Abstract
Previous studies demonstrated changes in urinary bladder neurotrophin content and upregulation of neurotrophin receptors, TrkA and the p75 neurotrophin receptor (p75NTR), in micturition reflex pathways after cyclophosphamide (CYP)-induced cystitis. p75NTR can bind nerve growth factor (NGF) and modulate NGF-TrkA binding and signaling. We examined p75NTR expression and the role of p75NTR in the micturition reflex in control and CYP-treated rats. p75NTR Immunoreactivity was present throughout the urinary bladder. CYP-induced cystitis (4 h, 48 h, chronic) increased (P ≤ 0.05) p75NTR expression in whole urinary bladder as shown by Western blotting. The role of p75NTR in bladder function in control and CYP-treated rats was determined using conscious cystometry and immunoneutralization or PD90780, a compound known to specifically block NGF binding to p75NTR. An anti-p75NTR monoclonal antibody or PD90780 was infused intravesically and cystometric parameters were evaluated. Both methods of p75NTR blockade significantly (P ≤ 0.05) decreased the intercontraction interval and void volume in control and CYP-treated rats. Intravesical infusion of PD90780 also significantly (P ≤ 0.001) increased intravesical pressure and increased the number of nonvoiding contractions during the filling phase. Control intravesical infusions of isotype-matched IgG and vehicle were without effect. Intravesical instillation of PD90780 significantly (P ≤ 0.01) reduced the volume threshold to elicit a micturition contraction in control rats (no inflammation) and CYP-treated in a closed urinary bladder system. These studies demonstrate 1) ubiquitous p75NTR expression in urinary bladder and increased expression with CYP-induced cystitis and 2) p75NTR blockade at the level of the urinary bladder produces bladder hyperreflexia in control and CYP-treated rats. The overall activity of the urinary bladder reflects the balance of NGF-p75NTR and NGF-TrkA signaling.
Keywords: PD90780, conscious cystometry, Western blotting, bladder hyperreflexia, immunoneutralization
nerve growth factor (NGF) is important in sensory and sympathetic neuronal development and maintenance (35); however, many recent studies suggest additional role(s) for NGF in painful somatic and visceral inflammatory conditions (1, 12, 28, 50). NGF upregulation occurs at sites of tissue injury and inflammation (40, 71) and changes in NGF levels in urine as well as urinary bladder have been documented in humans with painful bladder syndrome (PBS)/interstitial cystitis (IC) and rodents with bladder inflammation (43, 48, 49, 64). A number of addition and subtraction studies have begun to demonstrate the role(s) of NGF in inflammatory conditions of the urinary bladder. For example, intravesical infusion or intramuscular detrusor administration of exogenous NGF results in increased bladder activity, sensitization of bladder afferents (12, 20), increased expression of Fos protein in lumbosacral spinal cord in response to bladder distention, and increased expression of neuropeptides in lumbosacral spinal cord (73). Strategies to reduce NGF in micturition reflex pathways reduce or eliminate these effects (20, 34).
NGF signals through its specific receptor TrkA, as well as p75NTR. TrkA is a tropomyosin-related receptor tyrosine kinase receptor and p75NTR belongs to the tumor necrosis factor-α family of receptors (1, 4, 8). p75NTR binds all neurotrophins, including brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and NT-4/5. NGF-TrkA signaling is involved in some inflammatory effects of NGF (50), including sensitization of nociceptive afferents and the release of neuropeptides. TrkA expression is increased in bladder afferent cells in lumbosacral dorsal root ganglia (DRG) with acute and chronic cyclophosphamide (CYP)-induced bladder inflammation (51). In addition, p75NTR expression is also significantly upregulated in bladder afferent cells (2.4- to 2.8-fold) in lumbosacral DRG after CYP-induced cystitis (39).
The pan-neurotrophin receptor p75NTR exhibits a ubiquitous distribution and has many functions and multiple binding partners and ligands (4, 8). In the absence of Trk expression, p75NTR is suggested to be involved in apoptosis and regulation of neuronal growth (4, 27); however, in the presence of TrkA, p75NTR is suggested to function by enhancing Trk binding to neurotrophins (3, 4, 70). p75NTR expression has previously been identified in the urinary bladder (61, 66, 67) but few studies have examined the role of p75NTR in bladder function or in the context of urinary bladder inflammation (66). Although we do know that p75NTR expression is present in micturition reflex pathways in control rats and is regulated with CYP-induced cystitis (39), the potential role(s) of p75NTR in urinary bladder reflexes have not been explored. In this study, we determined 1) expression of p75NTR in the urinary bladder with immunohistochemistry and Western blot and regulation of p75NTR expression after CYP-induced cystitis, 2) the effect of immunoneutralization with an anti-p75NTR monoclonal antibody on bladder function in female rats without (noninflamed) and with bladder inflammation (48-h CYP treatment) using conscious cystometry, and 3) the effect of intravesical infusion of PD90780, a compound known to specifically block NGF binding to p75NTR (14, 55), in female rats without (noninflamed) and with bladder inflammation (48-h CYP treatment) using conscious cystometry.
MATERIALS AND METHODS
CYP-Induced Cystitis: Acute, Intermediate, or Chronic
Chemical cystitis was induced in adult (200–225 g), female Wistar rats by CYP treatment (39, 63). CYP was administered in one of the following ways: 1) 4 h (150 mg/kg ip) before euthanasia of the animals to elicit acute bladder inflammation, 2) 48 h (150 mg/kg ip) before euthanasia to examine an intermediate inflammation, or 3) administered every third day for 9 days to elicit chronic bladder inflammation (75 mg/kg ip). All injections of CYP were performed under isoflurane (2%) anesthesia. Control animals were gender matched to the experimental groups and received a corresponding volume of saline (0.9%) or distilled water injected intraperitoneally under isoflurane (2%) anesthesia. Animals were euthanized by isoflurane anesthesia (5%) plus thoracotomy at the indicated time points, and the urinary bladder was harvested and weighed. The University of Vermont Institutional Animal Care and Use Committee approved all experimental procedures (protocol number 06–014) involving animal use. Animal care was under the supervision of University of Vermont College of Medicine's Office of Animal Care in accordance with American Association for Accreditation of Laboratory Animal Care and National Institutes of Health guidelines. All efforts were made to minimize animal stress/distress and suffering and to use the minimum number of animals.
Whole Mount Bladder Preparation and Immunohistochemistry
The urinary bladder from control (n = 5) and CYP treatment (n = 5) was dissected and placed in Krebs solution. The bladder was cut open along the midline and pinned to a Sylgard-coated dish. The bladder was incubated for 3 h at room temperature in cold fixative (2% paraformaldehyde + 0.2% picric acid), and the urothelium was removed as previously described (74). Urothelium and bladder musculature were processed separately for p75NTR-immunoreactivity (IR). Control and CYP-treated tissues were incubated overnight at room temperature in rabbit anti-p75NTR antiserum [1:3,000; Advanced Targeting Systems (ATS), San Diego, CA] in 1% goat serum and 0.1 M KPBS (0.1 M PBS with potassium) and then washed (3 × 15 min) with 0.1 M KPBS, pH 7.4. After being washed, the tissue was incubated in a species-specific secondary antibody (1:500; Cy3-conjugated goat anti-rabbit; Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature, followed by washing and coverslipping with Citifluor (London, UK). Control tissues incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary antibody, no positive immunostaining was observed. The specificity of the p75NTR antiserum was previously established (42). Some whole mount preparations were stained with the pan neuronal marker, protein gene product 9.5 (Abcam, Cambridge, MA; 1:15), to visualize nerve fibers in the suburothelial plexus and to demonstrate that suburothelial nerve fibers expressed p75NTR-IR.
p75NTR Localization in Urinary Bladder Sections After Intravesical p75NTR Infusion
Immediately after cystometric analyses, urinary bladders were harvested from rats that had received intravesical infusion of monoclonal antibody to p75NTR and those that had received intravesical infusion of protamine sulfate. Animals were deeply anesthetized with isoflurane (5%) and euthanized via thoracotomy. Bladders were quickly removed and postfixed in 4% paraformaldehyde overnight. Tissues were cryoprotected by immersion in 30% sucrose (in 0.1 M PBS) overnight. Bladders were sectioned (20 μm) on a cryostat and directly mounted on gelled (0.5%) microscope slides. Tissue was incubated in secondary antibody (Cy2-conjugated goat anti-mouse; Jackson ImmunoResearch) for 2 h and washed (3 × 15 min) at room temperature with 0.1 KPBS (pH 7.4). Slides were coverslipped with Citifluor.
Assessment of Positive Staining in Urinary Bladder
Staining observed in experimental tissue was compared with that observed from experiment-matched negative controls. Tissues exhibiting immunoreactivity that was greater than the background level observed in experiment-matched negative controls were considered positively stained.
Imaging and Visualization of Bladder Sections
Tissues were examined under an Olympus fluorescence photomicroscope (Optical Analysis, Nashua, NH) for visualization of Cy2. Cy2 was visualized with a filter with an excitation range of 470–490 and an emission range from 510 to 530. Images of bladder sections were captured through a video camera attachment to the microscope with the exposure time, brightness, and contrast being held constant.
Imaging and Visualization of Bladder Whole Mounts
Tissue was examined and optical sections were acquired using a Zeiss LSM 510 confocal scanning system attached to a Zeiss LSM 510 microscope using a plan Fluor ×20 or ×10 objective. An excitation wavelength of 543 nm was used for visualization of p75NTR. Bladder whole mount images were captured through a video camera attachment to the microscope with the exposure time, brightness, and contrast held constant.
Western Blotting for p75NTR Expression in Whole Urinary Bladder
Whole urinary bladders were homogenized separately in tissue protein extraction agent with protease inhibitors (T-PER; Roche, Indianapolis, IN), and aliquots were removed for protein assay. Samples (23 μg) were suspended in sample buffer for fractionation on gels and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and efficiency of transfer was evaluated. Membranes were blocked overnight in a solution of 5% milk, 3% bovine serum albumin in Tris-buffered saline with 0.1% Tween. Membranes were incubated in rabbit anti-p75NTR (1:2,000; ATS) overnight at 4°C. Washed membranes were incubated in a species-specific secondary antibody (1:7,000; goat anti-rabbit horseradish peroxidase) for 2 h at room temperature for enhanced chemiluminescence detection (Pierce, Rockford, IL). Blots were exposed to Biomax film (Kodak, Rochester, NY) and developed. The intensity of each band was analyzed, and background intensities were subtracted using Un-Scan It software (Silk Scientific, Orem, UT). Western blot analysis of erk1 and erk2 (1:2,000; Cell Signaling Technology, Danvers, MA) in samples was used as a loading control. The specificity of the p75NTR antiserum was previously established (42). In pilot studies, the concentration of p75NTR antiserum used for Western blotting studies of urinary bladder was titrated. The p75NTR antiserum concentration selected for experimentation did not result in saturation in control tissues and permitted changes in p75NTR expression with CYP treatment to be evaluated semiquantitatively.
Intravesical Catheter Placement
A lower midline abdominal incision was performed under general anesthesia with 2–3% isoflurane using aseptic techniques. Polyethylene tubing (PE-50, Clay Adams, Parsippany, NJ) with the end flared by heat was inserted into the dome of the bladder and secured in place with a 6–0 nylon purse-string suture (33). The distal end of the tubing was sealed, tunneled subcutaneously, and externalized at the back of the neck, out of the animal's reach. Animals were maintained for 72 h after surgery to ensure complete recovery.
Cystometry
Continuous cystometry.
The effects of p75NTR blockade on bladder function in control (no inflammation) and CYP-treated rats (48 h) were evaluated by immunoneutralization with intravesical infusion of anti-p75NTR monoclonal antibody (ATS; 100 μg/ml) or PD90780 (Pfizer; 10–100 μM), known to specifically block NGF-p75NTR (14, 55) using conscious cystometry and continuous infusion of intravesical saline. Intravesical instillation of an isotype-matched IgG and saline (room temperature, 0.9%) were used as controls. Animals were placed conscious and unrestrained in recording cages with a balance and pan for urine collection and measurement was placed below (7, 34, 73). Intravesical pressure changes were recorded using a Small Animal Cystometry System (Med Associates). Saline at room temperature was infused at a rate of 10 ml/h to elicit repetitive bladder contractions. At least four reproducible micturition cycles were recorded after an initial stabilization period of 25–30 min. Voided saline was collected to determine voided volume. Intercontraction interval, maximal voiding pressure, pressure threshold for voiding, and baseline resting pressure were measured (44). The number of nonvoiding bladder contractions (NVCs) per voiding cycle during the filling phase was determined. For these studies, NVCs were defined as rhythmic intravesical pressure rises greater than 7 cmH2O from baseline pressure without a release of fluid from the urethra.
Exclusion Criteria
Rats were removed from study when adverse events occurred that included: ≥20% reduction in body weight postsurgery, a significant postoperative event, lethargy, pain, or distress not relieved by our IACUC-approved regimen of postoperative analgesics or hematuria in control rodents. In the present study, no rats were excluded from the study or from analysis due to any of these exclusion criteria. In addition, behavioral movements such as grooming, standing, walking, and defecation rendered bladder pressure recordings during these events unusable (58). Experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements (21). Rats were euthanized at the conclusion of the study and urinary bladder was harvested as described above.
Isovolumetric cystometry.
For isovolumetric cystometry, the bladder was exposed and cannulated as described above. The ureters were ligated and cut proximally, and the external urethral orifice was ligated to generate a closed urinary bladder system. The bladder cannula was connected to a pressure transducer and infusion pump via a three-way connector. The bladder was drained at the start of the study and saline was infused at a constant rate of 5 ml/h for up to 30 min per cycle. Saline was drained from the bladder between infusion cycles and the bladders were rested for 20 min before the start of the next infusion. The volume threshold for initiation of contraction and the effects of PD90780 (50 μM) on volume threshold were derived from these data in control (n = 4) and CYP-treated (48 h; n = 4) rats.
Drug Treatments
The effects of p75NTR blockade on bladder function in control (no inflammation) and CYP-treated rats (48 h) were evaluated by immunoneutralization with intravesical infusion of anti-p75NTR monoclonal antibody (ATS; 100 μg/ml) or PD90780 (Pfizer; 10–100 μM), known to specifically block NGF-p75NTR (14, 22, 55, 69) using conscious cystometry (continuous fill and isovolumetric). Immediately before cystometric analysis, rats were anesthetized (1–2% isoflurane) and bladders were manually emptied with the Credé maneuver. A solution of protamine sulfate (Sigma, St. Louis, MO; 10 mg/ml in sterile saline) was then infused into the bladder (≤1 ml) and maintained (45 min) in the bladder while the rat was anesthetized with isoflurane (1–2%) to prevent voiding and expulsion of bladder contents (15). Protamine sulfate is used to disrupt urothelial cell-to-cell contact and increase urothelial permeability (11, 25). This concentration of protamine sulfate does not affect voiding frequency (11, 25). The bladder was then flushed with 0.9% sterile saline and emptied. Rats were then infused (≤1 ml) with either anti-p75NTR monoclonal antibody (n = 9) or with PD90780 (n = 24) maintained (30 min) in the bladder while the rat was anesthetized (isoflurane, 1–2%) to prevent voiding and expulsion of bladder contents (7, 15). The concentrations and duration of PD90780 infusion chosen for this study were based on previous experiments with PD90780 (14, 22, 55, 69). The concentration anti-p75NTR monoclonal antibody used for intravesical infusion was based on previous studies using anti-p75NTR or anti-NGF in intrathecal infusion or cell culture applications (22, 36, 47, 52, 53).
Figure Preparation
Digital images were obtained using a charge-coupled device camera (MagnaFire SP, Optronics; Optical Analysis) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis). Exposure times were held constant when acquiring images from control and experimental animals processed and analyzed on the same day. Images were imported into Adobe Photoshop 8.0 (Adobe Systems, San Jose, CA) where groups of images were assembled and labeled.
Materials
All standard chemicals were obtained from Sigma or Fisher and were either analytical or laboratory grade. PD90780 was obtained through a compound transfer agreement with Pfizer (Groton, CT). PD90780 was made up as concentrated stock solutions stored in single use vials (to eliminate freeze thaw cycles) at −20°C until usage.
Statistical Analyses
All values represent means ± SE. Data were compared with one- or two-way ANOVA, where appropriate. For isovolumetric cystometry data, data were compared with repeated-measures ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), the Dunnett's post hoc test was used to compare group means.
RESULTS
p75NTR IR in Urinary Bladder
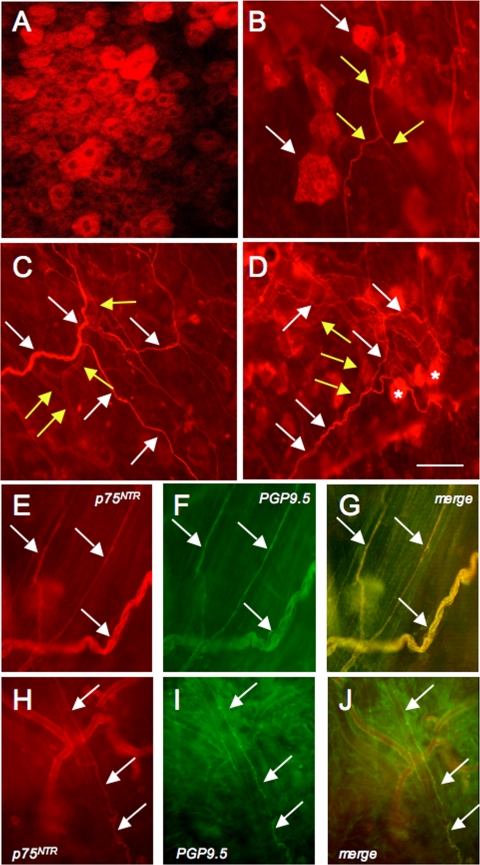
To determine the localization of p75NTR expression in the urinary bladder, whole mount preparations were prepared in control (Fig. 1, A–J) and 4-h (not shown) and chronic CYP-treated rats (not shown). p75NTR expression was present in the urothelium and neuronal fibers in the urinary bladder of control rats and CYP-treated rats. p75NTR-IR was seen in urothelial cells (Fig. 1, A, B, D), nerve fibers (Fig. 1, B–J) in the suburothelial plexus, and in nerve fibers in the detrusor in both control and CYP-treated rats. Nerve fibers adjacent to and encircling suburothelial vasculature also exhibited p75NTR-IR (Fig. 1, C, D, H–J). p75NTR-immunoreactive suburothelial nerve fibers and nerve fibers associated with the suburothelial vasculature also exhibited immunoreactivity for the pan neuronal marker, protein gene product (PGP9.5; Fig. 1, E–J). Given the widespread and overlapping nature of the p75NTR-IR in the defined structures, it was not possible to quantify changes in p75NTR-IR in individual structures with CYP-induced cystitis. Rather, we examined regulation of p75NTR protein expression in whole urinary bladder using Western blotting techniques.
Fig. 1.
p75NTR and pan-neuronal marker, protein gene product 9.5 (PGP9.5), immunoreactivity (IR) in urinary bladder whole mount preparations with the urothelium/suburothelium dissected from the detrusor smooth muscle. A: confocal image of p75NTR-IR in urothelial cells. B: epifluorescence image of p75NTR-IR suburothelial nerve fibers (yellow arrows) in close proximity to p75NTR-IR urothelial cells (white arrows). C: p75NTR-IR in suburothelial nerve fibers (white arrows) and vasculature (yellow arrows). D: p75NTR-IR in vasculature (yellow arrows), nerve fibers (white arrows), and urothelial cells out of the focal plane (*). p75NTR-immunoreactive suburothelial fibers (arrows E, H) also expressed PGP9.5-IR (arrows F, I). G and J: merged images of E, H and F, I, respectively. Calibration bar represents 100 μm.
p75NTR Protein Expression in Urinary Bladder with CYP-Induced Cystitis
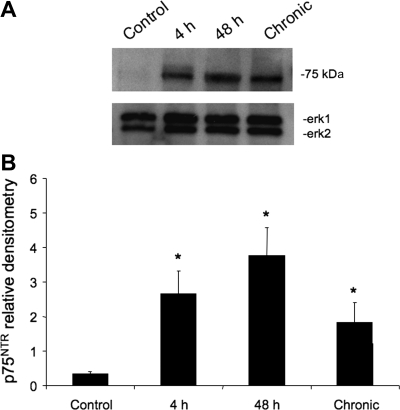
p75NTR protein expression in whole urinary bladder as determined by Western blot analysis significantly (P ≤ 0.05) increased after 4-h (7.7-fold), 48-h (11.1-fold), or chronic CYP treatment (3.3-fold; Fig. 2, A and B).
Fig. 2.
A: representative Western blot of whole urinary bladder (23 μg) for p75NTR expression in control rats and those treated with cyclophosphamide (CYP) for varying duration. Erk1 staining was used as a loading control. B: histogram of relative p75NTR band density in all groups examined normalized to Erk1 in the same samples. p75NTR expression in whole urinary bladder is significantly increased with acute (4 h), intermediate (48 h), and chronic CYP treatment. *P ≤ 0.05; n = 4 for all groups.
Effects of Immunoneutralization of p75NTR on Cystometry in Rats with and without CYP-Induced Cystitis with Intravesical Instillation of Anti-p75 Monoclonal Antibody
Control (no inflammation).
Intravesical infusion of an anti-p75NTR monoclonal antibody (100 μg/ml; Fig. 3, B and D) increased voiding frequency, with a decreased intercontraction interval (ICI; P ≤ 0.001) and decreased void volume (P ≤ 0.001) compared with control rats (Figs. 3A and 4, A and B) without intravesical anti-p75NTR instillation. Intravesical infusion of a monoclonal antibody to p75NTR did not affect baseline, micturition, or threshold pressure or nonvoiding contractions during the filling phase (Table 1).
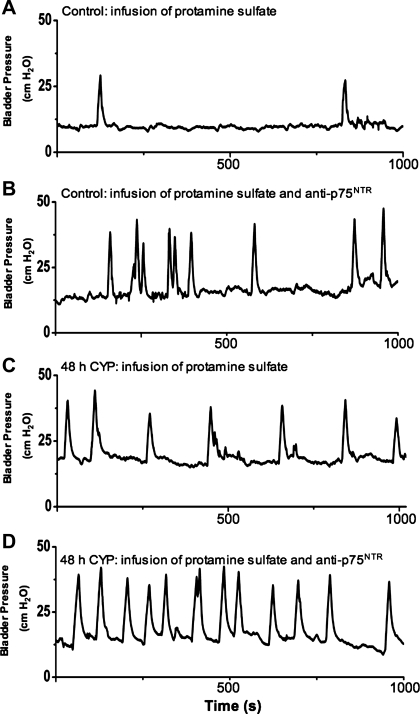
Fig. 3.
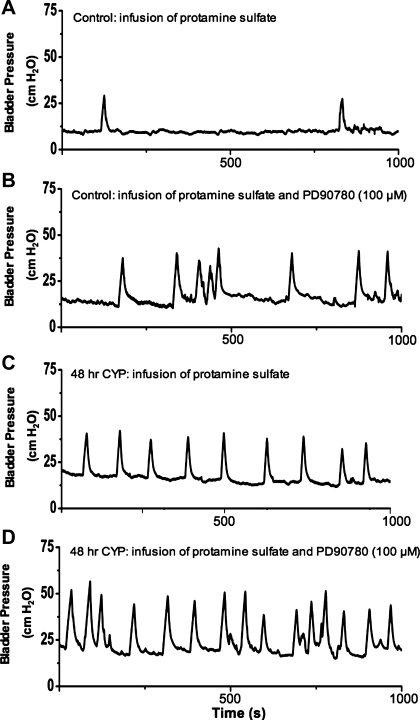
Intravesical infusion of anti-p75NTR monoclonal antibody in control and in CYP-treated (48 h) rats increased voiding frequency (decreased intercontraction interval). Representative continuous cystometrogram recordings in rats treated with intravesical protamine sulfate in control rats (A), protamine sulfate and anti-p75NTR monoclonal antibody (100 μg/ml) in control rats (B), protamine sulfate in CYP-treated (48 h) rats (C), and protamine sulfate and anti-p75NTR monoclonal antibody (100 μg/ml) in CYP-treated (48 h) rats (D).
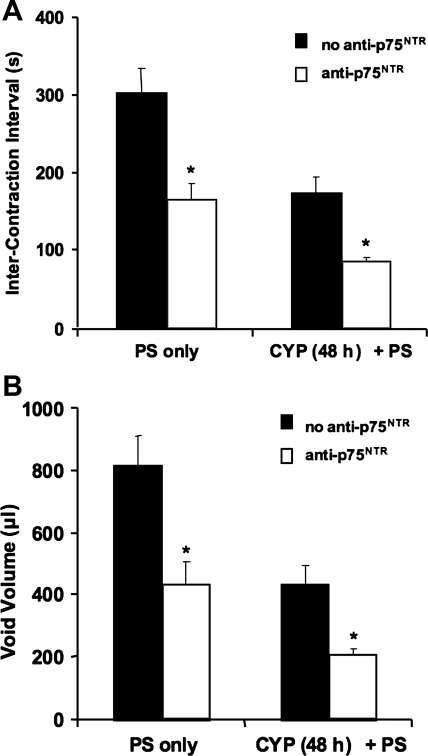
Fig. 4.
Intravesical infusion of anti-p75NTR monoclonal antibody (100 μg/ml) significantly increased voiding frequency in control and in CYP-treated (48 h) rats. Both intercontraction interval (A) and void volume (B) were significantly (*P ≤ 0.001) reduced with intravesical infusion of anti-p75NTR; n = 4–11 for all groups.
Table 1.
Effects of intravesical infusion of an anti-p75NTR monoclonal antibody on cystometric parameters
| Intravesical Infusion | Threshold Pressure, cmH2O | Micturition Pressure, cmH2O | Baseline Pressure, cmH2O | NVCs per Micturition Cycle | |
|---|---|---|---|---|---|
| Control (no CYP) | No antibody | 15.1±0.9 | 38.5±1.3 | 15.1±0.9 | 0.7±0.3 |
| Anti-p75NTR monoclonal antibody | 14.2±1.0 | 39.5±2.0 | 15.0±0.6 | 0.8±0.3 | |
| 48-h CYP | No antibody | 19.3±0.7* | 46.7±1.4* | 20.0±0.8* | 0.2±0.1* |
| Anti-p75NTR monoclonal antibody | 13.9±1.1 | 44.1±2.0 | 14.0±1.0 | 0.3±0.1 |
Values are means ± SE.
P ≤ 0.001 compared with isotype-matched IgG infusion in control rats; n = 4–11 rats in each group. Effects of intravesical infusion of an anti-p75NTR monoclonal antibody on baseline pressure, threshold pressure, micturition pressure, and numbers of nonvoiding contractions (NVCs) per micturition cycle are indicated. Threshold pressure and micturition pressure were significantly increased after 48-h cyclophosphamide (CYP) treatment. No effects of anti-p75NTR monoclonal antibody on threshold pressure, baseline pressure, or peak micturition pressure were observed. The no antibody group received intravesical infusion of protamine sulfate (PS; 10 mg/ml), which did not differ from rats receiving intravesical infusion of PS and isotype-matched IgG.
CYP treatment.
As previously demonstrated, CYP treatment (48 h) decreased ICI (P ≤ 0.001) and void volume (P ≤ 0.001; Fig. 4) and increased micturition (P ≤ 0.001) and threshold pressure (P ≤ 0.001; Table 1). Intravesical infusion of anti-p75NTR monoclonal antibody (100 μg/ml) in 48-h CYP-treated rats (Fig. 3D) resulted in an additional increase in voiding frequency with associated decreased ICI (P ≤ 0.001) and decreased void volume (P ≤ 0.005; Fig. 4, A and B, and Table 1). There were no significant changes in micturition pressure, threshold pressure, or nonvoiding contractions compared with rats with only 48-h CYP treatment (Fig. 3C; Table 1). Control experiments with intravesical infusion of an isotype-matched monoclonal IgG (100 μg/ml) or protamine sulfate (10 mg/ml) showed no effects on bladder function in control or CYP-treated rats (Figs. 3 and 4; Table 1) compared with rats without protamine sulfate or antibody infusion in control or CYP-treated rats. In pilot studies performed without the initial infusion of protamine sulfate, no changes in bladder function with subsequent intravesical infusion of the anti-p75NTR monoclonal antibody (100 μg/ml) were observed. Therefore, all subsequent experiments included protamine sulfate intravesical infusion. The duration of anti-p75NTR monoclonal antibody effects on all cystometric parameters evaluated in control and CYP-treated rats was 30–40 min.
p75NTR-IR in Urinary Bladder after Intravesical Instillation of Anti-p75NTR Monoclonal Antibody
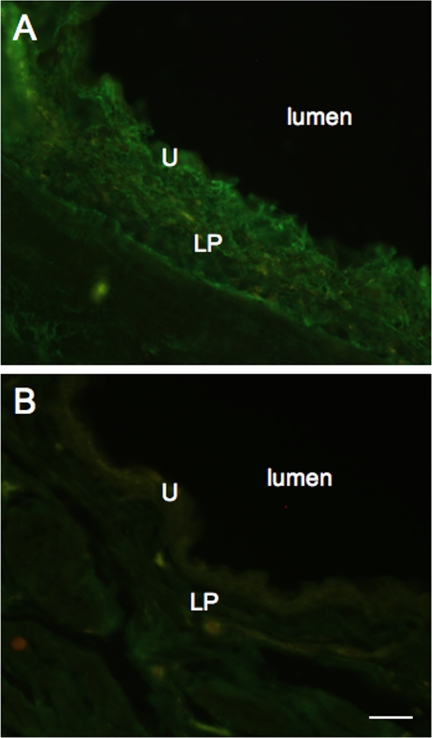
p75NTR-IR was examined after intravesical instillation of anti-p75NTR monoclonal antibody by application of a species-specific secondary antibody to cryostat sections (20 μm). p75NTR-IR was present in urothelium and lamina propria (Fig. 5A). p75NTR-IR decreased in intensity with increasing distance from the bladder lumen. Urinary bladder sections from rats not receiving intravesical instillation of anti-p75NTR monoclonal antibody exhibited no immunoreactivity above background levels (Fig. 5B).
Fig. 5.
p75NTR-IR in urinary bladder sections after intravesical infusion of anti-p75NTR and application of species-specific secondary antibodies to demonstrate penetration of the antibody into the urinary bladder with intravesical infusion. Epifluorescence images of rat urinary bladder after intravesical infusion of anti-p75NTR (100 μg/ml; A) and rat urinary bladder after only intravesical infusion of protamine sulfate (B). A: p75NTR-IR is observed in urothelium and lamina propria, with staining reduced with increasing distance from the bladder lumen. B: no p75NTR-IR was observed in rat urinary bladder in the absence of intravesical antibody infusion. U, urothelium; LP, lamina propria; l, lumen. Calibration bar represents 120 μm.
Effects of NGF-p75NTR Blockade on Cystometry in Rats with and without CYP-Induced Cystitis with Intravesical Instillation of PD90780
Control (no inflammation) rats.
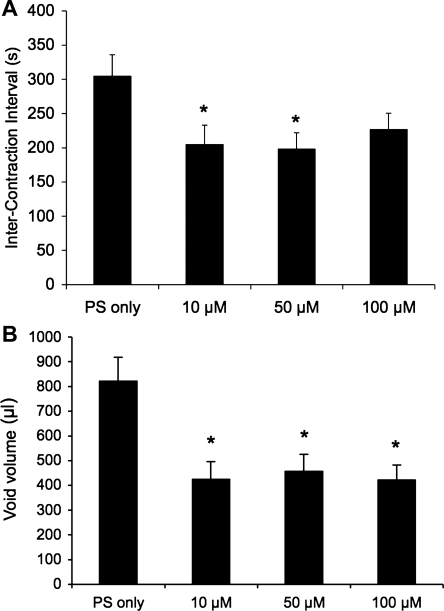
PD90780, a compound that specifically blocks NGF binding to p75NTR (14, 55), was infused intravesically (10, 50, and 100 μM) in rats without CYP-induced cystitis and the effects on cystometric parameters using continuous instillation were determined (Fig. 6, A–D). Intravesical instillation of PD90780 decreased void volume (P ≤ 0.002) at all concentrations evaluated, and decreased ICI at 10 μM (P ≤ 0.05; Fig. 7) and 50 μM (P ≤ 0.05; Figs. 6, A and B, and 7). PD90780 instillation increased threshold pressure at 50 μM (P ≤ 0.02) and increased micturition and baseline pressure at all concentrations (P ≤ 0.002; Table 2) evaluated. Effects of intravesical infusion of PD90780 in control rats (no inflammation) on void volume were more variable than that observed in CYP-treated rats (data not shown); there was a disparity between void volume and infused volume suggestive of potential PD90780 effects on urethral outlet function.
Fig. 6.
Intravesical infusion of PD90780, a compound that specifically blocks nerve growth factor (NGF) binding to p75NTR, results in decreased bladder capacity in control (no inflammation) and in 48-h CYP-treated rats. Representative continuous cystometrogram recordings in rats treated with intravesical protamine sulfate in control rats (A), protamine sulfate and PD90780 (100 μM) in control rats (B), protamine sulfate in CYP-treated (48 h) rats (C), and protamine sulfate and PD90780 (100 μM) in CYP-treated (48 h) rats (D).
Fig. 7.
Summary histograms of the effects of intravesical infusion of PD90780 in control rats (no CYP treatment). A: infusion of PD90780 (10, 50, and 100 μM) significantly (*P ≤ 0.05) reduced intercontraction interval. B: void volume in control rats was also significantly (*P ≤ 0.05) reduced at all PD90780 concentrations evaluated (10, 50, and 100 μM); n = 4–6 for all groups.
Table 2.
Effects of intravesical infusion of PD90780 on cystometric parameters
| Intravesical Infusion | Threshold Pressure, cmH2O | Micturition Pressure, cmH2O | Baseline Pressure, cmH2O | NVCs per Micturition Cycle | |
|---|---|---|---|---|---|
| No CYP | PS only | 15.1±0.9 | 38.5±1.3 | 15.1±0.9 | 0.7±0.3 |
| 10 μM PD90780 | 17.0±1.3 | 47.1±3.0* | 18.4±1.2* | 1.9±0.7 | |
| 50 μM PD90780 | 19.4±1.1* | 47.1±2.6* | 21.5±1.0* | 1.6±0.4 | |
| 100 μM PD90780 | 19.1±1.2 | 51.0±2.1* | 21.3±1.4* | 1.0±0.4 | |
| 48 h CYP | PS only | 19.3±0.7 | 46.7±1.4 | 20.0±0.8 | 0.2±0.1 |
| 10 μM PD90780 | 25.1±0.8* | 57.8±2.8* | 24.3±0.6* | 1.1±0.3* | |
| 50 μM PD90780 | 25.7±0.8* | 61.2±2.5* | 27.7±1.3* | 0.6±0.2* | |
| 100 μM PD90780 | 24.0±0.8* | 56.1±2.6* | 24.3±0.8* | 0.3±0.1 |
Values are means ± SE.
P ≤ 0.001 compared with PS (10 mg/ml) and saline infusion in control rats; n = 4–11 rats in each group. Effects of intravesical infusion of PD90780 on baseline pressure, threshold pressure, micturition pressure, and NVCs per micturition cycle are indicated. In control rats (no CYP treatment), intravesical infusion of PD90780 significantly increased micturition pressure and threshold pressure. In CYP-treated rats (48 h), PD90780 infusion significantly increased threshold pressure, micturition pressure, and NVCs.
CYP treatment.
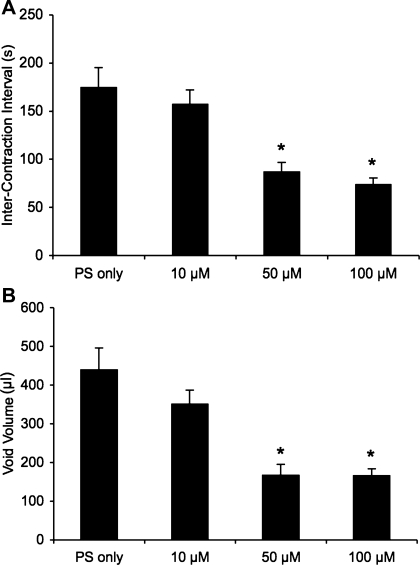
Intravesical infusion of PD90780 in CYP-treated (48 h) rats significantly increased voiding frequency (Fig. 6, C and D) with a decreased void volume (P ≤ 0.002; Fig. 8B) and ICI (P ≤ 0.002; Fig. 8A) compared with rats with CYP treatment (48 h) alone. PD90780 (10, 50, and 100 μM) significantly (P ≤ 0.001) increased threshold, baseline, and micturition pressure (Table 2). The numbers of nonvoiding contractions per micturition cycle during the filling phase were significantly (P ≤ 0.002) increased at 10 and 50 μM PD90780 concentrations (Table 2). The duration of PD90780 effects for all concentrations examined for all cystometric parameters evaluated in control and CYP-treated rats was 30–40 min.
Fig. 8.
Summary histograms of the effects of intravesical infusion of PD90780 in CYP-treated (48 h) rats. Rats were treated with CYP 48 h before intravesical infusion of PD90780 and analysis with conscious cystometry. A: intravesical infusion of PD90780 (50 and 100 μM) significantly (*P ≤ 0.002) reduced intercontraction interval in rats treated with CYP (48 h). B: void volume in CYP-treated (48 h) rats was also significantly (*P ≤ 0.002) reduced at these same concentrations; n = 5–11 for all groups.
Effects of NGF-p75NTR Blockade on Volume Threshold in Rats with and without CYP-Induced Cystitis with Intravesical Instillation of PD90780 Using Isovolumetric Cystometry
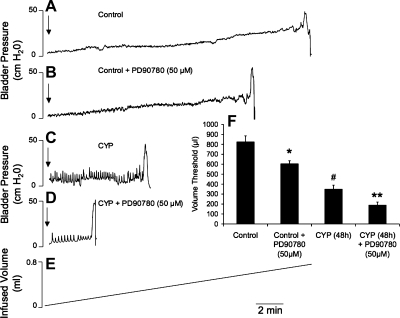
Intravesical instillation of PD90780 (50 μM) significantly (P ≤ 0.01) reduced the volume threshold to elicit a micturition contraction in control rats (no inflammation) in a closed urinary bladder system (Fig. 9, A, B, F). CYP treatment (48 h) reduced (P ≤ 0.01) the volume threshold to elicit a micturition contraction compared with control rats (no inflammation; Fig. 9, C and F). Intravesical instillation of PD90780 (50 μM) further reduced the volume threshold in CYP-treated (48 h) rats (Fig. 9, D and F).
Fig. 9.
Intravesical PD90780 (50 μM) instillation decreases volume threshold in conscious control and CYP-treated (48 h) rats determined with isovolumetric cystometry. A–D: representative cystometrograms with saline infusion into the bladder at a constant rate of 5 ml/h. F: summary histogram of volume threshold needed to elicit bladder contractions in control and CYP-treated rats with and without intravesical PD90780 (50 μM) instillation. The volume threshold was calculated as the volume infused between the start of the infusion and the first contraction. The infusion was turned off after the first contraction and the bladder was rested 20 min before repeating the infusion at least 2 more times. Volume threshold was calculated as the average of at least 3 trials. Volume threshold was significantly (P ≤ 0.01) reduced in control and CYP-treated (48 h) rats with intravesical PD90780 instillation; n = 4 for control and CYP-treated groups. *P ≤ 0.01 compared with control. #P ≤ 0.01 compared with control. **P ≤ 0.01 compared with CYP. Arrows indicate start of infusion.
DISCUSSION
We recently demonstrated upregulation of p75NTR in dye-labeled lumbosacral bladder afferent cells in the DRG and in central micturition reflex pathways after CYP-induced cystitis (39). Few studies examined the regulation of p75NTR in rat urinary bladder after nerve injury or urinary bladder inflammation (23, 37, 65, 66). We demonstrated several novel findings with respect to the expression and regulation of p75NTR in urinary bladder with CYP-induced cystitis and the role of p75NTR in urinary bladder function in control (no inflammation) and CYP-treated rats. p75NTR is expressed throughout the urinary bladder and is present in nerve fibers of the detrusor smooth muscle, the suburothelial nerve plexus, urothelial cells, and nerve fibers associated with the suburothelial bladder vasculature. Total urinary bladder p75NTR expression increased after acute (4 h), intermediate (48 h), and chronic CYP-induced cystitis. p75NTR blockade via immunoneutralization and PD90780, known to block NGF-p75NTR interactions (14, 55), resulted in bladder hyperreflexia in control rats and further enhanced bladder hyperreflexia in CYP-treated (48 h) rats associated with decreased void volume, volume threshold, and ICI and an increased number of nonvoiding contractions for PD90780. These studies suggest that p75NTR interactions can affect overall bladder function. Cystometric effects of p75NTR blockade with an anti-p75NTR monoclonal antibody may be due to blockade of other neurotrophins in addition to NGF. BDNF, NT-3, and NT-4 may also bind p75NTR and are also present in urinary bladder, and expression is regulated by CYP-induced cystitis (64). However, the PD90780 compound, which specifically blocks NGF binding to p75NTR (14, 55), produced similar and broader cystometric changes. Thus, we suggest that the observed cystometric effects are attributable to blockade of p75NTR-NGF interactions.
NGF binds to two receptors, the p75NTR receptor, a member of the tumor necrosis factor family of receptors, and TrkA, a receptor tyrosine kinase (1, 3, 28). However, little information exists concerning the underlying interactions between neurotrophins, TrkA, and p75NTR receptors in mediating inflammatory-induced changes in micturition pathways. The majority of NGF-responsive cells express both p75NTR and TrkA (9) and TrkA is exclusively expressed with p75NTR in rat DRG (72). NGF selectively binds TrkA, whereas p75NTR can bind all neurotrophins with the same affinity (35). p75NTR also binds neurotrophin precursor molecules with high affinity, including proNGF (35). The ability of NGF to bind two very distinct receptors and the resulting signaling via this receptor complex has puzzled researchers (3). Much of the recent attention on p75NTR has been focused on its role in apoptosis and neuronal growth inhibition. However, the original function attributed to p75NTR was as an accessory protein that modulates Trk receptors to regulate their affinity for their appropriate ligand (3, 10). The coexpression of TrkA and p75NTR in heterologous cells results in high-affinity NGF binding sites (4, 62). Thus, it has become a prevailing concept that p75NTR and TrkA combine to form a receptor complex that results in high-affinity NGF binding (10). However, a recent study (70) provides structural and mechanistic evidence for a ligand-passing model in which NGF rapidly associates with p75NTR and then is presented to TrkA (70). In the model presented, blocking NGF binding to p75NTR attenuates NGF binding to TrkA and subsequent TrkA activation (70). It is also suggested that convergent signaling pathways activated by p75NTR and TrkA could underlie the complex crosstalk between p75NTR and TrkA (3, 70).
It is well documented that NGF is important in a number of inflammatory conditions including urinary bladder, colon, and lung inflammation (18, 26, 56, 64). The exact contribution of NGF to bladder function is not known but a role for NGF in bladder hyperreflexia and bladder overactivity has been suggested (12, 13, 20, 30, 34, 48, 73). NGF or TrkA sequestration methods and Trk inhibitors reduce bladder hyperreflexia in rats with experimentally induced inflammation and improve animal well being (20, 34) (Vizzard et al., unpublished observations). Elevated levels of neurotrophins are detected in the urine of women with PBS/IC (49) or in the urothelium of individuals with neuropathic bladder (43). However, a recent study failed to demonstrate an association between increased urothelium/suburothelium NGF with detrusor overactivity or bladder sensation (6). More recently, it has been demonstrated that urinary NGF levels are increased in overactive bladder (OAB), urinary incontinence, and decreased in patients responding to botulinum toxin-A treatment (41, 42). Thus, NGF may be a potential biomarker for OAB.
Blockade of the NGF-TrkA pathway in respiratory inflammation models with k252a (45) or NGF blocking antibodies (30) decreased recruitment of inflammatory cells and bronchial hyperresponsiveness, respectively. Immunoneutralization of p75NTR in adult rat sensory neurons inhibits upregulation of substance P induced by NGF application (54). Studies using p75NTR knockout (KO) mice or immunoneutralization of p75NTR reduced bronchial hyperresponsiveness and substance P release in the airway (24, 38, 59). In contrast, the present studies demonstrate bladder hyperreflexia or enhanced hyperreflexia in control and CYP-treated rats following intravesical blockade of p75NTR by two different approaches. Immunoneutralization of p75NTR via intravesical instillation of an anti-p75NTR monoclonal antibody increased urinary bladder frequency (decreased ICI, decreased void volume) in both control (no inflammation) and CYP-treated rats, whereas intravesical pressures (baseline, micturition, threshold) were not affected. To confirm that the observed changes in bladder function were not associated with nonspecific actions of immunoglobulin, intravesical instillation of isotype-matched IgG was infused and was without effect on any cystometric parameter evaluated. Intravesical infusion of PD90780, known to block NGF-p75NTR interactions (14, 55), produced similar but also additional changes in the cystometric parameters evaluated. PD90780 increased voiding frequency (decreased ICI, decreased void volume) in control and CYP-treated rats, but also altered threshold, baseline, and micturition pressures; vehicle infusions were without effect. In continuous cystometry recording, voided volume and ICI may be affected by increased residual urine volume due to decreased voiding efficiency and changes in urethral outlet resistance. In a closed urinary bladder system, PD90780 significantly decreased the volume threshold to elicit a micturition contraction in both control (no inflammation) and CYP-treated (48 h) rats. Although the present studies cannot rule out the possibility of NGF-p75NTR actions at the urethra, the isovolumetric studies clearly demonstrate NGF-p75NTR actions at the level of the urinary bladder.
Intravesical infusion of PD90780 produced some differential effects between control (no inflammation) and CYP-treated rats in contrast to the effects produced by immunoneutralization of p75NTR that were similar in control and CYP-treated rats. In control rats treated with intravesical instillation of PD90780, there was evidence of incomplete voiding as rats exhibited small void volumes compared with infused volume. This mismatch between void and infused volumes was not observed in CYP-treated rats infused with PD90780 or in control or CYP-treated rats infused with anti-p75NTR monoclonal antibody. This mismatch between void volume and infused volume may suggest PD90780 effects on the urethral outlet. Given the ubiquitous expression of p75NTR throughout the urinary bladder demonstrated in the present studies and the peripheral and central nervous system in general (27, 28, 65, 66), it would not be surprising that the urethra expressed p75NTR. Why the potential effects of PD90780 on the urethra are exhibited in control but not CYP-treated rats may be related to p75NTR and NGF expression in the urinary bladder with CYP-induced cystitis. Previous and present studies demonstrated significant increases in total p75NTR and NGF bladder expression with CYP-induced cystitis (34, 39, 64). In control rats with less p75NTR and NGF expression in the urinary bladder, infusion of PD90790 may exert bladder and urethral effects because the drug may be more available and have broader effects and affect NGF-p75NTR actions at the urethra. In contrast, with CYP-induced cystitis and increased p75NTR and NGF expression in the urinary bladder, effects of PD90780 infusion may be less available and restricted to the urinary bladder. In the future, urethral pull-through studies (68) or urethrometry (53, 60) studies may be designed determine potential NGF-p75NTR actions at the level of the urethra. These future studies will necessitate changes in route of drug application from intravesical to systemic or intrathecal administration and the use of anesthetized rodents.
These studies demonstrate widespread p75NTR expression in many cell types in the urinary bladder and increased total p75NTR expression in the urinary bladder with CYP-induced cystitis. This makes it difficult to determine the exact site(s) of action in the urinary bladder of the p75NTR antibody. We do know that intravesical infusion of anti- p75NTR monoclonal antibody without infusion of protamine sulfate showed no cystometric effects. This likely indicates a site of p75NTR action deep to the urothelium, since protamine sulfate is used to disrupt cell-to-cell contact of urothelial cells (11, 57). Our demonstration of p75NTR-IR after intravesical infusion of anti-p75NTR supports this suggestion as p75NTR-IR is present in the lamina propria. Potential targets include the suburothelial nerve plexus, interstitial cells of Cajal, and myofibroblasts all distributed beneath the urothelium (2) as well as inflammatory cell infiltrates in bladders from CYP-treated rats and nerve fibers innervating the detrusor smooth muscle.
p75NTR receptor blockade at the level of the urinary bladder produced changes in ICI, void volume, changes in threshold and micturition pressure, and increases in the number of nonvoiding contractions during the filling phase. Changes in ICI and volume threshold to initiate micturition and changes in threshold pressure are suggestive of changes in the afferent limb of the micturition reflex. Changes in micturition pressure are thought to be dependent on the efficiency of neurotransmission of bladder efferents (44) or changes in the urethral outlet with continuous cystometry. Changes in nonvoiding contractions observed with PD90780 infusions in CYP-treated rats may have both myogenic and neurogenic components (29, 32, 58). For example, myogenic contractions could trigger afferent firing which then evokes a reflex efferent discharge that amplifies the myogenic contraction. Effects of intravesical anti-p75NTR infusion were limited to ICI and void volume without effects on intravesical pressures suggesting that the afferent limb of the micturition reflex is targeted. Greater concentrations of anti-p75NTR were not evaluated in the present study so it is not known whether additional cystometric changes would have emerged suggestive of effects on the efferent limb of the micturition reflex. In contrast, effects of PD90780 on ICI, volume threshold, void volume, and intravesical pressures suggest that both the afferent and efferent limbs of the micturition reflex are affected. In addition, changes in micturition pressures (e.g., baseline and peak micturition pressure) with continuous cystometry may suggest changes in the urethral outlet as a result of intravesical drug administration.
The present experiments show that p75NTR blockade at the level of the urinary bladder increases voiding frequency in control rats and further increases voiding frequency in CYP-treated rats. Thus, one function of p75NTR and NGF-p75NTR interactions in vivo may be to reduce bladder activity or to offset bladder hyperreflexia induced by CYP-induced cystitis. Whereas it is known that p75NTR expression can enhance TrkA-NGF binding in some systems (1, 9), p75NTR may also reduce NGF-TrkA signaling by sequestering NGF (19, 31, 46). In the latter instance, any decrease or perturbation in NGF/p75NTR binding may tip the homeostatic equilibrium, resulting in increased NGF bioavailability to enhance NGF/TrkA signaling. If NGF/TrkA signaling is favored, increased expression or function of ion channels such as the transient receptor potential (TRP) channels (e.g., TRPV1) and neuropeptides may contribute to urinary bladder hyperreflexia. A role for both TRPV1 expression in bladder afferents and urothelial cells in bladder hyperreflexia has been suggested (5). In addition, increased expression of TRPV1 in bladder nerves and urothelium has been demonstrated in neurogenic detrusor overactivity (5). Our previous studies demonstrated increased expression of substance P (63), calcitonin gene-related peptide (63), and pituitary adenylate cyclase activating polypeptide (PACAP) (7) in lower urinary tract tissues with CYP-induced cystitis. Furthermore, intravesical and intrathecal blockade of the PACAP receptor, PAC1, reduced bladder hyperreflexia induced by CYP treatment (7). These downstream regulatory events can be examined in TRPV1 null or PACAP null mice with p75NTR blockade. Conversely, if NGF/p75NTR signaling is favored, NGF/TrkA signaling in bladder function may be dampened. However, as p75NTR can partner not only with other Trk receptors but also with a large number of other membrane-associated proteins (4), such as TROY and sortilin, there may be multiple mechanistic intersections, the summation of which will determine bladder reflex responses.
Conclusions
The present studies suggest that one role of p75NTR and NGF-p75NTR interactions in vivo may be to reduce bladder activity or to offset bladder hyperreflexia induced by urinary bladder inflammation. Although perhaps less clinically relevant than OAB, detrusor overactivity, or bladder hyperreflexia, detrusor underactivity is a clinical problem that is seen most often in men, although women are also affected (16, 17). It consists of reduced muscle contraction, strength, or velocity and often results in incomplete voiding, postvoid residual, prolonged emptying, and reduced free uroflow (16, 17). Detrusor underactivity can be neurogenic, idiopathic, or a result of bladder outflow obstruction (16, 17). Whether blockade of p75NTR in this context would be beneficial or whether p75NTR receptors are regulated by detrusor underactivity awaits further study.
GRANTS
This work was funded by National Institutes of Health (NIH) Grants DK-051369, DK-060481, and DK-065989. NIH Grant P20-RR-16435 from the COBRE Program of the National Center also supported the project for research resources.
Acknowledgments
The authors gratefully acknowledge the technical expertise and support provided by the VT Cancer Center DNA Analysis Facility. The authors thank A. Dattilio, K. Schutz, and S. Malley for technical assistance. The authors also thank Dr. V. May for many helpful discussions of this work.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond) 110: 175–191, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Barker PA High affinity not in the vicinity? Neuron 53: 1–4, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barker PA p75NTR is positively promiscuous: novel partners and new insights. Neuron 42: 529–533, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Birder LA TRPs in bladder diseases. Biochim Biophys Acta 1772: 879–884, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birder LA, Wolf-Johnston A, Griffiths D, Resnick NM. Role of urothelial nerve growth factor in human bladder function. Neurourol Urodyn 26: 405–409, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290: R951–R962, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronfman FC, Fainzilber M. Multitasking by the p75 neurotrophin receptor: sortilin things out? EMBO Rep 5: 867–871, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci 18: 321–326, 1995. [PubMed] [Google Scholar]
- 10.Chao MV, Rajagopal R, Lee FS. Neurotrophin signaling in health and disease. Clin Sci (Lond) 110: 167–173, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Chuang YC, Chancellor MB, Seki S, Yoshimura N, Tyagi P, Huang L, Lavelle JP, De Groat WC, Fraser MO. Intravesical protamine sulfate and potassium chloride as a model for bladder hyperactivity. Urology 61: 664–670, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165: 975–979, 2001. [PubMed] [Google Scholar]
- 13.Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Regul Integr Comp Physiol 275: R1279–R1286, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Colquhoun A, Lawrance GM, Shamovsky IL, Riopelle RJ, Ross GM. Differential activity of the nerve growth factor (NGF) antagonist PD90780 {7-(benzolylamino)-4,9-dihydro-4-methyl-9-oxo-pyrazolo[5,1-b]quinazoline-2-carboxylic acid} suggests altered NGF-p75NTR interactions in the presence of TrkA. J Pharmacol Exp Ther 310: 505–511, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Cucchi A, Quaglini S, Guarnaschelli C, Rovereto B. Urodynamic findings suggesting two-stage development of idiopathic detrusor underactivity in adult men. Urology 70: 75–79, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Cucchi A, Quaglini S, Rovereto B. Development of idiopathic detrusor underactivity in women: from isolated decrease in contraction velocity to obvious impairment of voiding function. Urology 71: 844–848, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Delafoy L, Gelot A, Ardid D, Eschalier A, Bertrand C, Doherty AM, Diop L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 55: 940–945, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhanoa NK, Krol KM, Jahed A, Crutcher KA, Kawaja MD. Null mutations for exon III and exon IV of the p75 neurotrophin receptor gene enhance sympathetic sprouting in response to elevated levels of nerve growth factor in transgenic mice. Exp Neurol 198: 416–426, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78: 449–459, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Dorr W Cystometry in mice–influence of bladder filling rate and circadian variations in bladder compliance. J Urol 148: 183–187, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF. Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci 31: 366–375, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Elsasser-Beile U, Gutzeit O, Bauer S, Katzenwadel A, Schultze-Seemann W, Wetterauer U. Systemic and local immunomodulatory effects of intravesical BCG therapy in patients with superficial urinary bladder carcinomas. J Urol 163: 296–299, 2000. [PubMed] [Google Scholar]
- 24.Farraj AK, Haykal-Coates N, Ledbetter AD, Evansky PA, Gavett SH. Inhibition of pan neurotrophin receptor p75 attenuates diesel particulate-induced enhancement of allergic airway responses in C57/B16J mice. Inhal Toxicol 18: 483–491, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Fraser MO, Chuang YC, Tyagi P, Yokoyama T, Yoshimura N, Huang L, De Groat WC, Chancellor MB. Intravesical liposome administration–a novel treatment for hyperactive bladder in the rat. Urology 61: 656–663, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Freund-Michel V, Frossard N. The nerve growth factor and its receptors in airway inflammatory diseases. Pharmacol Ther 117: 52–76, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Friedman WJ, Greene LA. Neurotrophin signaling via Trks and p75. Exp Cell Res 253: 131–142, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Frossard N, Freund V, Advenier C. Nerve growth factor and its receptors in asthma and inflammation. Eur J Pharmacol 500: 453–465, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie JI Inhibitory actions of calcitonin gene-related peptide and capsaicin: evidence for local axonal reflexes in the bladder wall. BJU Int 95: 149–156, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Hannila SS, Kawaja MD. Nerve growth factor-mediated collateral sprouting of central sensory axons into deafferentated regions of the dorsal horn is enhanced in the absence of the p75 neurotrophin receptor. J Comp Neurol 486: 331–343, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol 284: R574–R585, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016–1021, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Huang CS, Zhou J, Feng AK, Lynch CC, Klumperman J, DeArmond SJ, Mobley WC. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem 274: 36707–36714, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Ito H, Nomoto H, Furukawa S. Growth arrest of PC12 cells by nerve growth factor is dependent on the phosphatidylinositol 3-kinase/Akt pathway via p75 neurotrophin receptor. J Neurosci Res 72: 211–217, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Jahed A, Kawaja MD. The influences of p75 neurotrophin receptor and brain-derived neurotrophic factor in the sympathetic innervation of target tissues during murine postnatal development. Auton Neurosci 118: 32–42, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Kerzel S, Path G, Nockher WA, Quarcoo D, Raap U, Groneberg DA, Dinh QT, Fischer A, Braun A, Renz H. Pan-neurotrophin receptor p75 contributes to neuronal hyperreactivity and airway inflammation in a murine model of experimental asthma. Am J Respir Cell Mol Biol 28: 170–178, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Klinger MB, Girard B, Vizzard MA. p75NTR expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507: 1379–1392, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in nonneuronal cells of rat sciatic nerve. Nature 330: 658–659, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor level could be a biomarker in the differential diagnosis of mixed urinary incontinence in women. BJU Int In press. [DOI] [PubMed]
- 42.Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology 72: 104–108, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79: 572–577, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods 15: 157–167, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Mohtasham L, Auais A, Piedimonte G. Nerve growth factor mediates steroid-resistant inflammation in respiratory syncytial virus infection. Pediatr Pulmonol 42: 496–504, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Nykjaer A, Willnow TE, Petersen CM. p75NTR–live or let die. Curr Opin Neurobiol 15: 49–57, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Obata K, Katsura H, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Noguchi K. Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J Neurosci 26: 11974–11986, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oddiah D, Anand P, McMahon SB, Rattray M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 9: 1455–1458, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161: 438–441, 1999. [PubMed] [Google Scholar]
- 50.Pezet S, Onteniente B, Jullien J, Junier MP, Grannec G, Rudkin BB, Calvino B. Differential regulation of NGF receptors in primary sensory neurons by adjuvant-induced arthritis in the rat. Pain 90: 113–125, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol 454: 200–211, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168: 2269–2274, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol 171: 478–482, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol 197: 430–436, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Spiegel K, Agrafiotis D, Caprathe B, Davis RE, Dickerson MR, Fergus JH, Hepburn TW, Marks JS, Van Dorf M, Wieland DM, Jaen JC. PD90780, a nonpeptide inhibitor of nerve growth factor's binding to the P75 NGF receptor. Biochem Biophys Res Commun 217: 488–494, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Stanzel RD, Lourenssen S, Blennerhassett MG. Inflammation causes expression of NGF in epithelial cells of the rat colon. Exp Neurol 211: 203–213, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Stein PC, Pham H, Ito T, Parsons CL. Bladder injury model induced in rats by exposure to protamine sulfate followed by bacterial endotoxin. J Urol 155: 1133–1138, 1996. [PubMed] [Google Scholar]
- 58.Streng T, Hedlund P, Talo A, Andersson KE, Gillespie JI. Phasic nonmicturition contractions in the bladder of the anaesthetized and awake rat. BJU Int 97: 1094–1101, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Tokuoka S, Takahashi Y, Masuda T, Tanaka H, Furukawa S, Nagai H. Disruption of antigen-induced airway inflammation and airway hyper-responsiveness in low affinity neurotrophin receptor p75 gene deficient mice. Br J Pharmacol 134: 1580–1586, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torimoto K, Fraser MO, Hirao Y, De Groat WC, Chancellor MB, Yoshimura N. Urethral dysfunction in diabetic rats. J Urol 171: 1959–1964, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Vaidyanathan S, Krishnan KR, Mansour P, Soni BM, McDicken I. p75 nerve growth factor receptor in the vesical urothelium of patients with neuropathic bladder: an immunohistochemical study. Spinal Cord 36: 541–547, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Verdi JM, Birren SJ, Ibanez CF, Persson H, Kaplan DR, Benedetti M, Chao MV, Anderson DJ. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron 12: 733–745, 1994. [DOI] [PubMed] [Google Scholar]
- 63.Vizzard MA Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Vizzard MA Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Wakabayashi Y, Maeda T, Aimi Y, Kwok YN. Increase of low-affinity neurotrophin receptor p75 and growth-associated protein-43 immunoreactivities in the rat urinary bladder during experimentally induced nerve regeneration. J Urol 160: 1513–1517, 1998. [PubMed] [Google Scholar]
- 66.Wakabayashi Y, Maeda T, Kwok YN. Increase of p75 immunoreactivity in rat urinary bladder following inflammation. Neuroreport 7: 1141–1144, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Wakabayashi Y, Tomoyoshi T, Tooyama I, Kitahama K, Kim SU, Maeda T. Low-affinity nerve growth factor receptor immunoreactivity in the human urinary bladder. Neurosci Lett 186: 9–12, 1995. [DOI] [PubMed] [Google Scholar]
- 68.Walters RD, McMurray G, Brading AF. Comparison of the urethral properties of the female guinea pig and rat. Neurourol Urodyn 25: 62–69, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Wang W, Dow KE, Riopelle RJ, Ross GM. The common neurotrophin receptor p75NTR enhances the ability of PC12 cells to resist oxidative stress by a trkA-dependent mechanism. Neurotox Res 3: 485–499, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron 53: 25–38, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 121: 417–424, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol 351: 329–338, 1995. [DOI] [PubMed] [Google Scholar]
- 73.Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zvarova K, Vizzard MA. Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: a developmental study. J Comp Neurol 489: 501–517, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]