine (GIBCO RL) at 26°C and transfected with 1.0 μg of the 200-bp PKD1 promoter construct, together with 0.5 μg of pPAC vector alone or 0.5 μg of pPAC-Sp1. The transfected cells were incubated with growth medium for 30 h. The cells were then treated by adding ethanol or 5 μM AT-RA (+) and were incubated for 6 h. Luciferase values were normalized to protein concentrations. The average RLU value obtained for the untreated cells (far left) was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. The 200-bp construct was induced by exogenous Sp1 (*P < 0.05) but was not induced by AT-RA in the absence of exogenous Sp1.
To determine in mammalian cells whether exogenous Sp1 in combination with exogenous RXRβ or AT-RA treatment can stimulate the 200-bp PKD1 promoter, we cotransfected Sp1-transfected HEK293T cells with RXRβ, or treated Sp1-transfected cells with AT-RA. As shown in Fig. 11A, additional Sp1 led to activation of the 200-bp promoter, and this induction by exogenous Sp1 was further augmented by RXRβ, and even more so by AT-RA. As shown in the Western blot (Fig. 11B, left), HEK293T cells contain endogenous Sp1, which was not increased in AT-RA-treated cells or in RXRβ-transfected cells, thus indicating that the responses of the PKD1 promoter to RXRβ and AT-RA did not involve upregulation of endogenous Sp1. In contrast, Sp1 was significantly increased in the Sp1-transfected cells (Fig. 11B, right), thus explaining the augmentation of promoter activity in Sp1-transfected cells.
Fig. 11.
Effect of exogenous Sp1 on basal and induced PKD1 promoter activity in HEK293T cells. A: cells were transfected with 0.5 μg of WT 200-bp PKD1 promoter construct, together with 0.4 μg of pcDNA3 alone (−), 0.2 μg pcDNA3+0.2 μg of Sp1, or 0.2 μg Sp1+0.2 μg RXRβ. Fifty nanograms of a β-galactosidase construct was included in each transfection. The transfected cells were incubated with growth medium containing 2% serum only or were supplemented with ethanol (−) or 1 μM AT-RA for 40 h. Luciferase values were normalized to β-galactosidase or to protein concentration. The average RLU value obtained for the untreated cells (far left) was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. The 200-bp construct was induced by exogenous Sp1 (*P < 0.05) and further induced by the combination of Sp1 and RXRβ (**P < 0.01) or Sp1 and AT-RA (***P < 0.001). B: Western blot showing expression of Sp1 in untransfected cells (Cells), cells transfected with vector alone (pcDNA3), untransfected cells treated with AT-RA (AT-RA), and cells transfected with RXRβ (RXRβ). Also shown are cells transfected with vector (pcDNA3) compared with cells transfected with Sp1 (Sp1). Equal amounts of protein (30 μg) were analyzed and compared with a β-actin loading control.
To further demonstrate the involvement of Sp1 in promoter activation by the RA receptor, siRNA knockdown experiments were carried out. As shown in Fig. 12, activation of the 200-bp PKD1 promoter by RXRβ was completely abolished by Sp1-specific siRNA knockdown. Western blot analysis of cells in parallel experiments showed a significant reduction of Sp1 compared with a β-actin control.
Fig. 12.
Knockdown of Sp1 blocks RXRβ induction of the PKD1 promoter. HEK293T cells were transfected with the 200-bp promoter-luciferase construct (0.5 μg), β-galactosidase, and pcDNA3 or RXRβ (0.4 μg), with or without Sp1-small interefering RNA (siRNA) duplex (80 pmol). The cells were harvested 40 h posttransfection and assayed for luciferase and β-galactosidase. The average luciferase value obtained for control transfected cells was set at 1.0. Each bar is the mean ± SD of a representative experiment done in triplicate. RXRβ-induced activity (*P < 0.05) was blocked by siRNA knockdown (#P < 0.05). Right: HEK293T cells transfected in parallel experiments with (+) or without (−) Sp1-siRNA were analyzed for the presence of Sp1 and β-actin (as a control) by Western blotting.
The PKD1 gene is upregulated by AT-RA and binds RXR.
To determine whether expression of the endogenous PKD1 gene responds to RA, total RNA was isolated from AT-RA-treated HEK293T cells and analyzed by RT-PCR. As shown in Fig. 13 (A–C), PKD1 mRNA levels were increased ∼1.6-fold, whereas the ribosomal protein L7 mRNA was not affected by the treatment. As shown in Fig. 13D, there was a dose-response to AT-RA which increased PKD1 mRNA levels up to 2.6-fold at the maximum dose of 5 μM AT-RA.
Fig. 13.
Retinoic acid increases endogenous PKD1 mRNA levels. A–C: RT-PCR analysis of HEK293T cells with ethanol (−) or 5 μM AT-RA (+) for 40 h in growth medium containing 2% serum. A: amplification of L7 and PKD1 was carried out separately: 17 PCR cycles for ribosomal protein L7 mRNA; 26 PCR cycles for PKD1 mRNA. B: amplification of L7 and PKD1 was carried out in the same reaction: 21 PCR cycles. The last lane on the right contains markers. C: quantitation of the bands from 3 independent experiments. Each bar is the average ± SD of 3 independent experiments. *P < 0.05. D: cells were treated with 0, 1, 2, or 5 μM AT-RA for 40 h. Amplification of L7 and PKD1 was carried out in the same reaction: 21 PCR cycles. Fold-increase was calculated using National Institutes of Health Image software, from the PKD1 bands normalized to the L7 bands, setting the PKD1 band 0 AT-RA to 1.0. M, size markers.
To determine whether RXR binds the proximal promoter in vivo, ChIP-PCR assays were carried out with an anti-RXR antibody. As shown in Fig. 14 (lane 3), a PCR product similar in size to that obtained with the input DNA was seen, indicating that RXR is bound to a region within the PKD1 proximal promoter. This 135-bp promoter region includes Sp1-C and Sp1-F (the locations of the forward and reverse primers are shown in Fig. 5). Because this promoter region does not contain an RAR/RXR element, it is likely that RXR is bound indirectly through Sp1. As shown in lane 4, Sp1 was also found present on this 135-bp promoter region (Fig. 14). These results suggest that the PKD1 gene is a target of retinoid activation mediated through Sp1.
Fig. 14.
RXR and Sp1 bind within the proximal promoter of the PKD1 gene. ChIP-PCR analysis was carried out in untransfected HEK293T cells, as described in materials and methods. The formaldehyde-cross-linked, sonicated DNA was immunoprecipitated before PCR, and the amplified products were analyzed by gel electrophoresis. Lane 1, cross-linked input DNA (not immunoprecipitated); lane 2, cross-linked, rabbit preimmune serum immunoprecipitated DNA; lane 3, cross-linked, anti-RXR immunoprecipitated DNA (RXR); lane 4, cross-linked, anti-Sp1 immunoprecipitated DNA (Sp1).
DISCUSSION
In the present study, we report that the human PKD1 promoter is a target for vitamin A metabolites. This response was localized to the proximal 200-bp region upstream of the transcription start site. A 200-bp PKD1 promoter-reporter construct was typically induced up to >10-fold by RA (AT-RA or 9C-RA) treatment in transient transfection assays using three different cell lines. Activation of the 200-bp promoter-reporter was also observed in response to cotransfected RARα, RARβ, and RXRβ (but not RARγ). These results suggested that activation of the proximal region of the PKD1 promoter involves activation of RA receptors and that the PKD1 gene may be a target of RAR/RXR. Sequence analysis of the 200-bp PKD1 proximal promoter region identified two Ets elements, two p53 elements (63), and six GC boxes (Sp1-A thru Sp1-F), but no canonical RARE. However, not all effects of RAs are directly regulated by interactions between RAR/RXR and RAREs, and a number of mechanisms through which the retinoids stimulate gene transcription have been reported. These include inactivation of AP-1 via a CREB-binding protein-regulated pathway (66), inhibition of JNK (66), modulation of histone acetylation (27), and Ets- or Sp1-mediated activation (30, 56, 59).
ChIP showed that both Sp1 and RXR were bound to the PKD1 promoter in a 135-bp region that lies within 170 bp of the transcription start site, suggesting that RXR interacts with DNA-bound Sp1 at the proximal PKD1 promoter. Mutation of three of the GC boxes in the Sp1-BCF triple mutant eliminated all basal promoter activity and completely abolished the response to both retinoids (AT-RA and 9C-RA) and to both RXRβ and RARβ. Since the Sp1-B mutation alone had no effect on basal or induced activity, whereas the individual Sp1-C and Sp1-F mutations decreased promoter activity and the Sp1-BCF triple mutation had no promoter activity, it is likely that both Sp1-C and Sp1-F are functional Sp1 sites that are responsive to retinoid activation.
Similar observations were described for the urokinase plasminogen activator promoter (56). This promoter contains three canonical GC boxes and one atypical GC box within a 42-bp proximal promoter sequence. Mutation of the distal-most (canonical) site had no effect on either basal or hormone-induced activity, whereas a combination of mutations in the other three sites caused elimination of most basal and receptor-stimulated activity. Thus not all canonical GC box motifs are necessarily functional. Furthermore, a number of studies (11, 33, 34, 56) have identified the importance of 3′-flanking sequences immediately adjacent to the hexanucleotide core GC box motif in determining functionality. The presence of five or more purines or pyrimidines in the 3′-flanking region may reduce or completely abolish function, as observed in transforming growth factor (TGF)-β1 (CTCCCC and CCCCC), TGF-β1 RI (AGGGGG), TGF-β RII (AGAGAGG), and MDR1 (GGAGCAG), as they may interfere with binding of Sp1 to the GC box (56). In contrast, a mixture of purines and pyrimidines in the 3′-flanking region may support Sp1 binding. In the PKD1 promoter, the 3′-flanking regions of Sp1-A (AGCTTCC) and Sp1-B (GGCCTCC) contain five continuous pyrimidines, while those of Sp1-C (AGCCTGCAC) and Sp1-F (ACGGGGCGA) have mixed purine and pyrimidine sequences consistent with functional GC box motifs. We did not directly test the combined Sp1-D/Sp1-E sequence. However, the fact that the Sp1-BCF triple mutant completely abrogated promoter activity argues that the Sp1-D/Sp1-E sequence is not functional in either basal regulation or in the RA response.
MthA blocks interactions between Sp1 and GC boxes by obscuring GC-rich sequences (7, 23). MthA treatment significantly reduced (∼70%) basal activity of the wild-type PKD1 promoter, suggesting that it does so by preventing the binding of Sp1 to functional GC box motifs, most likely Sp1-C and Sp1-F. However, the incomplete inhibition of basal activity suggests that MthA was not completely effective at these sites, since both basal and induced promoter activities were almost completely abolished in the Sp1-BCF triple mutant. MthA completely inhibited both AT-RA and RXRβ induced promoter activity, suggesting that the RA response of the PKD1 promoter is dependent on Sp1.
Further support for an involvement of Sp1 RA-induced PKD1 promoter activity was obtained using Drosophila SL2 cells, which lack Sp1 (12) but contain RAR/RXRs (37). Quite robust induction of the PKD1 promoter was obtained following transfection of these cells with Sp1, and this Sp1-dependent induction could be further augmented by AT-RA treatment. Exogenous Sp1 was also able to upregulate the PKD1 promoter in HEK293T cells. While it could be argued that the action of AT-RA and RXRβ are to upregulate Sp1, this was ruled out by showing that neither AT-RA nor RXRβ changed Sp1 protein levels (Fig. 11B). Furthermore, siRNA knockdown of Sp1 in HEK293T cells blocked RXRβ induction of the promoter (Fig. 12). Taken together, these results suggest that RA and its receptors facilitate Sp1 activation of the PKD1 promoter.
Transactivation of the PKD1 promoter by retinoids may occur in a similar manner to that seen for the urokinase transglutaminase, TGF-β, and TGF-β RI promoters, by mediation of the interaction between Sp1 and GC box motifs (11, 33, 34, 56). In those studies, it was found that RA and its receptors failed to increase Sp1 mRNA or protein but that a physical interaction between Sp1 and the RARα/RXRα receptor dimer is an intermediate to induction by RA. In this case, RA treatment first induced expression of RA receptors through RARE sites (8, 40, 42). The RARs then physically interacted with Sp1 together with RXR and enhanced Sp1 binding to GC box motifs, resulting in Sp1-mediated upregulation of gene transcription (56). Both types of receptors had the same ability to modulate Sp1 binding, and in vivo they appeared to act synergistically. Similar interactions between Sp1 and the estrogen receptor have also been observed (16, 55, 57, 64). In contrast, it has been shown that RA activation of thrombomodulin gene transcription can occur by induction of both Sp1 mRNA and protein in RA-treated cells (25).
Sp1 family transcription factors play a key role in regulating transcription initiation at TATA-less promoters (3), and they are temporally and spatially regulated during vertebrate morphogenesis and are important for normal organogenesis (67). Similarly, the retinoids have profound effects on many essential biological programs, including cellular growth, somatic cell differentiation, and morphogenesis (42). In situ hybridization and RNA protection analyses have indicated distinct and often mutually exclusive expression patterns of some of the RA receptor isoforms (14, 15). We have demonstrated that expression of the endogenous PKD1 gene was elevated in response to AT-RA treatment in HEK293T cells (Fig. 13), supporting the idea that the endogenous PKD1 gene is a target of vitamin A-derived metabolites. It has been reported that targeted disruption of RXRα causes cardiac conotruncal defects identical to the cardiac phenotype found in Pkd1 null mouse embryos (44), suggesting that the Pkd1 gene may be a critical target of RA signaling in the heart. It has also been shown that a retinoid, N-(4-hydroxyphenyl) retinamide, markedly inhibited cystogenesis of ADPKD cyst-derived cells, Madin-Darby canine kidney cells, and rat epithelial cells in collagen matrix cultures (1). As such, PKD therapies may include treatment with retinoic acid derivatives and could be beneficial in cases of PKD1 haploinsufficiency.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants P50-DK-057301 (J. P. Calvet) and R15-DK-069897 (M. R. Islam).
Acknowledgments
We thank Dr. Yu-Chung Yang (Dept. of Pharmacology and Cancer Center, School of Medicine, Case Western Reserve University) for the Sp1 constructs and Dr. Ronald M. Evans (Howard Hughes Medical Institute, the Salk Institute) for the RAR and RXR expression constructs, Kyle Jansson and Yvonne Wan for help with the RA-treatment experiments, and Dr. Mike Wolfe for critically reading the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Altieri P, Caridi G, Chiesa V, Ponzoni M, Ghiggeri GM. N-(4-hydroxyphenyl) retinamide inhibits cystogenesis by polycystic epithelial cell lines in vitro. Life Sci 64: 259–265, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Arnaout MA Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med 52: 93–123, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Blake MC, Jambou RC, Swick AG, Kahn JW, Azizkhan JC. Transcriptional initiation is controlled by upstream GC-box interactions in a TATAA-less promoter. Mol Cell Biol 10: 6632–6641, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci USA 98: 12174–12179, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasier JL, Henske EP. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest 99: 194–199, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin Nephrol 21: 107–123, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter ML, Marks JN, Fox KR. DNA-sequence binding preference of the GC-selective ligand mithramycin. Deoxyribonuclease-I/deoxyribonuclease-II and hydroxy-radical footprinting at CCCG, CCGC, CGGC, GCCC and GGGG flanked by (AT)n and An Tn. Eur J Biochem 215: 561–566, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Chambon P A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954, 1996. [PubMed] [Google Scholar]
- 9.Chauvet V, Qian F, Boute N, Cai Y, Phakdeekitacharoen B, Onuchic LF, Attie-Bitach T, Guicharnaud L, Devuyst O, Germino GG, Gubler MC. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am J Pathol 160: 973–983, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90: 569–580, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Cornwell MM, Smith DE. SP1 activates the MDR1 promoter through one of two distinct G-rich regions that modulate promoter activity. J Biol Chem 268: 19505–19511, 1993. [PubMed] [Google Scholar]
- 12.Courey AJ, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55: 887–898, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Curr Med Chem 9: 623–637, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Dolle P, Fraulob V, Kastner P, Chambon P. Developmental expression of murine retinoid X receptor (RXR) genes. Mech Dev 45: 91–104, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Dolle P, Ruberte E, Kastner P, Petkovich M, Stoner CM, Gudas LJ, Chambon P. Differential expression of genes encoding alpha, beta and gamma retinoic acid receptors and CRABP in the developing limbs of the mouse. Nature 342: 702–705, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Samudio I, Kladde MP, Vyhlidal C, Safe S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta-estradiol in breast cancer cells. J Biol Chem 274: 32099–32107, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Gabow PA Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Gidoni D, Dynan WS, Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. Nature 312: 409–413, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Gieteling EW, Rinkel GJ. Characteristics of intracranial aneurysms and subarachnoid haemorrhage in patients with polycystic kidney disease. J Neurol 250: 418–423, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Glass CK, Rose DW, Rosenfeld MG. Nuclear receptor coactivators. Curr Opin Cell Biol 9: 222–232, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Grantham JJ Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int 64: 1157–1162, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Greenwel P, Inagaki Y, Hu W, Walsh M, Ramirez F. Sp1 is required for the early response of alpha2(I) collagen to transforming growth factor-beta1. J Biol Chem 272: 19738–19745, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Hermanson O, Glass CK, Rosenfeld MG. Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol Metab 13: 55–60, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Horie S, Ishii H, Matsumoto F, Kusano M, Kizaki K, Matsuda J, Kazama M. Acceleration of thrombomodulin gene transcription by retinoic acid: retinoic acid receptors and Sp1 regulate the promoter activity through interactions with two different sequences in the 5′-flanking region of human gene. J Biol Chem 276: 2440–2450, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 319: 907–912, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402: 93–96, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest 104: 1459–1468, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Karperien M, Farih-Sips H, Lowik CW, de Laat SW, Boonstra J, Defize LH. Expression of the parathyroid hormone-related peptide gene in retinoic acid-induced differentiation: involvement of ETS and Sp1. Mol Endocrinol 11: 1435–1448, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem 274: 4947–4953, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci USA 97: 1731–1736, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SJ, Glick A, Sporn MB, Roberts AB. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem 264: 402–408, 1989. [PubMed] [Google Scholar]
- 34.Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, Kim SJ, Friedman SL. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem 273: 33750–33758, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Lania L, Majello B, De Luca P. Transcriptional regulation by the Sp family proteins. Int J Biochem Cell Biol 29: 1313–1323, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, Peters DJ. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet 13: 3069–3077, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann JM, Zhang XK, Pfahl M. RAR gamma 2 expression is regulated through a retinoic acid response element embedded in Sp1 sites. Mol Cell Biol 12: 2976–2985, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu W, Fan X, Basora N, Babakhanlou H, Law T, Rifai N, Harris PC, Perez-Atayde AR, Rennke HG, Zhou J. Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nat Genet 21: 160–161, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet 17: 179–181, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell 83: 835–839, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marill J, Idres N, Capron CC, Nguyen E, Chabot GG. Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab 4: 1–10, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signalling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 46: 451–480, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Maser RL, Magenheimer BS, Zien CA, Calvet JP. Transient transfection assays for analysis of signal transduction in renal cells. Methods Mol Med 86: 205–217, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Muto S, Aiba A, Saito Y, Nakao K, Nakamura K, Tomita K, Kitamura T, Kurabayashi M, Nagai R, Higashihara E, Harris PC, Katsuki M, Horie S. Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum Mol Genet 11: 1731–1742, 2002. [DOI] [PubMed] [Google Scholar]
- 45.O Bukanov N, Husson H, Dackowski WR, Lawrence BD, Clow PA, Roberts BL, Klinger KW, Ibraghimov-Beskrovnaya O. Functional polycystin-1 expression is developmentally regulated during epithelial morphogenesis in vitro: downregulation and loss of membrane localization during cystogenesis. Hum Mol Genet 11: 923–936, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Ong AC Polycystin expression in the kidney and other tissues: complexity, consensus and controversy. Exp Nephrol 8: 208–214, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Peters DJ, Breuning MH. Autosomal dominant polycystic kidney disease: modification of disease progression. Lancet 358: 1439–1444, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Pirson Y, Chauveau D, Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 13: 269–276, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Pritchard L, Sloane-Stanley JA, Sharpe JA, Aspinwall R, Lu W, Buckle V, Strmecki L, Walker D, Ward CJ, Alpers CE, Zhou J, Wood WG, Harris PC. A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum Mol Genet 9: 2617–2627, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Puri S, Rodova M, Islam MR, Magenheimer BS, Maser RL, Calvet JP. Ets factors regulate the polycystic kidney disease-1 promoter. Biochem Biophys Res Commun 342: 1005–1013, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 61: 1000–1005, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87: 979–987, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Qian Q, Harris PC, Torres VE. Treatment prospects for autosomal-dominant polycystic kidney disease. Kidney Int 59: 2005–2022, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Rodova M, Islam MR, Maser RL, Calvet JP. The polycystic kidney disease-1 promoter is a target of the beta-catenin/T-cell factor pathway. J Biol Chem 277: 29577–29583, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Salvatori L, Ravenna L, Felli MP, Cardillo MR, Russo MA, Frati L, Gulino A, Petrangeli E. Identification of an estrogen-mediated deoxyribonucleic acid-binding independent transactivation pathway on the epidermal growth factor receptor gene promoter. Endocrinology 141: 2266–2274, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Shimada J, Suzuki Y, Kim SJ, Wang PC, Matsumura M, Kojima S. Transactivation via RAR/RXR-Sp1 interaction: characterization of binding between Sp1 and GC box motif. Mol Endocrinol 15: 1677–1692, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Stoner M, Wang F, Wormke M, Nguyen T, Samudio I, Vyhlidal C, Marme D, Finkenzeller G, Safe S. Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor alpha and Sp3 proteins. J Biol Chem 275: 22769–22779, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Sutters M, Germino GG. Autosomal dominant polycystic kidney disease: molecular genetics and pathophysiology. J Lab Clin Med 141: 91–101, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki Y, Shimada J, Shudo K, Matsumura M, Crippa MP, Kojima S. Physical interaction between retinoic acid receptor and Sp1: mechanism for induction of urokinase by retinoic acid. Blood 93: 4264–4276, 1999. [PubMed] [Google Scholar]
- 60.Thivierge C, Kurbegovic A, Couillard M, Guillaume R, Cote O, Trudel M. Overexpression of PKD1 causes polycystic kidney disease. Mol Cell Biol 26: 1538–1548, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres VE Hypertension, proteinuria, and progression of autosomal dominant polycystic kidney disease: where do we go from here? Am J Kidney Dis 35: 547–550, 2000. [DOI] [PubMed] [Google Scholar]
- 62.van Adelsberg J Polycystin-1 interacts with E-cadherin and the catenins–clues to the pathogenesis of cyst formation in ADPKD? Nephrol Dial Transplant 15: 1–2, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Van Bodegom D, Saifudeen Z, Dipp S, Puri S, Magenheimer BS, Calvet JP, El-Dahr SS. The polycystic kidney disease-1 gene is a target for p53-mediated transcriptional repression. J Biol Chem 281: 31234–31244, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Wang W, Dong L, Saville B, Safe S. Transcriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol 13: 1373–1387, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Wilson PD Polycystic kidney disease. N Engl J Med 350: 151–164, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev 9: 140–147, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Zhao C, Meng A. Sp1-like transcription factors are regulators of embryonic development in vertebrates. Dev Growth Differ 47: 201–211, 2005. [DOI] [PubMed] [Google Scholar]