Abstract
We carried out LC-MS/MS-based proteomic profiling of differential centrifugation fractions from rat inner medullary collecting duct (IMCD): 1) to provide baseline knowledge of the IMCD proteome and 2) to evaluate the utility of differential centrifugation in assessing trafficking of the water channel aquaporin-2 (AQP2). IMCD suspensions were freshly prepared from rat kidneys using standard methods. Homogenized samples were subjected to sequential centrifugations at 1,000, 4,000, 17,000, and 200,000 g. These samples, as well as the final supernatant, were subjected to LC-MS/MS analysis. Preliminary immunoblotting confirmed that the ratio of AQP2 in the 17,000-g fraction to the 200,000-g fraction underwent an increase in response to the vasopressin analog dDAVP, largely due to a reduction in the 200,000-g fraction. Immunoblotting for the major phosphorylated forms of AQP2 revealed that phosphorylated AQP2 was present in both the 17,000- and 200,000-g fractions. LC-MS/MS analysis showed that markers of “intracellular vesicles,” chiefly endosomal markers, were present in both the 17,000- and the 200,000-g fractions. In contrast, plasma membrane proteins were predominantly present in the 4,000- and 17,000-g fractions. Proteins associated with several multiprotein complexes (e.g., actin-related protein 2/3 complex and proteasome complex) were virtually exclusively present in the 200,000-g fraction. Overall, we identified 656 proteins, including 189 not previously present in the IMCD database. The data show that both the 17,000- and 200,000-g fractions are highly heterogeneous and cannot be equated with “plasma membrane” and “intracellular vesicle” fractions, respectively, leading us to propose an alternative approach for use of differential centrifugation to assess vesicular trafficking to the plasma membrane.
Keywords: aquaporin-2, phosphorylation, vasopressin, membrane trafficking
the renal collecting duct is the final segment of the renal tubule and its main function is to transport water and various solutes. The key extracellular signaling molecule is the peptide hormone arginine vasopressin. Vasopressin acts to regulate water permeability of the collecting duct by altering the trafficking of vesicles containing the water channel aquaporin-2 (AQP2) to and from the apical membrane of collecting duct and connecting tubule cells. To study vesicular trafficking of AQP2, many techniques, including immunogold labeling, fluor-escence imaging, surface biotinylation, and differential centrifugation, have been used.
Differential centrifugation is a common technique in which different elements contained within a cell can be separated based on density. It is frequently used to enrich certain organelles that may be the focus of a particular study. Cells are initially homogenized and then the homogenate is centrifuged at a series of increasing speeds. Denser cellular components pellet more rapidly than less dense ones. The pellets obtained with successive spins are typically collected for biochemical analysis.
A method for investigation of AQP2 trafficking using differential centrifugation was introduced by Marples and colleagues (12). In most papers that followed, the cell fraction obtained from a 17,000-g spin (17K) has been referred to as the “plasma membrane” fraction (for example, see Refs. 6, 7, 9, 10, 19), while the fraction obtained from a 200,000-g spin (200K) has been called the “intracellular vesicle” fraction. Marples and colleagues demonstrated in rat kidneys that the ratio of AQP2 in the 17K pellet to that in the 200K pellet increased in response to exposure to the V2 receptor-selective vasopressin analog dDAVP (12). This finding correlated with immuno-EM and other results showing translocation of AQP2 to the apical plasma membrane.
For this differential centrifugation approach to successfully measure AQP2 translocation, it must be assumed that the AQP2 in the 17K fraction is predominantly in plasma membrane and that the AQP2 in the 200K fraction is predominantly in intracellular vesicles. However, these fractions are likely to be heterogeneous. To investigate the composition of such fractions when differential centrifugation is applied to inner medullary collecting duct (IMCD) cells, we have now carried out large-scale LC-MS/MS-based proteomic profiling of all fractions from homogenized IMCDs.
MATERIALS AND METHODS
Animals.
Male pathogen-free Sprague-Dawley rats (Taconic Farms, Germantown, NY) were maintained on drinking water and ad libitum rat chow (NIH-07; Zeigler, Gardners, PA) in the Small Animal Facility, National Institutes of Health, Intramural Research Program. All experiments were conducted following the animal protocol H-0110 as approved by the Animal Care and Use Committee, National Heart, Lung, and Blood Institute.
Antibodies (listed by gene symbol).
Rabbit polyclonal antibodies against AQP2 (8), AQP2 phosphorylated at S264 (5), AQP2 phosphorylated at S269 (8), ATP1A1 (17), VAMP2 (15), STX4 (11), SCNN1B (13), SCNN1G (13), and SLC14A2 (2) were generated in our laboratory. Phosphospecific antibodies targeting AQP2 phosphorylated at S256 (rabbit #1697) and S261 (rabbit #1028) were newly generated using appropriate phosphopeptide antigens (PhosphoSolutions, Aurora, CO). The rabbit polyclonal antibody against STX12 was newly prepared using a synthetic peptide (sequence: YRNPGRRSLRDFSSIIQTC) conjugated to keyhole limpet hemocyanin. All antibodies from our laboratory were affinity purified using the appropriate immunizing peptide. The commercial antibodies against RAB11 (610656), RALA (610221), CDH1 (610181), and GOLGA2 (610822) (16) were from BD Transduction Laboratories (San Jose, CA). The antibodies against RAB5 (sc-598), AKR1B1 (sc-17735), HSPA5 (sc-1050), and BRG1 (sc-17796) were from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody against STX7 (110072) was from Synaptic Systems GmBH (Goettingen, Germany). The antibody against CANX (spa-860) was from Stressgen Bioreagents (Ann Arbor, MI). The antibody against IMMT was from Millipore (Charlottesville, VA). The species-specific secondary antibodies conjugated with fluorophores were obtained from Rockland Immunochemicals (Gilbertsville, PA).
IMCD cell suspension.
IMCD cell suspensions were prepared as described previously (3). Adult rats ranging from 200–250 g were injected with furosemide (5 mg/rat, 30 min before euthanization), which dissipates the medullary osmotic gradient, thereby preventing osmotic shock to the cells upon isolation. The rats were decapitated and both inner medullas were dissected from the kidneys. Inner medullas were minced into cubes ∼1 mm3 in size and digested in isolation solution [2,000 U/ml hyaluronidase and 3 mg/ml collagenase B in sucrose buffer (250 mM sucrose, 10 mM Tris, pH 7.4)] for 90 min at 37°C. The resulting solution was centrifuged for 20 s at 60 g to separate the non-IMCD segments from IMCD cells. The cells were washed three times in sucrose buffer and spun as outlined above. Finally, the cells were resuspended in homogenization solution [sucrose buffer plus protease inhibitor cocktail (Complete Mini, Roche, Indianapolis, IN) and phosphatase inhibitors (Halt Phosphatase Inhibitor, Pierce, Rockford, IL)].
Homogenization and differential centrifugation.
IMCD cells were homogenized following the protocol of Marples et al. (12) with slight modifications, since the Marples protocol dealt with whole IM tissue. Briefly, suspensions were homogenized for 10 slow strokes using a motor-driven Potter-Elvehjem homogenizer. The cells were then centrifuged at 1,000 g for 10 min to pellet nuclei and unbroken cells. The supernatant was collected for the next step. To increase protein yield, the pellet was rehomogenized in fresh buffer for another 10 strokes and respun as described above. The supernatants were further spun at 4,000 g, and the resulting supernatants were extracted and spun at 17,000 g (both spins at 4°C for 20 min). The supernatants from the 17,000-g spin were then spun in an ultracentrifuge at 200,000 g at 4°C for 1 h.
The pellets obtained from the 1,000-, 4,000-, 17,000-, and 200,000-g spins were suspended in 1× Laemmli buffer (1.5% SDS, 50 mM Tris, pH 6.8) and labeled “1K,” “4K,” “17K,” and “200K,” respectively. The remaining cytosol from the 200,000-g spin was labeled “Sup” and concentrated using centrifugal filters with a cut-off of 10,000 Da (Microcon, Millipore, Billerica, MA) to achieve a concentration between 1–4 μg/μl in 1× Laemmli buffer. The protein concentration of each fraction was determined using a BCA assay.
In-gel trypsin digestion.
One-hundred micrograms of protein from each fraction were loaded into a 4–15% gradient SDS-PAGE mini-gel (Bio-Rad Life Science, Hercules, CA) and electrophoresed at 200 V for 45 min. The gel was then washed in ultrapure water and stained with Coomassie blue (Imperial Protein Stain, Pierce). After being destained with water for 1 h, each lane was cut into 10 separate gel pieces which were then minced and transferred into 1.5-ml Eppendorf tubes. These gel pieces were destained, reduced, alkylated, and trypsin-digested using the same protocol as previously described (18).
LC-MS/MS protein identification and analysis.
Cell fractions were analyzed using mass spectrometry as outlined previously (21). Peptides recovered from gel slices were injected into a reverse-phase liquid chromatographic (LC) column (PicoFritTM, Biobasic C18, New Objective, Woodburn, MA) for further separation, and then delivered into an LTQ tandem mass spectrometer (Thermo Electron, San Jose, CA) via electrospray. Spectra with ion currents larger than 10,000 were used to search for matching peptides by using Sequest with a concatenated RefSeq database. We used target-decoy analysis to set the false discovery rate to 2%. To assess relative protein abundance, we applied spectral counting normalized by molecular weight to correct for the higher probability of peptide identification in larger proteins.
Bioinformatics.
Clustering of proteins according to normalized spectral counting was achieved using the Cluster program (4). Protein groups that formed groups of interest were then analyzed for Gene Ontology Cell Components. Heat maps were generated using the TreeView program (4).
Dot-blot analysis.
Newly generated anti-pS256 and anti-pS261 AQP2 phospho-specific antibodies (used at 1:3,000 dilution) were tested by spotting 0.15 μg of various synthetic, biotinylated AQP2 COOH-terminal peptides onto a nitrocellulose membrane and detected using a fluorescently conjugated goat anti-rabbit secondary antibody [see Supplemental Materials (the online version of this article contains supplemental data)]. Both antibodies recognized the appropriate phosphopeptide but not the nonphosphorylated peptide.
Immunoblotting.
After solubilization in Laemmli's reagent, samples were loaded onto 4–15% gradient polyacrylamide gels, resolved, and transferred onto a nitrocellulose membrane using electrophoresis. The membrane was blocked for 1 h using Odyssey Blocking Buffer (LI-COR, Lincoln, NE) and incubated overnight in primary antibody. Following a wash with 1× PBS buffer containing 0.1% Tween, the membrane was bathed for an hour in appropriate secondary antibody. After another wash in PBS, bands were visualized using a LI-COR Odyssey Scanner and analyzed with Odyssey 2.1. All immunoblots were loaded to compare equal percentages of the total protein in the individual differential centrifugation fractions.
dDAVP treatment.
For dDAVP treatment, IMCD pellets were washed in sucrose buffer as above, but the final solution was instead 290 mOsm bicarbonate buffer (in mM: 118 NaCl, 25 NaHCO3, 5 KCl, 4 Na2HPO4, 2 CaCl2, 1.2 MgSO4, 5.5 glucose) containing 1 nM dDAVP. Controls were identical except that dDAVP was not added. dDAVP-treated and control IMCD samples were incubated for 30 min. All experiments were performed in a pH-/temperature-controlled chamber with gentle mixing under an atmosphere of 95% air-5% CO2 at 37°C. After incubation, the cells were pelleted and resuspended in homogenization solution.
RESULTS
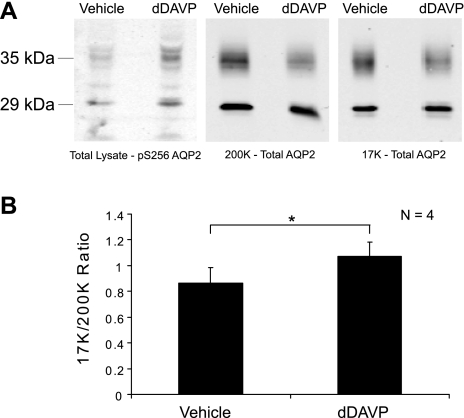
Immunoblots of differential centrifugation fractions from rat IMCDs exposed to either dDAVP (1 nM) or vehicle for 30 min are shown in Fig. 1A. Figure 1A, left, shows that dDAVP increased AQP2 phosphorylation at Ser-256, a phosphorylation event already known to occur in response to vasopressin (16), thereby confirming the effectiveness of the dDAVP. Figure 1A, middle, shows the effect of dDAVP on the AQP2 abundance in the 200K fraction. As previously shown (9), the AQP2 band density decreased in this fraction in response to agonist. Figure 1A, right, shows the effect of dDAVP on the AQP2 abundance in the 17K fraction. There was no significant change in this value, as demonstrated previously (9). [These immunoblots in Fig. 1A, middle and right, used an AQP2 antibody (8) upstream from the polyphosphorylated region in the COOH terminus and therefore the band densities obtained would not be expected to be influenced by dDAVP-induced phosphorylation.] Thus, we confirmed the observation of Marples that vasopressin induces an increase in the 17K/200K ratio (Fig. 1B), but also show in accord with the findings of Inoue et al. (9) that the change is largely due to a decrease in the AQP2 contained in the 200K fraction, at least in isolated IMCD suspensions. These experiments demonstrated that the IMCD cells in our suspensions were viable and responsive to dDAVP and that the differential centrifugation technique used successfully reproduced prior findings.
Fig. 1.
Aquaporin-2 (AQP2) response to vasopressin in inner medullary collecting duct (IMCD) suspensions. A: immunoblots of differential centrifugation fractions from rat IMCDs exposed to either dDAVP (1 nM) or vehicle for 30 min. Left: immunoblot incubated with phospho-specific antibody to Ser-256 of AQP2. Substantial increase of phosphorylation confirms efficacy of dDAVP (vasopressin) [normalized band density values (means ± SE) of vehicle and dDAVP = 0.44 ± 0.10 and 1.56 ± 0.10, respectively]. Middle: immunoblot incubated with an antibody to total AQP2. AQP2 abundance is reduced in the 200K fraction upon exposure to dDAVP [normalized band density values (means ± SE) of vehicle and dDAVP = 1.15 ± 0.15 and 0.93 ± 0.01, respectively]. Right: same antibody as in middle. 17K fraction does not show a corresponding increase of AQP2 [normalized band density values (means ± SE) of vehicle and dDAVP = 0.93 ± 0.05 and 0.99 ± 0.11, respectively]. B: graph showing average ratio of AQP2 band intensities in the 17K fraction to the 200K fraction, with or without vasopressin. *Statistical significance (n = 4).
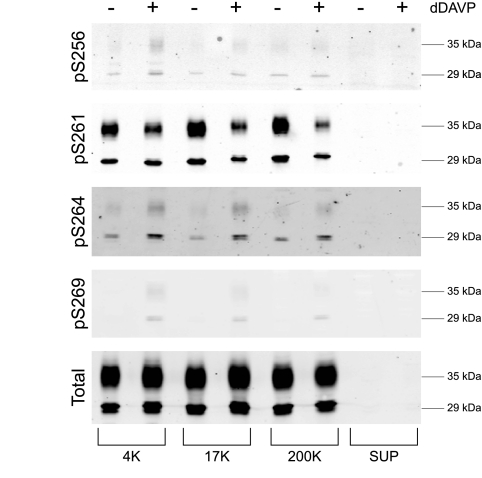
AQP2 is known to be phosphorylated at four sites in its COOH-terminal tail, Ser-256, Ser-261, Ser-264, and Ser-269 (8a). To determine which fractions from differential centrifugation contain AQP2 phosphorylated at each site, we carried out immunoblotting of all fractions using phospho-specific antibodies as well as an antibody that recognizes total AQP2 (Fig. 2). All phosphorylated forms of AQP2 were identified in all membrane fractions (4K, 17K, and 200K), but not in the supernatant. As previously observed, the vasopressin analog dDAVP increased phosphorylation at Ser-256, Ser-264, and Ser-269, while decreasing phosphorylation at Ser-261 (8).
Fig. 2.
Distribution of phosphorylated AQP2 after differential centrifugation. Immunoblot stained with various phospho-specific antibodies to AQP2 (pS256, pS261, pS264, and pS269) as well as total AQP2. Samples from 4,000-g (4K), 17,000-g (17K), 200,000-g (200K) pellets, and 200,000-g supernatant (Sup) were exposed to either dDAVP or vehicle.
To discover what structures are present in the 4K, 17K, and 200K membrane fractions and the supernatant fraction (cytosol), we carried out protein mass spectrometry using liquid chromatography combined with tandem mass spectrometry (LC-MS/MS). This analysis identified a total of 656 proteins, including 266 proteins in the 4K fraction, 243 proteins in the 17K fraction, 233 proteins in the 200K fraction, and 297 proteins in the supernatant (see Supplemental Table 1). (Note that some proteins were identified in multiple fractions.) Among the identified proteins, 189 were proteins not previously identified in the IMCD proteome. These newly identified proteins have been added to the “IMCD Proteome Database” (the updated version can be found at http://dir.nhlbi.nih.gov/papers/lkem/imcd/).
Table 1 reports normalized spectral counts for all proteins previously identified in AQP2-immunoisolated vesicles from IMCD (1) and also present in membrane fractions from this analysis. (We make no assumption that these proteins are exclusively present in intracellular vesicles.) Here, “normalized spectral counts” means the number of spectra identified for a given protein divided by the unmodified molecular weight calculated from the primary sequence. The table is divided into three groups based on the calculated ratio of normalized spectral counts in the 17K to the 200K fraction (last column). Focusing on the first group, i.e., proteins found disproportionately in the 17K fraction, several endosomal marker proteins are present, including Rab25 (recycling endosomes), Rab5b (early endosomes), and Rab5c (early endosomes). In addition, several proteins believed to be predominantly associated with the plasma membrane are included in this group, such as Na-K-ATPase subunits, integrin α2, integrin β1, annexin IV, and annexin V. The middle group, i.e., proteins present in both 17K and 200K fractions, include AQP2 and markers of a variety of structures, e.g., syntaxin 7 (late endosomes), Rab11a (recycling endosomes), Rab10 (Golgi apparatus), and calnexin (endoplasmic reticulum). In addition, it is not surprising that this group contains cytoskeletal proteins which could be attached to both plasma membrane and intracellular vesicles. The third group, i.e., proteins disproportionately present in the 200K fraction, includes ribosomal elements as well as the AP-1 and AP-2 clathrin adaptor complexes. From this, we conclude that many endosomal proteins, including recycling endosome proteins, are present in multiple fractions, not just the 200K fraction. In contrast, plasma membrane predominant proteins are found mainly in the low-speed fractions, including the 17K fraction.
Table 1.
List of proteins identified in this study that are known to be present in AQP2-containing vesicles
| Protein Name | Gene Symbol | Accession Number | 4K | 17K | 200K | Sup. | 17K/200K |
|---|---|---|---|---|---|---|---|
| Histone cluster 1, H4b | Hist1 h4b | NP_073177.1 | 26.39 | 17.59 | 0.00 | 0.00 | > 10 |
| Proteolipid protein 2 | Plp2 | NP_997484.1 | 0.00 | 12.08 | 0.00 | 0.00 | > 10 |
| ATPase-Na+-K+ transporting, beta 1 polypeptide | Atp1b1 | NP_037245.2 | 11.36 | 8.52 | 0.00 | 0.00 | > 10 |
| RAB5C, member RAS oncogene family | Rab5c | NP_001099310.1 | 7.15 | 7.15 | 0.00 | 0.00 | > 10 |
| Histone cluster 1, H2bl | Hist1 h2bl | NP_072173.1 | 0.00 | 7.15 | 0.00 | 0.00 | > 10 |
| Cytochrome b-5 | Cyb5 | NP_071581.1 | 0.00 | 6.51 | 0.00 | 0.00 | > 10 |
| Tumor-associated calcium signal transducer 1 | Tacstd1 | NP_612550.1 | 0.00 | 5.68 | 0.00 | 0.00 | > 10 |
| Integrin beta 1 | Itgb1 | NP_058718.2 | 4.53 | 5.66 | 0.00 | 0.00 | > 10 |
| Annexin A5 | Anxa5 | NP_037264.1 | 0.00 | 5.60 | 0.00 | 16.79 | > 10 |
| Annexin A4 | Anxa4 | NP_077069.3 | 2.79 | 5.57 | 0.00 | 16.72 | > 10 |
| Heat shock protein 5 | Hspa5 | NP_037215.1 | 4.15 | 5.53 | 0.00 | 0.00 | > 10 |
| Guanine nucleotide-binding protein, beta-1 subunit | Gnb1 | NP_112249.2 | 0.00 | 5.35 | 0.00 | 0.00 | > 10 |
| Transmembrane emp24 protein transport domain containing 4 | Tmed4 | NP_001100708.1 | 0.00 | 5.11 | 0.00 | 0.00 | > 10 |
| Myosin light chain, regulatory B | Mrlcb | NP_059039.1 | 0.00 | 5.04 | 0.00 | 0.00 | > 10 |
| Histone cluster 1, H1d | Hist1 h1d | NP_579819.1 | 4.55 | 4.55 | 0.00 | 0.00 | > 10 |
| RAB25, member RAS oncogene family | Rab25 | NP_001101157.2 | 0.00 | 4.27 | 0.00 | 0.00 | > 10 |
| RAB2A, member RAS oncogene family | Rab2a | NP_113906.1 | 0.00 | 4.25 | 0.00 | 0.00 | > 10 |
| RAB5B, member RAS oncogene family | Rab5b | NP_001073405.1 | 0.00 | 4.22 | 0.00 | 0.00 | > 10 |
| ATP synthase, H+ transporting, mitochondrial F1 complex, beta subunit | Atp5b | NP_599191.1 | 7.10 | 3.55 | 0.00 | 0.00 | > 10 |
| Prolyl 4-hydroxylase, beta polypeptide | P4 hb | NP_037130.1 | 12.31 | 3.52 | 0.00 | 1.76 | > 10 |
| Ceruloplasmin | Cp | NP_036664.1 | 0.83 | 3.31 | 0.00 | 0.00 | > 10 |
| Voltage-dependent anion channel 1 | Vdac1 | NP_112643.1 | 3.25 | 3.25 | 0.00 | 0.00 | > 10 |
| N-ethylmaleimide-sensitive fusion protein attachment protein alpha | Napa | NP_542152.1 | 0.00 | 3.01 | 0.00 | 0.00 | > 10 |
| DEAD (Asp-Glu-Ala-Asp) box polypeptide 5 | Ddx5 | NP_001007614.1 | 0.00 | 2.89 | 0.00 | 0.00 | > 10 |
| Leucyl/cystinyl aminopeptidase isoform 1 | Lnpep | NP_001106874.1 | 0.00 | 2.56 | 0.00 | 0.00 | > 10 |
| Guanine nucleotide binding protein (G protein), alpha inhibiting 3 | Gnai3 | NP_037238.1 | 0.00 | 2.47 | 0.00 | 0.00 | > 10 |
| Hexose-6-phosphate dehydrogenase (glucose 1-dehydrogenase) | H6pd | NP_001100168.1 | 0.00 | 2.23 | 0.00 | 1.11 | > 10 |
| Alpha-1,3-mannosyltransferase ALG2 | Alg2 | XP_001055088.1 | 2.11 | 2.11 | 0.00 | 0.00 | > 10 |
| Protein disulfide isomerase associated 6 | Pdia6 | NP_001004442.1 | 2.05 | 2.05 | 0.00 | 0.00 | > 10 |
| Aldehyde dehydrogenase family 3, subfamily A2 | Aldh3a2 | NP_113919.2 | 1.85 | 1.85 | 0.00 | 0.00 | > 10 |
| Protein kinase C substrate 80K-H | Prkcsh | NP_001100276.1 | 3.38 | 1.69 | 0.00 | 1.69 | > 10 |
| carboxylesterase 2 | Ces2 | NP_598270.1 | 1.61 | 1.61 | 0.00 | 0.00 | > 10 |
| ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2 isoform a | Atp2a2 | NP_001104293.1 | 0.00 | 0.87 | 0.00 | 0.00 | > 10 |
| Membrane-bound C2 domain containing protein | Mbc2 | NP_058945.2 | 0.83 | 0.83 | 0.00 | 0.00 | > 10 |
| Integrin alpha-2 precursor | Itga2 | XP_001075558.1 | 0.00 | 0.78 | 0.00 | 0.00 | > 10 |
| UDP-glucose ceramide glucosyltransferase-like 1 | Ugcgl1 | NP_598280.1 | 0.00 | 0.57 | 0.00 | 0.00 | > 10 |
| ATPase-Na+-K+ transporting, alpha 1 polypeptide | Atp1a1 | NP_036636.1 | 10.61 | 7.96 | 0.88 | 0.00 | 9.00 |
| Calnexin | Canx | NP_742005.1 | 2.97 | 7.43 | 1.49 | 0.00 | 5.00 |
| Heat shock protein 8 | Hspa8 | NP_077327.1 | 2.82 | 5.64 | 1.41 | 15.52 | 4.00 |
| Aquaporin 2 | Aqp2 | NP_037041.2 | 0.00 | 10.37 | 3.46 | 0.00 | 3.00 |
| Annexin A2 | Anxa2 | NP_063970.1 | 15.51 | 25.85 | 10.34 | 15.51 | 2.50 |
| RAB10, member RAS oncogene family | Rab10 | NP_059055.2 | 8.87 | 8.87 | 4.44 | 0.00 | 2.00 |
| Signal peptidase complex subunit 2 homolog | Spcs2 | XP_001066421.1 | 4.00 | 8.01 | 4.00 | 0.00 | 2.00 |
| Calreticulin | Calr | NP_071794.1 | 6.25 | 4.17 | 2.08 | 4.17 | 2.00 |
| Basal cell adhesion molecule | Bcam | NP_113940.1 | 2.96 | 2.96 | 1.48 | 0.00 | 2.00 |
| Reticulon 4 | Rtn4 | NP_114019.1 | 1.58 | 1.58 | 0.79 | 0.00 | 2.00 |
| Keratin 19 | Krt19 | NP_955792.1 | 0.00 | 15.68 | 8.96 | 4.48 | 1.75 |
| Clathrin, heavy polypeptide (Hc) | Cltc | NP_062172.1 | 0.52 | 5.22 | 3.13 | 19.83 | 1.67 |
| Aldehyde reductase 1 | Akr1b1 | NP_036630.1 | 19.55 | 16.76 | 11.17 | 33.52 | 1.50 |
| Myosin, heavy polypeptide 9 | Myh9 | NP_037326.1 | 5.74 | 3.98 | 2.65 | 3.53 | 1.50 |
| Heat shock protein 90 kDa alpha (cytosolic), class B member 1 | Hsp90ab1 | NP_001004082.3 | 4.80 | 3.60 | 2.40 | 26.42 | 1.50 |
| Actin, beta | Actb | NP_112406.1 | 9.58 | 9.58 | 7.19 | 11.98 | 1.33 |
| Protein disulfide isomerase associated 3 | Pdia3 | NP_059015.1 | 10.60 | 7.07 | 5.30 | 3.53 | 1.33 |
| Myosin, heavy polypeptide 10, nonmuscle | Myh10 | NP_113708.1 | 8.30 | 6.11 | 5.68 | 5.24 | 1.08 |
| Ribosomal protein L13 | Rpl13 | NP_112363.1 | 0.00 | 4.13 | 4.13 | 0.00 | 1.00 |
| RAB11a, member RAS oncogene family | Rab11a | NP_112414.1 | 4.10 | 4.10 | 4.10 | 0.00 | 1.00 |
| Syntaxin 7 | Stx7 | NP_068641.2 | 3.35 | 3.35 | 3.35 | 0.00 | 1.00 |
| Crystallin, alpha B | Cryab | NP_037067.1 | 9.96 | 9.96 | 14.93 | 9.96 | 0.67 |
| Keratin 8 | Krt8 | NP_955402.1 | 7.40 | 5.55 | 11.11 | 5.55 | 0.50 |
| 14-3-3 zeta | Ywhaz | NP_037143.2 | 3.60 | 3.60 | 7.20 | 7.20 | 0.50 |
| Gelsolin | Gsn | NP_001004080.1 | 1.16 | 1.16 | 2.32 | 8.13 | 0.50 |
| Spectrin beta 3 | Spnb3 | NP_062040.1 | 4.80 | 1.48 | 3.69 | 5.17 | 0.40 |
| Adaptor protein complex AP-1, beta 1 subunit | Ap1b1 | NP_058973.1 | 0.00 | 0.00 | 1.91 | 1.91 | 0.00 |
| Adaptor protein complex AP-2, alpha 2 subunit | Ap2a2 | NP_112270.2 | 0.96 | 0.00 | 2.88 | 0.00 | 0.00 |
| Collagen alpha-1(XVIII) chain precursor | Col18a1 | XP_001079576.1 | 0.00 | 0.00 | 0.70 | 0.00 | 0.00 |
| Hypothetical protein LOC365554 | RGD1560646 | XP_001061392.1 | 0.00 | 0.00 | 5.93 | 0.00 | 0.00 |
| 60S ribosomal protein L7 | RGD1564216 | XP_001080627.1 | 0.00 | 0.00 | 2.38 | 0.00 | 0.00 |
| Ribosomal protein L6 | Rpl6 | NP_446423.2 | 0.00 | 0.00 | 5.96 | 0.00 | 0.00 |
| Ribosomal protein S11 | Rps11 | NP_112372.1 | 0.00 | 0.00 | 5.43 | 5.43 | 0.00 |
| Ribosomal protein S13 | Rps13 | NP_569116.1 | 0.00 | 0.00 | 5.81 | 0.00 | 0.00 |
| Ribosomal protein S18 | Rps18 | NP_998722.1 | 0.00 | 0.00 | 5.64 | 0.00 | 0.00 |
| Ribosomal protein S26 | Rps26 | NP_037356.1 | 0.00 | 0.00 | 7.68 | 0.00 | 0.00 |
| Ribosomal protein S3 | Rps3 | NP_001009239.1 | 0.00 | 0.00 | 3.75 | 0.00 | 0.00 |
| Ribosomal protein S4, X-linked | Rps4x | NP_001007601.1 | 0.00 | 0.00 | 3.38 | 0.00 | 0.00 |
| Ribosomal protein S8 | Rps8 | NP_113894.1 | 0.00 | 0.00 | 4.13 | 0.00 | 0.00 |
| Ribosomal protein S9 | Rps9 | NP_112370.2 | 0.00 | 0.00 | 8.85 | 0.00 | 0.00 |
| Valosin-containing protein | Vcp | NP_446316.1 | 1.12 | 0.00 | 3.36 | 1.12 | 0.00 |
Values shown are normalized spectral counts from mass spectrometry. Final column shows ratio of normalized protein spectral count in the 17K fraction to the 200K fraction. The proteins in bold are present in both fractions.
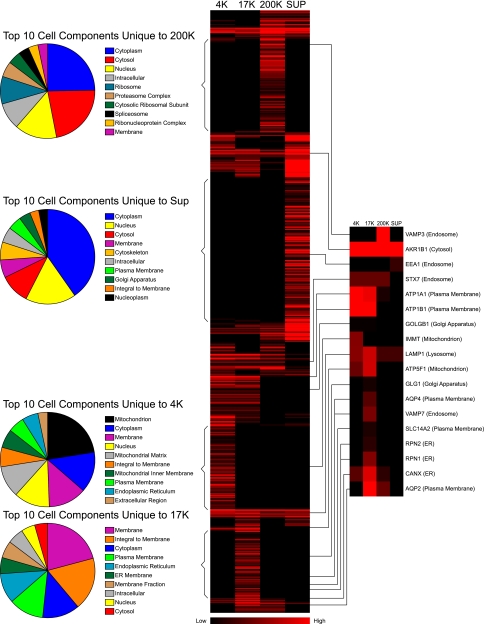
The large number of proteins identified in each fraction allows bioinformatic analysis to identify the structures enriched in each fraction. Figure 3 gives a synopsis. The central portion of this figure shows a heat map summarizing normalized spectral counts for every protein found in each fraction. Red represents high values and black represents low values. The proteins found are clustered according to the normalized spectral counts. Note that some proteins appear to be unique to a given fraction (bracketed). The pie charts on the left summarize the Gene Ontology Cell Component terms for these proteins, listing the 10 most frequent terms in each biochemical fraction. As seen for the 200K fraction, the 10 most frequent cell component terms include several protein complexes, namely “ribosome,” “proteasome complex,” “spliceosome,” and “ribonucleoprotein complex.” Although not explicitly listed in the pie chart, proteins present in other complexes could be culled from the overall 200K list, namely “T-complex protein 1,” “coatomer protein complex,” “actin-related protein 2/3 complex,” and “adaptor protein complexes 1 and 2.” Note that as discussed above, endosomes are not included in this pie chart because they are not unique to the 200K fraction. As seen for the 17K fraction (Fig. 3, bottom), the 10 most frequent cell component terms include “membrane,” “integral to membrane,” “plasma membrane,” and “endoplasmic reticulum.” As seen for the 4K fraction, the 10 most frequent cell component terms include “mitochondrion,” “membrane,” “nucleus,” and “endoplasmic reticulum.” For the supernatant fraction, the 10 most frequent cell component terms include “cytoplasm,” “nucleus,” “cytosol,” and “membrane.”
Fig. 3.
Summary of proteomic data. Middle: heat map of the spectral counts of proteins identified by mass spectrometry. After being normalized by molecular weight, data were clustered and colors were generated using Cluster and TreeView programs (4). Brighter red represents a higher normalized spectral count. Left: pie graphs of the top 10 cell components unique to each fraction. Right: blown up heat maps for selected marker proteins, listed by gene name: vesicle-associated membrane protein-3 (VAMP3), aldehyde reductase-1 (AKR1B1), early endosome antigen-1 (EEA1), syntaxin-7 (STX7), alpha and beta subunits of the Na/K pump (ATP1A1 and ATP1B1), macrogolgin-1 (GOLGB1), mitofilin (IMMT), lysosome-associated membrane protein-1 (LAMP1), ATP synthase (ATP5F1), golgi apparatus protein-1 (GLG1), AQP4, vesicle-associated membrane protein-7 (VAMP7), urea transporter UT-A1 (SLC14A2), ribophorins-II and -I (RPN2 and RPN1), calnexin (CANX), and AQP2. Associated cell component is listed in parenthesis.
Figure 3, right, also shows the distribution of selected marker proteins, culled from the comprehensive heat map in the middle of the figure. Note that the 17K fraction includes not only plasma membrane proteins, but also marker proteins from endosomes, lysosomes, Golgi, endoplasmic reticulum, and mitochondria. Thus, we conclude that reference to the 17K fraction as the “plasma membrane fraction” is inaccurate. Although plasma membrane proteins are clearly enriched in this fraction, a very large part of this fraction is derived from other structures, probably well more than half of the total protein (see discussion).
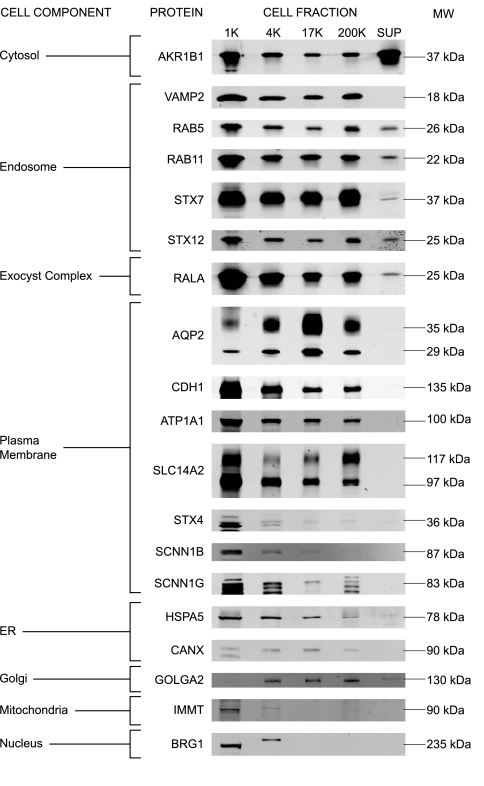
Figure 4 shows immunoblotting for 19 marker proteins in all of the fractions. In general, the immunoblotting confirmed the high heterogeneity of each membrane fraction. Note that the loading of these immunoblots was by equal volume rather than equal protein, and therefore cannot be compared quantitatively with the data in Table 1.
Fig. 4.
Immunoblotting of marker proteins. Immunoblots of marker proteins, listed by gene name and grouped by cell component: AKR1B1, VAMP2, RAS oncogene family member-5a (RAB5), RAS oncogene family member-11a (RAB11), STX7, STX12, v-ral simian leukemia viral oncogene homolog A (RALA), AQP2, E-cadherin (CDH1), Na-K-ATPase (ATP1A1), SLC14A2, STX4, epithelial sodium channel beta and gamma subunits (SCNN1B and SCNN1G), heat shock protein-5 (HSPA5), CANX, cis-Golgi matrix protein-130 (GOLGA2), IMMT, and brahma-related gene protein-1 (BRG1).
DISCUSSION
A major objective of these studies was to expand the known proteome of the native IMCD in rat. Prefractionation by differential centrifugation provides a means of “decreasing the complexity” of samples delivered to the LC-MS/MS system, thereby allowing less abundant proteins to be identified. In this study, we identified 266 proteins in the 4K fraction, 243 proteins in the 17K fraction, 233 proteins in the 200K fraction, and 297 proteins in the 200K supernatant (cytosolic fraction), giving a total of 656 identifications. Note that target-decoy analysis was used to limit the false discovery rate to less than 2%. Among the proteins identified were 189 new proteins, which have been added to the existing IMCD Proteome Database (http://dir.nhlbi.nih.gov/papers/lkem/imcd/). This database now contains 4,685 proteins, more than half of the number of mRNA transcripts detectable in the IMCD in Affymetrix array studies (IMCD Transcriptome Database, http://dir.nhlbi.nih.gov/papers/lkem/imcdtr/) (20).
A second objective of this work was to evaluate the utility of a technique that has been used in many papers to investigate trafficking of the AQP2 water channel (6, 7, 9, 10, 12, 19). Typically, in such studies, the ratio of the AQP2 in the 17K fraction to that in the 200K has been used to assess the distribution of AQP2 between intracellular vesicles (represented by the 200K fraction) and plasma membrane (represented by the 17K fraction). Preliminary studies demonstrated that dDAVP exposure in IMCD causes results in the 17K/200K ratio for AQP2 to increase. As we previously demonstrated (9), this change results chiefly from a fall of AQP2 abundance in the 200K fraction (Fig. 1). The present proteomic study allows us to evaluate the assumptions of this approach from the point of view of the proteins in each fraction. Since many of the identified proteins are considered markers for specific organelles, we can draw conclusions about what organelles were isolated in what fractions.
Based on our proteomic analysis, it appears that the 17K and the 200K membrane fractions are both very heterogeneous. The 17K fraction did contain many proteins that are believed to be present predominantly in the plasma membrane (e.g., MAL2, the UT-A1/3 urea transporter, and integrins). However, this fraction appears to contain proteins characteristic of endoplasmic reticulum, Golgi, lysosomes, mitochondria, and endosomes as well. Among the endosomal markers found in the 17K fraction were Rab25, Rab5b, Rab5c, and syntaxin-7. Calculation of normalized spectral counts for plasma membrane-predominant proteins vs. all proteins in the 17K fraction indicated that plasma membrane proteins account for well less than 50% of the total (see calculation in Supplemental Materials). This is probably one of the reasons why the reduction of AQP2 in the 200K fraction in response to vasopressin was not matched by an increase in the 17K fraction, as an increase of AQP2 in the plasma membrane may be obscured by the presence of proteins from many non-plasma membrane structures. For example, since recycling endosomes (Rab11- and Rab25-containing endosomes), which are thought to represent storage vesicles for AQP2 (14), are present in the 17K fraction, an increase in AQP2 in plasma membrane could be “cancelled out” by a corresponding decrease in the AQP2 in recycling endosomes.
Proteomic analysis of the 200K fraction was similarly informative. A large amount of the total protein in this fraction appears to be representative of various multiprotein complexes including ribosomes, proteasomes, ribonucleoprotein complexes, the coatomer complex, the actin-related protein 2/3 complex, and clathrin adaptor protein complexes 1 and 2 (AP-1 and -2). At the same time, endosomes are clearly represented as indicated by the presence of endosomal marker proteins such as syntaxin-7, vesicle-associated membrane protein 3, and Rab11a. Furthermore, proteins that we expect to be present predominantly in plasma membrane are not abundant in this fraction (<2% of total protein, see Supplemental Materials for calculation). Furthermore, Ser-269-phosphorylated AQP2 was also found in this fraction. Previous studies demonstrated that this phospho form of AQP2 is confined to the apical plasma membrane (8). In addition, significant numbers of marker proteins for endoplasmic reticulum and Golgi apparatus were identified in the 200K fraction. We conclude that the reduction in AQP2 in the 200K fraction seen in Fig. 1 is compatible with endosomal trafficking and fusion with plasma membranes in response to vasopressin. However, it is possible that a change in the amount of AQP2 in endoplasmic reticulum and/or Golgi could contribute to changes seen in the 200K fraction. A reduction in AQP2 in this fraction, therefore, could be seen with an acute reduction in AQP2 transcription.
Overall, based on these results, we recommend a change in the use of differential centrifugation in assessment of AQP2 trafficking. Since plasma membranes make up such a small fraction of the 17K membrane pellet, analysis of this fraction provides no information about the amount of AQP2 in plasma membranes. Therefore, such a measurement lacks utility. However, the analysis of the 200K pellet is of potential value, since much of the AQP2 is associated with endosomes. For AQP2 trafficking, we therefore recommend the analysis of the AQP2 content in the 200K pellet in combination with normalization using the total homogenate.
GRANTS
This work was supported by the intramural budget of the National Heart, Lung, and Blood Institute (Project number Z01-HL-01285-KE).
Supplementary Material
Acknowledgments
We thank G. Ma for maintaining the IMCD Proteome and IMCD Transcriptome Databases and Dr. W. Wu for mass spectrometry analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, Knepper MA. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics 4: 1095–1106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford AD, Terris JM, Ecelbarger CA, Klein JD, Sands JM, Chou CL, Knepper MA. 97- And 117-kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am J Physiol Renal Physiol 281: F133–F143, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Chou CL, DiGiovanni SR, Luther A, Lolait SJ, Knepper MA. Oxytocin as an antidiuretic hormone. II. Role of V2 vasopressin receptor. Am J Physiol Renal Fluid Electrolyte Physiol 269: F78–F85, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA 105: 3134–3139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel M, Sinkins WG, Zuo CD, Hopfer U, Schilling WP. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am J Physiol Renal Physiol 293: F1476–F1488, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Gooch JL, Guler RL, Barnes JL, Toro JJ. Loss of calcineurin Aα results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J Cell Sci 119: 2468–2476, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at ser-269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue T, Terris J, Ecelbarger CA, Chou CL, Nielsen S, Knepper MA. Vasopressin regulates apical targeting of aquaporin-2 but not of UT1 urea transporter in renal collecting duct. Am J Physiol Renal Physiol 276: F559–F566, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Klein JD, Frohlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol 17: 2680–2686, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Mandon B, Chou CL, Nielsen S, Knepper MA. Syntaxin-4 is localized to the apical plasma membrane of rat renal collecting duct cells: possible role in aquaporin-2 trafficking. J Clin Invest 98: 906–913, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marples D, Knepper MA, Christensen EI, Nielsen S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol Cell Physiol 269: C655–C664, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedvetsky PI, Stefan E, Frische S, Santamaria K, Wiesner B, Valenti G, Hammer JA III, Nielsen S, Goldenring JR, Rosenthal W, Klussmann E. A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 8: 110–123, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen S, Marples D, Mohtashami M, Dalby NO, Trimble W, Knepper M. Expression of VAMP2-like protein in kidney collecting duct intracellular vesicles: colocalization with aquaporin-2 water channels. J Clin Invest 96: 1834–1844, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, Nairn AC. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol Renal Physiol 276: F254–F259, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Pisitkun T, Bieniek J, Tchapyjnikov D, Wang G, Wu WW, Shen RF, Knepper MA. High-throughput identification of IMCD proteins using LC-MS/MS. Physiol Genomics 25: 263–276, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamma G, Klussmann E, Procino G, Svelto M, Rosenthal W, Valenti G. cAMP-induced AQP2 translocation is associated with RhoA inhibition through RhoA phosphorylation and interaction with RhoGDI. J Cell Sci 116: 1519–1525, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu MJ, Pisitkun T, Wang G, Aranda JF, Gonzales PA, Tchapyjnikov D, Shen RF, Alonso MA, Knepper MA. Large-scale quantitative LC-MS/MS analysis of detergent-resistant membrane proteins from rat renal collecting duct. Am J Physiol Cell Physiol 295: C661–C678, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.