Abstract
Neurons in the Doppler-shifted constant frequency processing (DSCF) area in the primary auditory cortex of mustached bats, Pteronotus parnellii, are multifunctional, responding both to echolocation and communication sounds. Simultaneous presentation of a DSCF neuron's best low and high frequencies (BFlow and BFhigh, respectively) facilitates its response. BFlow corresponds to a frequency in the frequency-modulated (FM) component of the first harmonic in the echolocation pulse, and BFhigh corresponds to the constant frequency (CF) component in the second harmonic of the echo. We systematically varied the slopes, bandwidths, and central frequencies of FMs traversing the BFhigh region to arrive at the “best FM” for single DSCF neurons. We report that nearly half (46%) of DSCF neurons preferred linear FMs to CFs and average response magnitude to FMs was not significantly less (P = 0.08) than that to CFs at BFhigh when each test stimulus was paired with a CF at BFlow. For linear FMs ranging in slope from 0.04 to 4.0 kHz/ms and in bandwidth from 0.44 to 7.88 kHz, the majority of DSCF neurons preferred upward (55%) to downward (21%) FMs. Central frequencies of the best FMs were typically close to but did not always match a neuron's BFhigh. Neurons exhibited combination-sensitivity to “call fragments” (calls that were band-pass filtered in the BFhigh region) paired with their BFlow. Our data show a close match between the modulation direction of a neuron's best FM and that of its preferred call fragment. These response properties show that DSCF neurons extract multiple parameters of FMs and are specialized for processing both FMs for communication and CFs for echolocation.
INTRODUCTION
Naturally produced sounds are generally complex, containing multiple harmonics and many types of frequency modulations (FMs). Examples of complex sounds include the sounds used for echolocation and communication among conspecifics. Recognition and discrimination between communication sounds or “calls” is important for the survival of the members of a species. How such critical tasks are accomplished by the auditory system within a fraction of a second remains unclear despite a vast amount of literature on auditory processing. Much of our understanding regarding the response profiles of auditory neurons is based on excitatory tuning to constant frequencies (i.e., pure tones or CFs), a class of stimuli that have no spectral dynamics. CFs have been useful for addressing psychoacoustical and neurophysiological questions such as the cochleotopic mapping of the auditory cortex (Imig et al. 1977; Merzenich et al. 1975; Reale and Imig 1980; Woolsey and Walzl 1942) and the study of pitch perception (Fishman et al. 2000; Zatorre et al. 1994). However, since communication sounds usually contain FMs, the exclusive use of CFs as stimuli in the majority of these earlier studies is a weakness from the viewpoint of understanding auditory communication. An increasing number of studies are using complex acoustic stimuli, such as FMs, to study the responses of neurons at various levels of the auditory system, including the cortex (Atencio et al. 2007; Godey et al. 2005; Heil and Scheich 1992; Heil et al. 1992a,b; Liang et al. 2002; Mendelson et al. 1993; Nelken et al. 2003; Nelken and Versnel 2000; Shamma et al. 1993; Zhang et al. 2003). Here we report results of a detailed study of the response properties of neurons within the primary auditory cortex (A1) of the mustached bat, Pteronotus parnellii, using FM stimuli as well as simple syllabic calls and salient components of calls.
Studies of the spectral composition of calls show that the majority of bat (Clement et al. 2006; Kanwal et al. 1994; Ma et al. 2006), bird (Margoliash 1983; Marler and Pickert 1984), frog (Fuzessery and Feng 1983; Mudry and Capranica 1987), monkey (DiMattina and Wang 2006; Hauser 1991), rat (Boinski and Mitchell 1995; Brudzynski 2005), and whale (Payne and McVay 1971) communication sounds contain FMs. Likewise, formant transitions, which simulate FMs, are integral components of consonants in human speech sounds (Liberman et al. 1967). Conspecifics recognize a particular call type despite variations in its pitch and timbre. CFs alone do not capture the acoustic complexity within calls and may therefore be inadequate to predict neural responses to calls. Accordingly, earlier studies of excitatory frequency tuning of cortical neurons in squirrel monkeys failed to predict whether a neuron would or would not respond to conspecific calls (Newman and Wollberg 1973; Winter and Funkenstein 1973). One aspect of the acoustic structure of calls that may play an important role in determining their identity is the pattern of FMs that are predominant components of many social calls. In contrast to CFs and noise bursts (NBs) that are spectrally static over time, FMs are spectrally dynamic changes in the structure of a sound. They can be acoustically specified by their slope and bandwidth. For a given slope and bandwidth, FMs may be further characterized by their location on the frequency axis (i.e., an FM's frequency range). For FMs of the same bandwidth, an FM's frequency range is determined by the value of its central frequency.
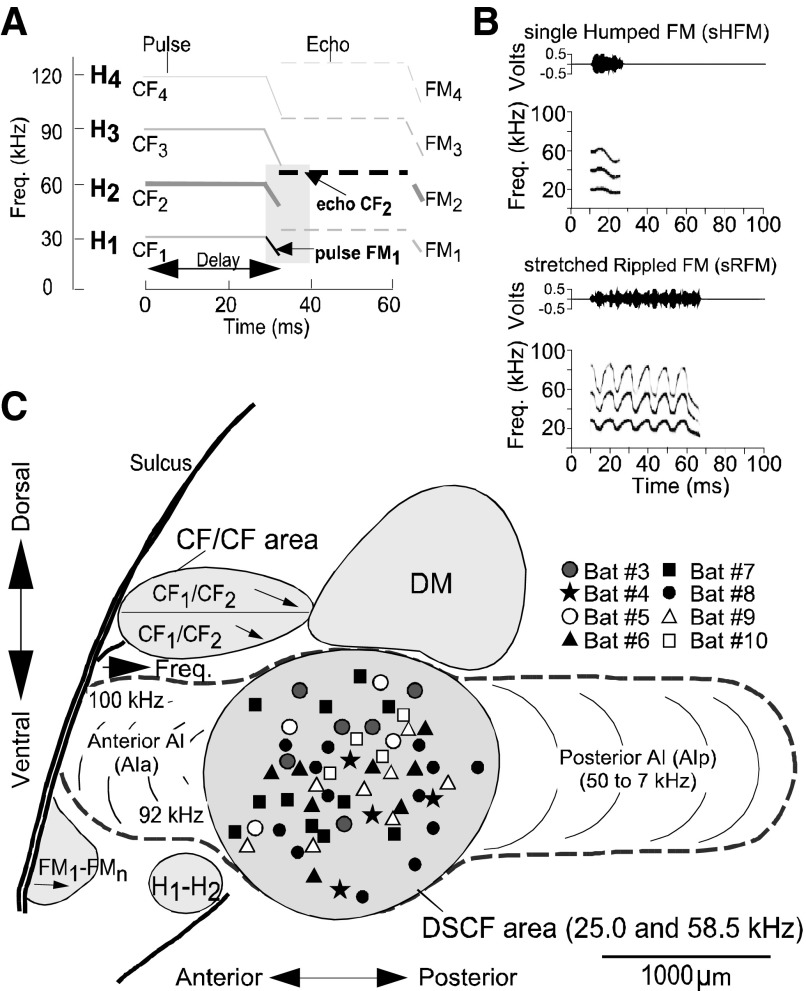
The auditory cortex of mustached bats has been studied in great detail to describe its functional organization and to elucidate the computational mechanisms involved in echolocation (Suga 1978). These early studies showed that the Doppler-shifted constant frequency processing (DSCF) area within the A1 of mustached bats contains an expanded representation of a narrow range of frequencies, which corresponds to the Doppler-shifted echo of the second harmonic of the CF component (CF2) in the echolocation signal (Fig. 1). The best excitatory frequency of a DSCF neuron corresponding to the echo CF2 (between 57 and 60 kHz) component is labeled as the BFhigh for that neuron (Kanwal et al. 1999; Xiao and Suga 2002). A pulse FM1 paired with the BFhigh triggers a robust response in neurons within the DSCF area. This facilitated response is larger than the sum of the responses to each stimulus presented alone. In other words a single FM1 frequency (between 23 and 27 kHz) or CF, termed the BFlow, paired with the BFhigh elicits a nonlinearly enhanced response from single DSCF neurons (Kanwal et al. 1999).
FIG. 1.
A: schematized spectrogram of the mustached bat's echolocation signal. H1-4 refer to harmonics 1-4 of the echolocation pulse or echo. Note the constant frequency (CF) and frequency-modulated (FM) components present in the pulse and echo. Shaded region shows the signal components (indicated by arrows) that trigger a facilitative response in the Doppler-shifted constant frequency processing (DSCF) area. B: amplitude envelopes (top) and spectrograms (bottom) for 2 examples of social calls emitted by mustached bats that contain different patterns within them. C: lateral view of the mustached bat brain showing the location of the DSCF area (shown in gray) as defined based on its role in computing biosonar signals. Anatomical landmarks (blood vessels shown by thick lines) and tuning properties of neuronal responses were used to identify the DSCF area in each animal. Other areas, such as the dorsomedial (DM) area, CF/CF area, a ventral FM-FM area, and H1-H2 areas, are also shown (adapted from Suga 1983). Symbols in the DSCF area represent 55 penetrations from the left and right hemispheres of 8 animals.
Our goal in this study was to test whether FM sounds sweeping through the BFhigh of a DSCF neuron can also trigger robust and facilitated responses when paired with the BFlow. If so, we were curious as to how these responses compared with responses elicited by simultaneous presentations of the BFhigh and BFlow. We also wanted to know the shape of the response areas obtained to these FMs traversing the BFhigh, and the distribution of best FMs in an unbiased sample of DSCF neurons. Finally, we wanted to know whether the FM response characteristics of a DSCF neuron are consistent with its call selectivity, including its response to a whole call and/or appropriate spectro-temporally filtered “call fragments.” We performed electrophysiological recordings from single neurons in the DSCF area of awake mustached bats and examined the characteristics of their responses to FMs. We determined the best FM (i.e., the FM that elicited the maximum response) for each DSCF neuron by systematically varying several FM parameters—namely slope, bandwidth, central frequency, modulation direction—while also pairing each manipulated FM with the neuron's BFlow to elicit a facilitated response.
METHODS
Animal acquisition and maintenance and data recording procedures were similar to those previously described (Kanwal et al. 1999; Medvedev and Kanwal 2004) and are only briefly described below. Stimulation presentation and data analysis procedures unique to these experiments are described in greater detail.
Surgery and recording of neural activity
Wild-caught Trinidadian mustached bats (Pteronotus parnellii rubiginosus) were transported to and housed in the Research Resource Facility at Georgetown University. Under isoflurane/air mixture (medical grade, Anaquest) anesthesia, a skin incision was made along the midline of the head, and a 2-mm-diam metal post was glued (cyanoacrylic, Loctite 411) immediately caudal to the intersection of the sagittal and coronal sutures. Bats were allowed to recover for >3 days before the first recording session.
Each bat was restrained during electrophysiological recordings by clamping the metal post, and the body was suspended in a Styrofoam mold by elastic bands in a heated (31°C), sound-proof, and echo-attenuated chamber (IAC 400A). A video camera was used to monitor the bat during recording procedures. The electrophysiological activity of single neurons was recorded using custom-made, sharpened, vinyl-coated tungsten-microelectrodes with tip diameters of ∼10 μm and impedances of >1 MΩ. Electrodes were inserted into the cortex perpendicular to the skull to a depth of 300–650 μm through a 50- to 200-μm hole. A second tungsten microelectrode (<1 MΩ impedance) was inserted into a second 50- to 200-μm hole made through the dura mater above a nonauditory region of cortex, and this second electrode acted as a reference for differential recordings. Electrical signals acquired by the recording electrode were amplified by an AC preamplifier and band-pass filtered between 300 and 3,000 Hz. All methods and procedures used in this study were approved by the Georgetown University Animal Care and Use Committee.
Experimental design and data acquisition
To test neural responses, three types of stimuli were used: CFs, linear FMs, and conspecific, simple syllabic social calls. CF stimuli were tone-bursts that we generated using custom-made analog function generators, whereas we digitally synthesized the FMs and calls. Frequency-shifted variants of prerecorded social calls (Clement et al. 2006) were presented using SIGNAL software (SIGNAL 3.0, Engineering Design) running on a personal computer (Pentium, Intel) with an A/D-D/A board (DT2821G). All manipulations of social calls and FMs (including filtrations and deletions) were performed using SIGNAL software. All CFs were 30 ms in duration. FMs ranged in duration between 0.5 and 200 ms. The duration of social calls ranged from 4 to 89 ms. CFs were presented from two condenser speakers that were flat (±6 dB SPL) between 20 and 120 kHz. FMs and social calls were delivered via a leaf-tweeter speaker that was flat (±3 dB SPL) between 5 and 100 kHz. All stimuli had a 10-ms onset delay relative to the acquisition of data on each trial. CF and FM stimuli were presented at a rate of 4/s. Social calls and related stimuli, such as filtered “call fragments,” were presented at a rate of 2/s. The initial and terminal 0.5 ms of CF and FM stimuli were tapered in amplitude unless an FM's duration was <2 ms. When an FM's duration was <2 ms, it was not tapered in amplitude.
Subsequent examples of experimental stimuli are presented here as amplitude envelopes (amplitude in V vs. time in ms) and spectrograms (frequency in kHz vs. time in ms). Amplitude envelopes are in volts because this is the default unit in SIGNAL 3.0. We represent the amplitude envelopes of FM and of CFs at BFhigh by solid filled rectangles and those of BFlow by an unfilled, outlined rectangle. The same convention is used for spectrograms. Where the two sounds overlap, the amplitude envelope is filled solid, but each component is visible in the spectrogram.
Call selectivity and responsiveness were determined using methods previously described (Kanwal et al. 1994; Ohlemiller et al. 1994). Briefly, bats were presented seven pitch-shifted variants (mean plus 3 up-shifted and 3 down-shifted variants) of each of the 14 call types. During these presentations, each call variant was attenuated from 46 to 91 dB SPL in steps of 15 dB SPL. These initial presentations were aimed at determining a set of best call variants for each of the 14 types. The best call variants of each of the 14 call types were attenuated in steps of 10 dB SPL and ranged in amplitude from −9 to 91 dB SPL.
Single DSCF neurons were isolated by identifying neuronal activity that displayed similar spike-waveform characteristics when plotted across multiple dimensions (e.g., a spike's peak height vs. its peak time; its peak amplitude vs. its overall width, etc.). Clusters of waveforms were indicative of multiple, single neuron responses to search stimuli relative to spontaneous activity. Pairs of CFs in the range of the FM1 and CF2 were presented to elicit excitatory responses from DSCF neurons. The frequencies were systematically increased and decreased at successively decreasing amplitude levels to obtain the facilitatory response areas of single DSCF neurons. This procedure also provided the values for the BFlow and BFhigh for each neuron. The lowest threshold was determined by decreasing the amplitude to levels at which the response was barely above the spontaneous level of activity. This was determined by observing the response on an on-line dot raster plot of the responses. Final determination of the value of BFhigh and its best amplitude (BA) for facilitation was made with the simultaneous presentation of BFlow at 10 dB SPL above threshold. CF pairs (BFlow+ BFhigh) were presented with each CF at its BF and BA. All data were acquired using SciWorks 3.0 software (Data Wave).
Responses to the BFlow, BFhigh, and their combinations were recorded in the form of peristimlulus time histograms (PSTHs). These histograms, calculated on-line by summation of spike trains over repeated trials, were used to measure the neuronal response that represents a stimulus-locked change in the peak response magnitude. This peak in the PSTH obtained in response to the reference stimulus was used to monitor the stability of the preparation when studying the response to various FMs. Peak response magnitude was used as the primary measure of a neuron's stimulus preference because DSCF neurons in general have short, stimulus-locked onset responses that are not likely to encode information via response duration. Responses to FMs presented within an array were calculated using a bin width of 10 ms compared with the individually presented stimuli. For the latter, peak responses were calculated using bin widths of 5 ms for comparisons at a higher resolution.
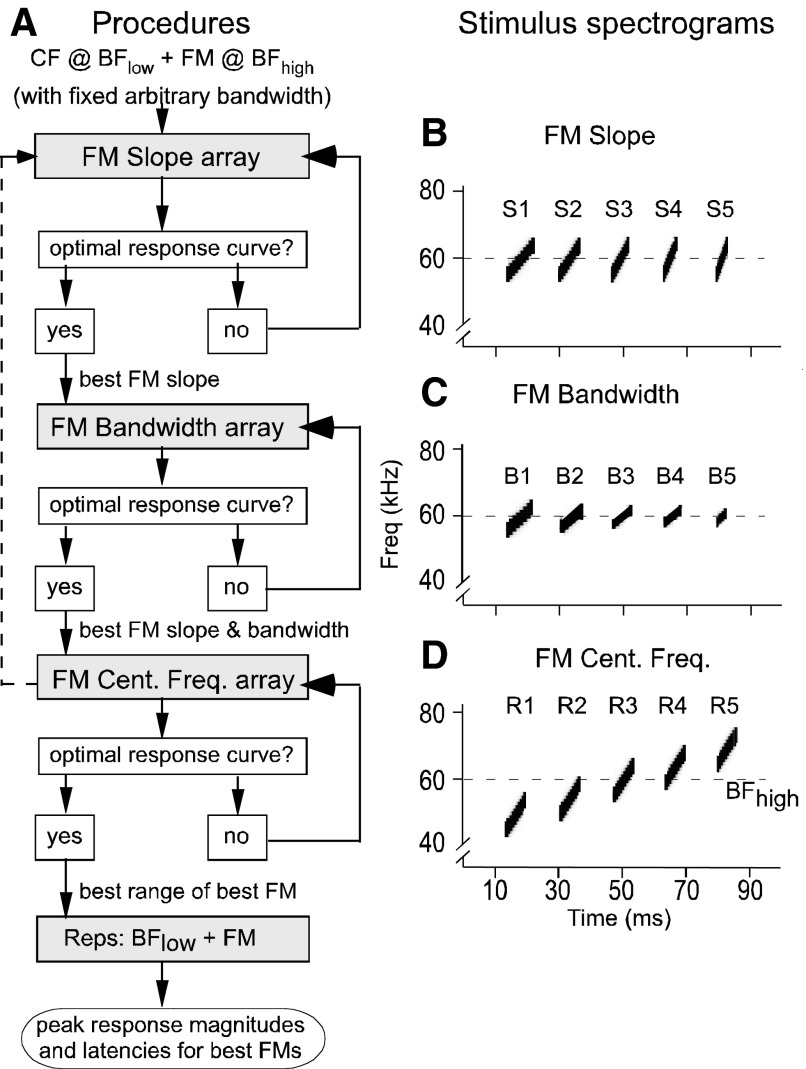
FM stimuli were linear modulations of frequency in the BFhigh range (∼57–60 kHz). The procedure for studying FM response characteristics is shown in the form of a flow chart in Fig. 2. Essentially, three parameters, namely FM slope (or rate of frequency change, in kHz/ms), FM bandwidth (or the difference between the highest and lowest frequencies included in the FM, in kHz), and FM central frequency (defining the range on the frequency axis, in kHz), can fully define a linear FM in either the upward or downward direction. Our procedure involved fixing two FM parameters and systematically varying the third. Initially, the BFhigh of the neuron under study served as the central frequency for each FM. Each sequence of FM stimuli (or FM array) was presented 100 times and decreased in amplitude by 10 dB SPL every 10 repetitions. Experimenter input into a customized SIGNAL script for FM generation was used to generate the eighth (or middle) FM in the array (Washington and Kanwal 2004). Based on this input, the preceding six FMs had successively lower slope, bandwidth, or central frequency values and the succeeding six FMs in the array had higher values of each parameter. This array of 14 stimuli were preceded by a no-stimulus control during each trial.
FIG. 2.
A: flow chart depicting the experimental design to determine the parameters for the best FM stimulus at each recording location. Each FM array was composed of 15 stimuli: a no-stimulus control, 13 or 14 FMs, and (when the array had only 13 FMs) a CF in the BFhigh range. Each FM array was repeated 100 times. Per every 10 repetitions, the stimulus level of each FM was attenuated by 10 dB SPL over a total range of −9 to 91 dB SPL. FM array presentations were repeated until we obtained optimal response curves that were characterized by stimulus-locked responses to FMs that were discernable from noise. B: spectrograms of 5 upward FMs (S1–S5) with equal bandwidths (10 kHz) and center frequencies (60 kHz) but different durations and thus different slopes. Actual FM slope array contained 14 FMs. C: spectrograms of 5 upward FMs (B1–B5) with equal slopes (1 kHz/ms) and center frequencies (60 kHz) but different durations and thus different bandwidths. The actual FM bandwidth array contained 13 FMs. D: spectrograms of 5 upward FMs (R1–R5) with equal slopes (2 kHz/ms), bandwidths (10 kHz), and durations (5 ms), but different center frequencies and thus different locations on the frequency axis. The actual FM central frequency array contained 13 FMs. All spectrograms were generated by the same SIGNAL script that generated the FM arrays used in this study.
The FMs in each array were manipulated in the following ways. First, for the FM slope array, we varied the slopes of 14 linear FMs between 0.04 and 4.0 kHz/ms at a constant bandwidth of 3.5 kHz that matched the mean width (varying from 0.2 to 5.4 kHz) of facilitatory tuning to the BFhigh at BA (see Kanwal et al. 1999). In later experiments, we expanded this bandwidth to 5.25 kHz. We determined the best FM slope for a given neuron by observing the peak response magnitudes in response to FMs in the FM slope array, and this slope was used as the slope for all FMs when generating an array of FM bandwidths. For the FM bandwidth array, we incremented the middle bandwidth in equal steps to generate six successive FMs above the middle bandwidth (3.5 or 5.25 kHz), and likewise we decremented the middle bandwidth to generate six successive FMs below the middle bandwidth. This procedure thus generated 13 FMs in the FM-bandwidth array and established the best FM slope and bandwidth for the given neuron. The bandwidths in the FM bandwidth array spanned a range of 0.66–7.88 kHz when the mid-value was 5.25 kHz and 0.44–5.25 kHz when the mid-value was 3.5 kHz. Finally, we incremented increased the central frequencies of six successive FMs above the BFhigh and decremented the central frequencies of another six successive FMs below the BFhigh by steps equal to one half of the “best bandwidth.”
The last stimulus in the FM bandwidth and central frequency arrays consisted of a 30-ms CF at the neuron's BFhigh. An exception was made for the FM slope array such that the last stimulus was an FM with a slope of 4.0 kHz/ms. All stimuli within the arrays (the control, the FMs, and the BFhigh) were paired at onset with a 30-ms CF presented at the neuron's BFlow. This method ensured both facilitation of FM responses and provided a direct comparison between the FM responses, the BFhigh in the presence of BFlow, and the BFlow presented alone.
Peak response magnitudes from PSTHs (bin width = 10 ms) were used to determine which FM in an array was the “best FM.” Each stimulus within the FM array was presented 10 times at each amplitude level that was successively attenuated in steps of 10 dB. Since we summed the responses of each stimulus across multiple amplitude levels (ranging from −9 to 91 dB SPL), we refer to the plot of the peak response magnitudes to successive FMs in the array as a “response curve” for the FM array. The response curve's peak corresponded to the best FM in an array. We plotted an FM response curve for each FM parameter tested and obtained values for the slope, bandwidth, and central frequency parameters of the prospective best FM for the neuron under study. Starting with this best FM, a second iteration of this series of tests for each parameter was sometimes performed to confirm the final parameters of the best FM.
Data generated by presenting FM arrays also provided the best amplitude (BA) of facilitation of a neuron's best FM in either the upward or downward modulation direction. Time permitting, the procedure for determining the optimal FM in one modulation direction was repeated for FMs in the opposite direction. More commonly, the best FM of a neuron was simply time-reversed to change its modulation direction and used to obtain a neuron's response to the reversed best FM. The reversed FM was otherwise identical to the best FM in terms of slope, bandwidth, and center frequency. These data were used to calculate a neuron's direction selectivity index (DSI) as described below.
The best CF, FM, and call stimuli were usually individually presented 200 times either at BA or at 10 dB SPL > threshold, and most of our calculations that related to neural responses were based on responses to this number of repetitions (bin width = 5 ms). For determining latency, a 1-ms bin width was used to improve temporal resolution. This individual presentation procedure ensured that 1) the effects of latency shifts caused by stimulus amplitude included in the response estimation from the FM array presentations were controlled for and 2) any potential effects of stimulus order in the sequence of FMs in the array were nullified. Repetition of CF and FM stimuli within or outside of the context of stimulus arrays did not result in any observable habituation or reduction of peak response magnitude. Previous studies have not shown habituation or other order effects in DSCF or other A1 neurons when bats were sequentially presented a series of CFs (Kanwal et al. 1999) or calls (Kanwal 2006; Medvedev and Kanwal 2004).
Data analysis
Peak response latencies were manually extracted from each PSTH by measuring the delay between the onset of the stimulus and the 1-ms bin containing the neuron's peak response to that stimulus. Acoustic transmission delays were <1 ms and was not subtracted from the reported latencies. If more than one bin had the same response magnitude (as is the case with a biphasic response in a PSTH), the first of these bins was used for the latency calculation. Peak response latencies were measured only from PSTHs elicited by responses to 200 repetitions of a stimulus at BA or at 10 dB SPL > threshold. Unless otherwise stated, all peak response latencies reported here are at onset responses. For FMs shorter than the average peak response latencies to a CF, a distinction between onset and offset responses could not be made.
We generated population response curves for each FM parameter by first normalizing (to their own peak values) the individual response curves for each neuron and then averaging together only normalized curves that were elicited by the same FM array types (i.e., slope, bandwidth, or frequency range arrays). The values comprising each of the resultant mean curves were mean peak response magnitudes for each stimulus contained in the FM arrays. Normalization ensured that, when averaged, both strongly and weakly responsive neurons equally contributed to the resultant mean curves. In addition to generating mean response curves, we performed repeated-measures ANOVAs using Greenhouse-Geisser correction on the collection of individual FM slope, bandwidth, and central frequency response curves. Repeated-measures ANOVAs allowed us to determine whether changing the stimulus parameters of slope, bandwidth, or central frequency of either an upward FM (UFM) or downward FM (DFM) caused significant changes (P < 0.05) in the peak response magnitudes of DSCF neurons.
We defined a neuron's direction selectivity for FMs using the DSI. DSI = (RUFM –RDFM)/(RUFM + RDFM), where RUFM is a neuron's peak response magnitude to a UFM presented at its BA and RDFM is the same neuron's peak response magnitude to a DFM presented at its respective BA (Mendelson et al. 1993). Our criterion for declaring directional preference was a ||DSI|| > 0.14. Values of DSI that are <−0.14 indicate a preference for DFM and those that are >0.14 indicate a preference for UFM and those in between −0.14 and 0.14 indicate no directional preference. This translated into a 25% greater response magnitude in one FM direction (UFM or DFM) than in the other.
RESULTS
Representation of CF versus FM sweeps
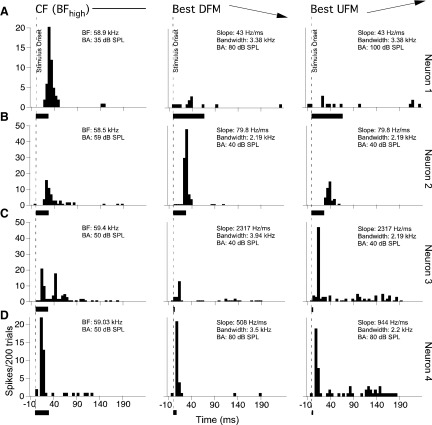
Extracellular single unit recordings were obtained from 227 DSCF neurons in 10 bats (6 males and 4 females). Peak response magnitudes were measured from PSTHs obtained in response to 200 presentations of either the BFhigh or the best FM stimulus paired with the neuron's BFlow. Figure 3 shows the responses of four typical DSCF neurons to FMs in the form of PSTHs. Some neurons responded best to CFs (Fig. 3A), other neurons responded best to either a DFM or a UFM stimulus (Fig. 3, B and C), and many neurons responded almost equally well to both CF and FM stimuli (Fig. 3D). Peak response magnitudes were unaffected by stimulus duration so that FM stimuli that were <30 ms elicited peaks that were similar to those elicited by FMs and CFs that were ≥30 ms (Fig. 3, C and D). DSCF neurons responded in a stimulus-locked manner with the response being mostly triggered by stimulus onset, although some offset responses for CF and/or FM stimuli were observed. The predominant response lasted for <30 ms, although tonic responses lasting for either the duration of the stimulus and/or up to 100 ms were also sometimes observed.
FIG. 3.
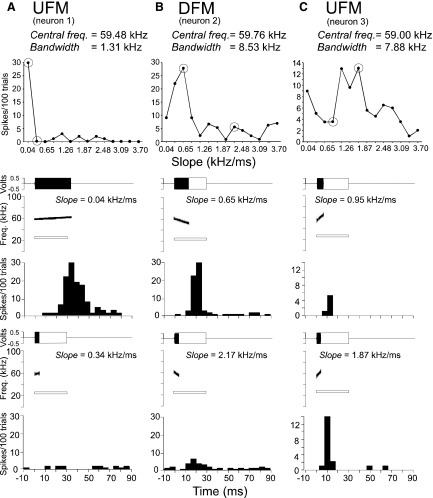
Peristimulus time histograms (PSTHs) depicting 3 different categories of DSCF neurons. All CF and FM stimuli described here were centered on each neuron's BFhigh and paired with its BFlow. Each row (A–D) represents the responses of a different neuron. From left to right, the PSTHs in each column represent single neuron responses to 200 repetitions of their BFhigh, best downward FM (DFM), and best upward FM (UFM). A: responses of a neuron that was selective for its BFhigh and did not respond to FMs. B: responses of a DFM selective neuron. C: responses of a UFM selective neuron. D: responses of a bi-directional neuron that responded with near equal magnitude to both FM and CF stimuli. Each FM stimulus was presented at its best amplitude (BA) of facilitation and at its best slope and bandwidth. Stimulus parameters are listed in the top right of each PSTH graph. Dashed lines represent stimulus onset (10 ms), and the black bars at the bottom of each graph represent stimulus duration. The horizontal axis is time in milliseconds, and the vertical axis is the number of spikes per 200 trials in a 5-ms-wide bin.
The peak response magnitude of 12% (28/227) of neurons was roughly equal for both CF and FM stimuli (±10% of the best CF normalized response). Forty-six percent (105/227) of neurons responded better to FMs than to CF stimuli and 41% (94/227) responded better to CFs compared with the best FM stimulus. Thus a slight majority of neurons in the DSCF area preferred FMs to CF stimuli. Figure 4A is a scatterplot of the peak response to UFMs and to DFMs plotted against each neuron's BFhigh. A log scale is used for each axis to minimize the disparity caused by the large differences in the peak firing rate among DSCF neurons. As seen in the plot, each BFhigh corresponds to several different response values for the best FM or vice versa. Also, each data point sitting on the horizontal axis represents a neuron that was highly selective for CFs and each data point sitting on the vertical axis represent a neuron that was highly selective for FMs. Approximately 25% of the neurons responded to UFMs with a peak that was within 25% of the peak response to DFMs. A similar number responded only slightly better to DFMs. These neurons were labeled as “bidirectional” and are plotted as gray circles in the scatter plot in Fig. 4A. Other neurons showed a clear preference for either a DFM or a UFM with ≤10-fold greater response magnitudes. The mean peak response to FM stimuli, however, was not significantly different (P = 0.08; paired t-test) from that to CFs (Fig. 4B).
FIG. 4.
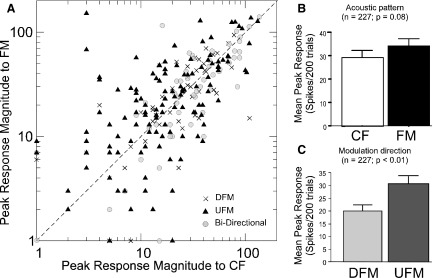
A: scatterplot for peak response magnitudes (spikes/200 trials in 5-ms bin) of 227 DSCF neurons to DFM (crosses) and UFM (black triangles) stimuli plotted against of their peak responses to CFs. Bidirectional responses are plotted as gray circles. Bidirectionality was estimated by computing a direction selectivity index (DSI) for each response. Axes use a logarithmic scale to more easily display the distribution of rasters. B: bar plot showing a pairwise comparison of the peak response magnitudes elicited by each neuron's best FM, regardless of direction, and its peak response magnitude to its best CF response (paired t-test; P = 0.08). C: bar graph showing the peak response magnitudes elicited by UFM vs. DFM stimuli. The majority of neurons responded significantly more to UFM than to DFMs (paired t-test, P < 0.01).
Directional preference
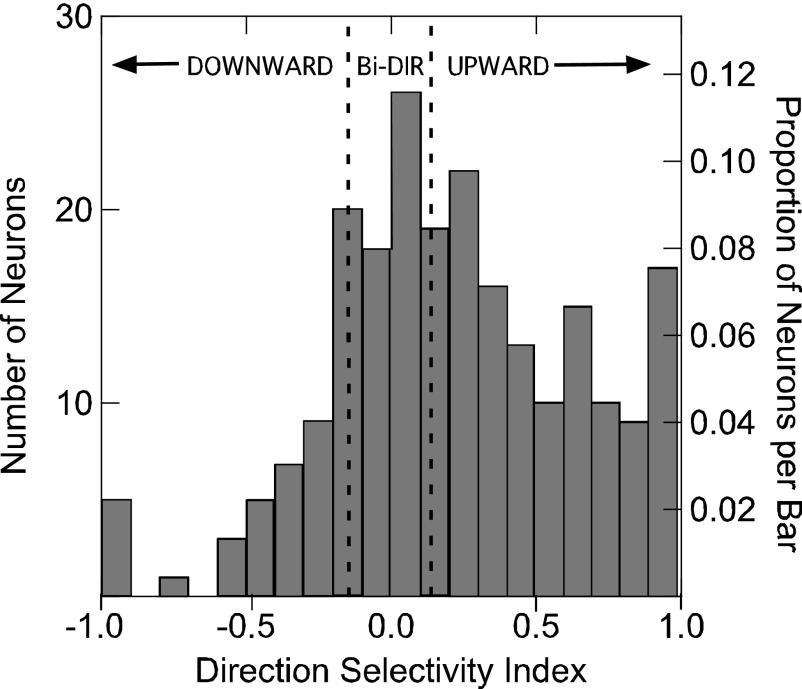
Over the total population of DSCF neurons, 75% (171/227) exhibited a preference for either the upward or downward direction of FM. Fifty-five percent (124/227) preferred UFMs, 21% (47/227) preferred DFMs, and 25% (56/227) were bidirectional. Of the 105 neurons that preferred FMs over CFs, 63% (66/105) preferred UFMs, 23% (24/105) preferred DFMs, and 14% (15/105) were bidirectional. The mean peak response of all neurons to UFMs was also significantly greater (paired t-test, P < 0.01) than for DFMs (Fig. 4C). The distribution of DSI values for all neurons is shown as a density plot in Fig. 5. Forty-seven percent of the DSCF neurons sampled (107/227) responded with a 50% greater peak response magnitude to FMs in their preferred direction than to FMs in the nonpreferred direction. It should be noted that directional preference was observed even when amplitude tapers were excluded from FMs that were <2 ms in duration (Fig. S1).1
FIG. 5.
Density histogram of DSI values of 227 DSCF neurons. The leftmost vertical axis shows the number of neurons represented by each bar and the rightmost vertical axis shows the percentage of the population represented by each bar. DSI values ranging from 1 to −1 are shown on the horizontal axis.
Distributions of FM slope, bandwidth, and central frequency
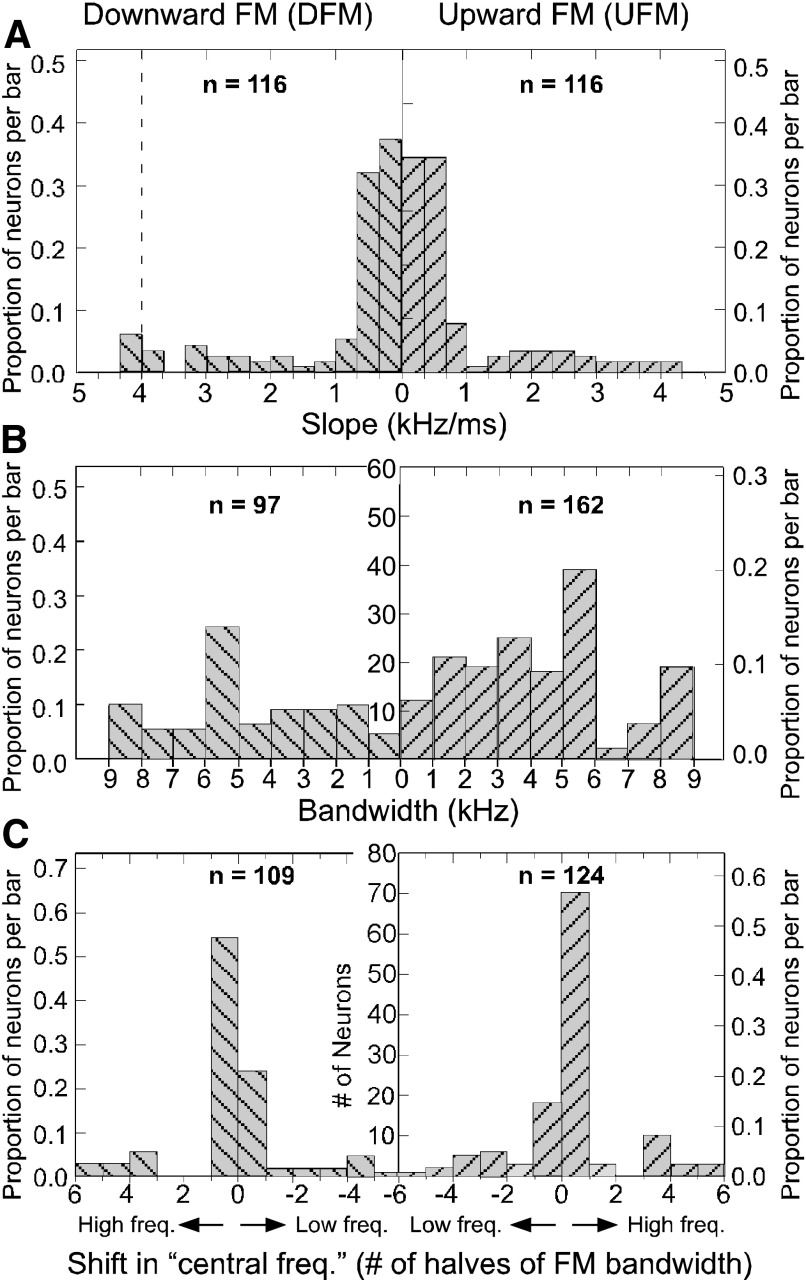
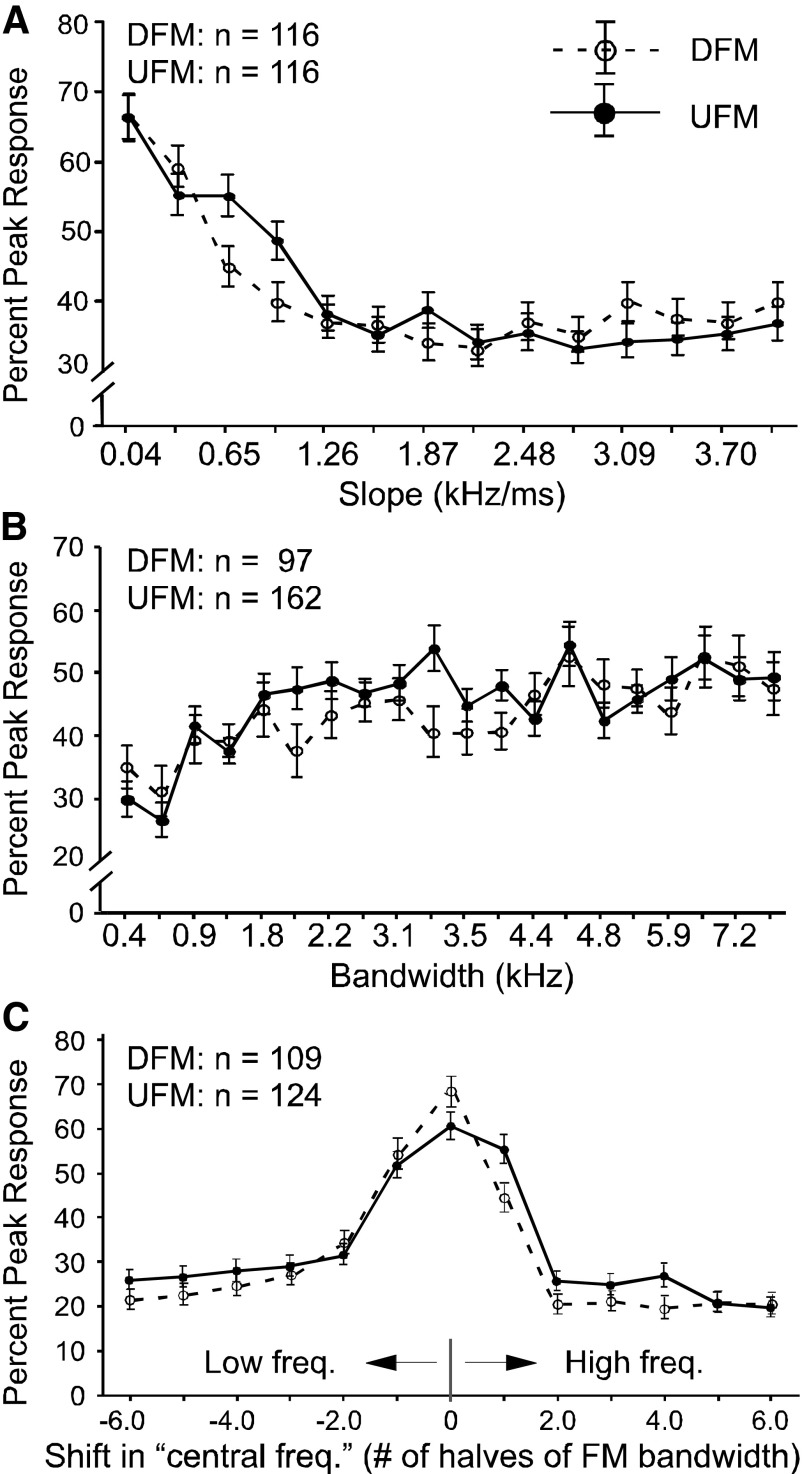
The distribution of the best FM slopes across all neurons tested is shown as a density histogram in Fig. 6A. Across all slopes tested for the full sample of DSCF neurons, best FM slopes were within the range of 0.04 to 4.0 kHz/ms. For both UFMs and DFMs, DSCF neurons showed a general preference for the lower end of the 0.04 to 4.0-kHz/ms slope range. For both UFMs and DFMs, a slope of 0.34 kHz/ms was nearly as likely to be the best slope of a neuron as 0.04 kHz/ms. Only a small proportion of neurons exhibited best slopes matching the slope of FM2 component of the echolocation pulse (indicated by the vertical dashed line). Across all bandwidths tested, the mean best bandwidth was 5.35 ± 2.49 (SD) kHz for UFMs and 5.60 ±2.36 kHz for DFMs (Fig. 6B). These preferred FM bandwidths are in contrast to the 1.35 ± 0.18 kHz excitatory frequency tuning (or the excitatory tuning to the BFhigh alone) and the 1.71 ± 0.25 kHz facilitatory frequency tuning (or the excitatory tuning to the BFhigh when paired with BFlow) width of DSCF neurons (Kanwal et al. 1999). The preferred frequency range for the FM with the best slope and bandwidth is designated by its central frequency. Because the best bandwidth was not the same for all neurons, the frequency range (horizontal axis) of this plot is best plotted as the number of steps (halves of best FM bandwidth) by which the central frequency of an FM was offset from a neuron's BFhigh (Fig. 6C). For both DFMs and UFMs, the distribution of FM slopes in the DSCF area was skewed toward slopes between 0.04 and 1 kHz/ms. The distribution of best FM bandwidths was relatively flat with a mode between 5 and 6 kHz, and the distribution of the best FM central frequency was the closest to a normal distribution.
FIG. 6.
A: density histograms of the best UFM (bars upward hatching) and DFM (bars with downward hatching) slopes of the 116 DSCF neurons. The leftmost vertical axis shows the percentage of the population represented by each bar for DFMs and the rightmost vertical axis shows this same information for UFMs. The central vertical axis, which shows the actual number of neurons per bar (labeled in B) applies to both UFMs and DFM plots. The horizontal axis is an integer scale of FM slopes (kHz/ms). The left side of the horizontal integer scale, which measures the slopes of DFMs, is the reverse of the right side, which measures the slopes of UFMs. A dashed line in the DFM plot shows where the echolocation FM2 would be located on the slope scale. B: density histogram of the best UFM (n = 162) and DFM (n = 97) bandwidths of DSCF neurons. The horizontal axis represents the range of the best DFM and UFM and bandwidths. C: density histogram of the best UFM (n = 124) and DFM (n = 109) center frequencies for DSCF neurons. The horizontal axis represents the number of halves of the “best bandwidth” by which the central frequency was shifted. Zero on the x-axis corresponds to each neuron's BFhigh.
Response curves for FM parameters
FM SLOPE.
The top panel in Fig. 7 shows three DSCF neurons with different patterns of response curves when presented with an array of systematically increasing slopes. Responses to FM slopes across all stimulus amplitudes are summed for each slope (Fig. 7), as they are for the FM bandwidth (Fig. 8), and FM central frequency examples (Fig. 9) as well. Peaks in the response curves in Fig. 7 show tuning to FM slopes. The FM slopes that elicited the responses shown in the bottom row of PSTHs in Fig. 7, A–C, are greater than the FM slopes that elicited responses in the PSTHs on the top row. The FMs that elicited the responses from the neurons in the top row differed from those in the bottom rowonly by slope and duration.
FIG. 7.
Top: line plots to show the response curves of 3 DSCF neurons (A, B, C) to the slope of an FM. Neurons show tuning (for peak responses) as FM slope increases. Bottom: amplitude envelopes (top), spectrograms (middle), and PSTH (bottom) obtained for 2 data points (encircled) on the response curve. The FM is shown by a solid, filled rectangle and the spectrogram as generated by SIGNAL software. The amplitude envelope of the CF at BFlow is shown by an unfilled, outlined rectangle and the spectrogram shown by an outlined white bar. Response curves in each column represent a single neuron's response to FMs that vary in slope between 0.04 and 4.0 kHz/ms. PSTHs in each column represent a single neuron's responses to the FMs depicted in the amplitude envelopes and spectrograms. FMs in each column are shallower in the upper panel (1st data point) than those in the lower panel (2nd data point). FM direction and stimulus parameters are indicated at the top of each panel. PSTHs (bin width = 5 ms) are based on 100 repetitions of the stimulus.
FIG. 8.
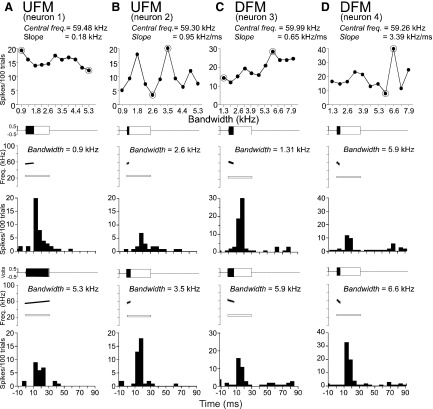
Top panel: line plots to show the response curves of 4 DSCF neurons (A–D) to shifts in the bandwidth of uFMs (A, B) and DFMs (B, D). Neurons show either a gradual decline, or an overall gain, or sharp tuning (for peak responses) to increases in FM bandwidth. Bottom panels: amplitude envelopes (top), spectrograms (middle), and PSTH (bottom) obtained in 2 data points (encircled) on the response curve. The amplitude envelope of the FM is shown as a solid, filled rectangle and the spectrogram is plotted using SIGNAL software. The amplitude envelope of the CF at BFlow is shown as an unfilled, outlined rectangle and the spectrogram is added as a white outlined bar. Response curves in each column represent 1 neuron's response to FMs that increase in bandwidth either from 0.9 to 5.3 or from 1.3 to 7.9 kHz. PSTHs in each column represent a single neuron's responses to the FMs depicted in the amplitude envelopes and spectrograms. FMs in each column have a relatively narrow bandwidth in the top panel (1st data point) and a broader bandwidth in the bottom panel (2nd data point). FM direction and stimulus parameters are indicated at the top of each column. PSTHs (bin width = 5 ms) are based on 100 repetitions of the stimulus.
FIG. 9.
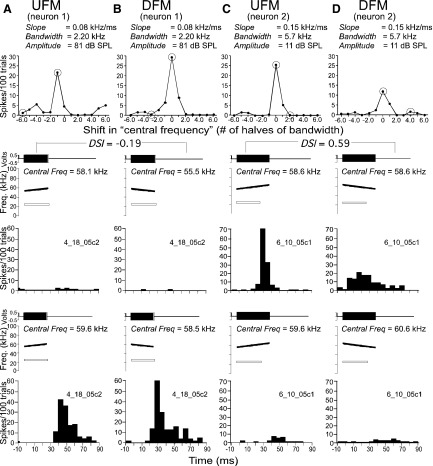
Top: line plots to show the response curves of 2 DSCF for neurons to a stepwise shift of the central frequency of a UFM (A, C) and a DFM (B, D) from each neuron's BFhigh. Neurons show tuning (for peak responses) to higher frequencies from left to right. Bottom: amplitude envelopes (top), spectrograms (middle), and PSTH (bottom) related to 2 data points (encircled) on the response curve. The amplitude envelope of the FM is shown as a solid, filled rectangle and the spectrogram is plotted using SIGNAL software. CF at BFlow is shown as an unfilled, outlined rectangle and the spectrogram is added as a white outlined bar. Response curves in each column represent a single neuron's response to FMs that differ in central frequency between 3 steps (1/2 best bandwidth) above and 3 in steps below BFhigh. PSTHs in each column represent a single neuron's responses to the FMs paired with the CF at BFlow. FMs in each column have a lower center frequency in the top panel (1st data point) and a higher center frequency in the bottom panel (2nd data point). FM direction and stimulus parameters are indicated at the top of each column. PSTHs (bin width = 5 ms) are based on 100 repetitions of the stimulus.
FM BANDWIDTH.
The patterns of response curves elicited by different bandwidths of FMs were more heterogeneous than those obtained for FM slopes. Neurons exhibited a variety of response patterns based on peak response magnitudes summed across stimulus amplitudes ranging from −9 to 91 dB SPL (Fig. 8, A–D). Two basic patterns were observed: response curves were either relatively flat (Fig. 8, A and C) or showed one or more sharp peaks in response to particular bandwidths (Fig. 8, B and D) within the range tested. Monotonically increasing response functions at either two extremes, such as those obtained for changes in slope, were not observed with respect to increasing FM bandwidth. PSTH plots show the response patterns of neurons for encircled values of the FM bandwidth on the response curve together with amplitude envelopes and spectrograms corresponding to each FM.
FM CENTRAL FREQUENCY.
Similar to the previous two examples, Fig. 9 shows examples of response curves to changes in “FM central frequency” or location on the frequency axis of the FM being tested. Zero on the horizontal axis corresponds to a neuron's BFhigh and is different for each neuron. Each step or interval in this shift always equaled one half the best bandwidth of the neuron. For both UFMs and DFMs, the response curves exhibit bell-shaped tuning with the peak centered at or close to 0. PSTH plots show response patterns of spiking in 5-ms bins for each neuron. The PSTHs shown in Fig. 9, A and B, responded better to DFMs than to UFMs presented at their best FM central frequency. In Fig. 9, C and D, the neuron's response to CFs (data not shown) and UFMs was better than that to DFMs.
Population response functions
The mean response curves of a total of 227 DSCF neurons to UFMs and DFMs of varying slope, bandwidth, and central frequency are shown in Fig. 10, A–C, respectively. For all three manipulations of the stimulus, FM response curves of single neurons were normalized to their peak responses and relative response magnitudes for each were averaged together to obtain a mean response curve.
FIG. 10.
A: average response curves of 116 DSCF neurons in response to arrays of 14 UFMs (solid) and DFMs (dashed) ranging in slope from 0.04 to 4.0 kHz/ms. The horizontal axis is slope (kHz/ms). Each response is based on 100 stimulus trials (bin width = 10 ms) for each of 14 FMs at several different intensities ranging from −9 to 91 dB SPL. Response curves were normalized to their absolute maxima before averaging. FMs were attenuated by 10 dB SPL per every 10 trials and these cumulative individual and averaged response curves include responses summed overall amplitude intervals. B: average response curves of 162 DSCF neurons in response to arrays of UFMs and 97 DSCF neurons in response to DFMs ranging in bandwidth between 0.44 and 7.88 kHz. These average response curves resulted from the interleaving of FM bandwidth response functions elicited by 2 different FM arrays, one of which ranged from 0.44 to 5.25 kHz and another ranged from 0.66 to 7.88 kHz. All other aspects of this representation are the same as those in A. C: average response curves of 124 DSCF neurons to UFMs (solid) and 109 neurons to DFMs (dashed) that vary in central frequency by 6 (from −3 to 3) times their bandwidths in steps of one half.
The mean best slope for DFMs was 0.829 ± 1.05 and 0.803 ± 1.28 kHz/ms for UFMs. However, many of these neurons responded best to FMs at different modulation slopes as seen in Fig. 7. The large value for the SD of the mean best DFM and UFM underscores the variability in the best FM slope for responses of DSCF neurons. Some neurons responded best to the maximum slope (4 kHz/ms) routinely presented in our study. In a few cases, when tested, neurons responded best to slopes as great as 8 kHz/ms in both the upward and downward directions, indicating that a saturation of response magnitudes was not always achieved.
Repeated-measures ANOVAs showed a significant effect (P < 0.01) of FM slope on DSCF neuron peak response magnitude. Thus, across 116 neurons, at least 1 of the 14 FM slopes elicited a change in peak response magnitude that significantly differed from the response magnitude changes elicited by other FMs. This result was significant whether or not the FMs presented were UFMs (F13,1495 = 23.97, P < 0.01), DFMs (F13,1495 = 21.84, P < 0.01), or modulated in the best direction for each neuron (F13,1547 = 31.39, P < 0.01).
To determine whether the lower or higher range of slope values had a greater effect on peak response magnitude, we ran one set of repeated-measures ANOVAs on neural responses elicited by FM slopes <2 kHz/ms and another on neural responses elicited by FM slopes >2 kHz/ms. The lower slope range (<2 kHz/ms) had a significant effect on peak response magnitude for UFMs (F6,690 = 20.16, P < 0.01), DFMs (F6,690 = 27.06, P < 0.01), and best direction (F6,714 = 28.05, P < 0.01). The higher slope range (>2 kHz/ms), however, was significant for DFMs (F6,690 = 2.292, P < 0.05) but not for either UFMs (F6,690 = 0.48, P = 0.83) or for best direction (F6,714 = 0.56, P = 0.75).
Based on a minimum threshold of 60% of peak response for local maxima and a maximum threshold of 80% of peak response for local minima, 48% of neurons in our sample showed multipeaked response curves to FM slopes within the range of 0.04 to 4.0 kHz/ms. Neurons with multipeaked FM slope response curves to within the range of 0.04 to 4.0 kHz/ms generally had 2 to 4 peaks. When compared with the directional preference data, these slope data show that 98% of the neurons thus far observed in DSCF respond to linear FMs that do not resemble the FM2, the stereotyped 4-kHz/ms DFM in the second harmonic of the echolocation pulse.
Repeated-measures ANOVAs showed that the significance of the effect of FM bandwidth on DSCF neuron responses depended on the range of bandwidths presented. When bandwidths ranged between 0.66 and 7.88 kHz, FM bandwidth had a significant effect on DSCF neuron peak response magnitude when FMs were modulated in the best direction for each neuron (F10,630 = 2.94, P < 0.01), as well as when the FMs were all UFMs (F10,580 = 2.68, P < 0.01) or DFMs (F10,330 = 2.33, P < 0.05). When bandwidths ranged between 5.25 and 0.44 kHz, FM bandwidth had a less significant effect on firing if the FMs were modulated in the best direction for each neuron (F10,1430 = 2.17, P < 0.05). Furthermore, UFMs (F10,210 = 0.94, P = 0.47) and DFMs (F10,200 = 1.68, P = 0.156) with bandwidths between 5.25 and 0.44 kHz had no significant effects on peak response magnitude.
To determine whether higher or lower bandwidth values had a greater effect on peak response magnitude, we ran one set of repeated-measures ANOVAs on the neural responses elicited by the higher range (>5 kHz) and another on neural responses elicited by the lower range (<5 kHz) of FM bandwidths in the 0.66- to 7.88-kHz range. Although DSCF neurons robustly respond to the higher range of FM bandwidths, FMs in this range did not significantly affect the peak response magnitudes of DSCF neurons regardless of whether they were UFMs (F4,232 = 0.51, P = 0.71), DFMs (F4,132 = 0.78, P = 0.52), or best FMs, regardless of direction, for each neuron (F4,252 = 0.45, P = 0.75). The lower range of FM bandwidths, on the other hand, showed a significant effect on response magnitude whether they were UFMs (F5,290 = 4.89, P < 0.01), DFMs (F5,165 = 4.38, P < 0.01), or modulated in the best direction for each neuron (F5,315 = 5.25, P < 0.01).
Although response curves to FM bandwidth differed between neurons, for both UFMs and DFMs, the population of DSCF neurons we tested responded with equal if not slightly greater magnitude to FMs with greater bandwidths than to those with lesser bandwidths. Specifically, DSCF neurons tended to respond best to FMs that had bandwidths that were at or near the greatest bandwidth tested, whether that bandwidth was 7.88 (over the 0.66- to 7.88-kHz range) or 5.25 kHz (over the 0.44- to 5.25-kHz range). When bandwidth decreased, peak response magnitudes decreased as well (Fig. 10B). This result shows that, despite their narrowband excitatory frequency tuning, DSCF neurons may prefer FMs with greater bandwidths to those with lesser bandwidths.
Averaging response curves elicited by shifting the central frequency in the best FM in a stepwise fashion toward lower and higher frequencies resulted in bell-shaped mean response curves. For many individual neurons (79.0% of UFMs and 80.7% of DFMs) as well as the average for the whole population, this manipulation yielded a bell-shaped distribution for both directions of FM (Fig. 10C). In 46.8% of cases for DFMs and 29.8% of cases for UFMs, the best central frequency of the FM corresponded to the BFhigh of the neuron under study. However, in 71.0% of cases for UFMs and 79.8% of cases for DFMs, the best FM contained the BFhigh.
Repeated-measures ANOVAs showed that FM central frequency had a more significant effect (P < 0.01) on DSCF neuron peak response magnitude than either FM bandwidth or slope. Results were highly significant whether the FMs were UFMs (F12,1476 = 37.13, P < 0.01), DFMs (F12,1269 = 41.88, P < 0.01), or modulated in the best direction for each neuron (F12,1428 = 50.67, P < 0.01).
Finally, to determine whether or not UFM and DFM selective neurons differ in how they encode three FM parameters (slope, bandwidth, and central frequency), we examined the effects that variations in these parameters had on the peak response magnitudes of DSCF neurons where ||DSI|| > 0.14. The peak response magnitudes of UFM selective (DSI > 0.14) DSCF neurons were significantly affected by FM slope (F13,728 = 15.53, P < 0.01) and FM central frequency (F12,600 = 20.55, P < 0.01), but not FM bandwidth (F10,330 => 1.98, P = 0.07). Likewise, the peak response magnitudes of DFM selective (DSI < −0.14) DSCF neurons were also significantly affected by FM slope (F13,481 = 16.52, P < 0.01) and FM central frequency (F12,168 = 24.01, P < 0.01) but not FM bandwidth (F10,90 = 0.622, P = 0.64).
Peak response latency to FM versus CF stimuli
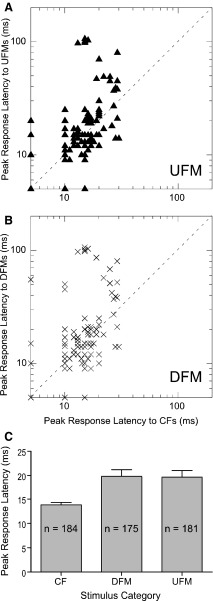
Peak response latencies were measured for 188 DSCF neurons in response to their best CFs and FMs presented 200 times at BA. Latency analyses were restricted to those neurons that had clear, robust, measurable responses when measured with a 1-ms bin width. Based on the aforementioned criteria (see methods), onset latencies accounted for 98% (184/188) of responses to CFs, 93% (175/188) of responses to DFMs, and 96% (181/188) of responses to UFMs. The scatterplots in Fig. 11, A and B, show the peak response latencies for onset responses elicited by the best UFMs and DFMs of DSCF neurons, respectively, plotted against the peak response latencies for onset responses elicited by their BFhigh. Responses to UFMs and DFMs had significantly longer latencies than responses to BFhigh (P < 0.01, paired t-test), but UFMs and DFMs did not significantly differ from one another (P = 0.93, paired t-test). The relatively long peak response latencies elicited by FM stimuli could be a function of overall FM duration.
FIG. 11.
A: scatter-diagram of peak response latencies of UFMs vs. CFs. B: scatter-diagram of peak response latencies of DFMs vs. CFs. C: bar plots showing comparisons of neuron peak response latencies to CFs, DFMs, and UFMs. All latencies shown here are for responses that were obtained either before stimulus offset or that did not exceed the mean peak response latency for CFs.
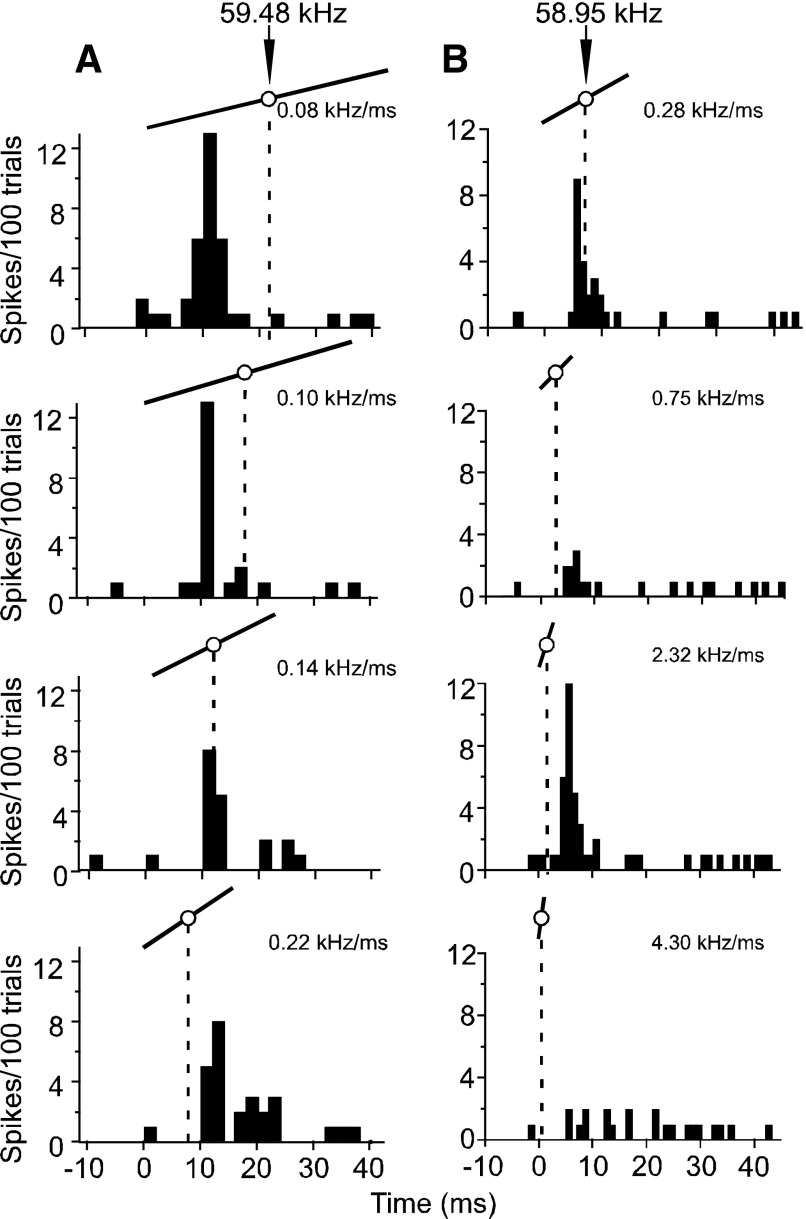
To determine whether response latency is a function of the central frequency as opposed to the FM as a whole, we presented four different FMs 100 times. As in Fig. 7, these FMs differed only in slope and duration. We examined the initial and peak response latencies in several neurons within each set of PSTHs to determine whether latency was dependent on changes in FM slope. Figure 12A shows changes in a neuron's response latency to an FM as a function of its central frequency as its slope was increased from 0.08 to 0.22 kHz/ms. Such an increase in slope would bring the central frequency closer to the FM's onset and, if the neuron were primarily responsive to the BFhigh (which was synonymous with the central frequency during FM slope array presentations), would cause a decrease in latency. This neuron, like the majority of neurons in our sample, showed no significant change in latency as the FM slope was either increased or decreased. Neurons with multipeaked FM slope response curves also showed a similar lack of effect on response latency (Fig. 12B). For this second exemplar neuron, not only did the latency remain largely unchanged with increasing slopes, but also two slopes that differed by an order of magnitude gave similarly robust responses. Other slopes, however, did not elicit responses from this neuron.
FIG. 12.
PSTHs (bin width = 2 ms) obtained for 2 typical DSCF neurons to show the temporal relationship between response peaks and the center frequency of FMs at increasing slopes. A: 4 PSTHs were elicited by an array of FMs and represent responses to 100 presentations at 10 different amplitude levels. Above each PSTH is an illustration of the FM that elicited it. The white circle in the center of the UFM represents its central frequency (here, 59.48 kHz) and the dashed line that drops from the central frequency shows the time (ms) that the central frequency occurred relative to the peak response. B: 4 PSTHs elicited by an array of FMs from another DSCF neuron. Note that the slopes of these UFMs increase more rapidly than those in A.
Responses to calls versus FMs
DSCF neurons are also responsive to and selective for calls (Kanwal 1999, 2006; Kanwal and Rauschecker 2007). The purpose of this section is to show the responses of specific DSCF neurons to calls and/or call-related stimuli. The individual examples are meant to show how the FM response characteristics detailed above play a role in DSCF neuron call selectivity. Data of this type are difficult to quantify for a large population of neurons because 1) this requires one to record from the same single unit for several hours and 2) calls are categorically distinct from each other and are not amenable to quantitative comparison.
ROLE OF COMBINATION-SENSITIVITY.
DSCF neurons respond to calls by nonlinearly integrating the spectral energy of call harmonics that enter their BFlow and BFhigh excitatory frequency ranges (Kanwal 1999, 2006; Kanwal and Rauschecker 2007). Figure 13, A–J, shows how combination-sensitivity affects the responses of two DSCF neurons to CFs and calls. The top row (Fig. 13, A–E) shows the responses of a neuron that had a BFlow of 25.25 kHz, a BFhigh of 59.22 kHz, and responded selectively to the single humped FM (sHFM) call. Similarly, the bottom row (Fig. 13, F–J) shows the responses of a different neuron that had a BFlow of 23.72 kHz, a BFhigh of 59.24 kHz, and responded selectively to the stretched rippled FM (sRFM) call. We band-pass filtered the best calls of these neurons such that the only remaining frequencies were a 5-kHz band centered on the BFhigh. In both cases, the remaining “call fragments” contained one or more FMs. Both neurons responded to simultaneous presentations of their BFlow and frequency modulated call fragments with greater peak response magnitudes than they did to simultaneous presentations of their BFlow and BFhigh or even to their best calls. Like their best CF pairs, responses to call fragments were facilitated by simultaneous presentations of the BFlow such that the response to both was greater than the sum of the response of each component presented alone. Although the responses in Fig. 13I were elicited by a series of FM “call fragments,” a single FM from that series was sufficient to elicit a stimulus-locked response of nearly equal magnitude (Fig. S2).
FIG. 13.
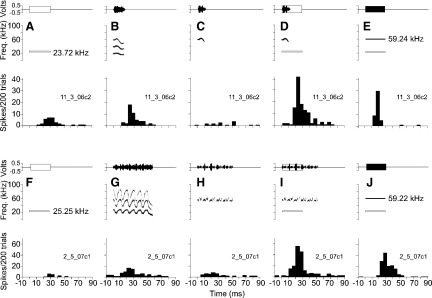
A–E: responses to calls and CF stimuli obtained from a single DSCF neuron. F–J: responses to calls and EF stimuli obtained for a second DSCF neuron. Top: amplitude envelopes (top) and spectrograms (bottom) for sounds presented to awake mustached bats. Bottom: PSTHs (bin width = 5 ms) representing the sum of neural responses to 200 repetitions of the sound in the corresponding top panel. The amplitude envelope and spectrogram of the CF at BFlow are shown, respectively, as unfilled, outlined rectangles and with outlined bars: amplitude envelopes of all other CF stimuli are shown as solid, filled rectangles and spectograms are shown as black bars. A: top: the BFlow (23.72 kHz) of a single DSCF neuron presented at 91 dB SPL. Bottom: the neuron's response to the CF at BFlow. B: top: the best call (sHFM) of the neuron, presented at 91 dB SPL (BA). Bottom: the neuron's response to the sHFM call. C: top: band-pass filtered sHFM call “fragment” with a 5-kHz bandwidth (61.74–56.74 kHz) presented at 91 dB SPL. The sHFM call fragment is predominantly downward and centered on the BFhigh (59.24 kHz). Bottom: response of the neuron to the sHFM call fragment. D: top: the sHFM call fragment paired with CF at BFlow. Bottom: response of the neuron to the sHFM call fragment paired with CF at BFlow. E: top: a CF at BFhigh paired with another at BFlow, each presented at BA (41 and 91 dB SPL, respectively). Bottom: response of the neuron to CFs at BFhigh and BFlow. F: top: the BFlow (25.25 kHz) of the 2nd DSCF neuron presented at 91 dB SPL. Bottom: the 2nd neuron's response to the CF at BFlow. G: top: the best call (sRFM) of the 2nd neuron, presented at 91 dB SPL (BA). Bottom: the 2nd neuron's response to the sRFM call. H: top: band-pass filtered sRFM call “fragment” with a 5-kHz bandwidth (61.72–56.72 kHz) presented at 91 dB SPL. The sRFM call fragment is a series of curvilinear FMs that is centered on the BFhigh (59.22 kHz). Bottom: response of the neuron to the sRFM call fragment. I: top: the sRFM call fragment paired with BFlow. Bottom: response of the neuron to the sRFM call fragment paired with the CF at BFlow. J: top: the CF at BFhigh paired with the another BFlow, each presented at BA (61 and 91 dB SPL, respectively). Bottom: response of the neuron to a CF at BFhigh paired with another at BFlow.
DIRECTION SELECTIVITY.
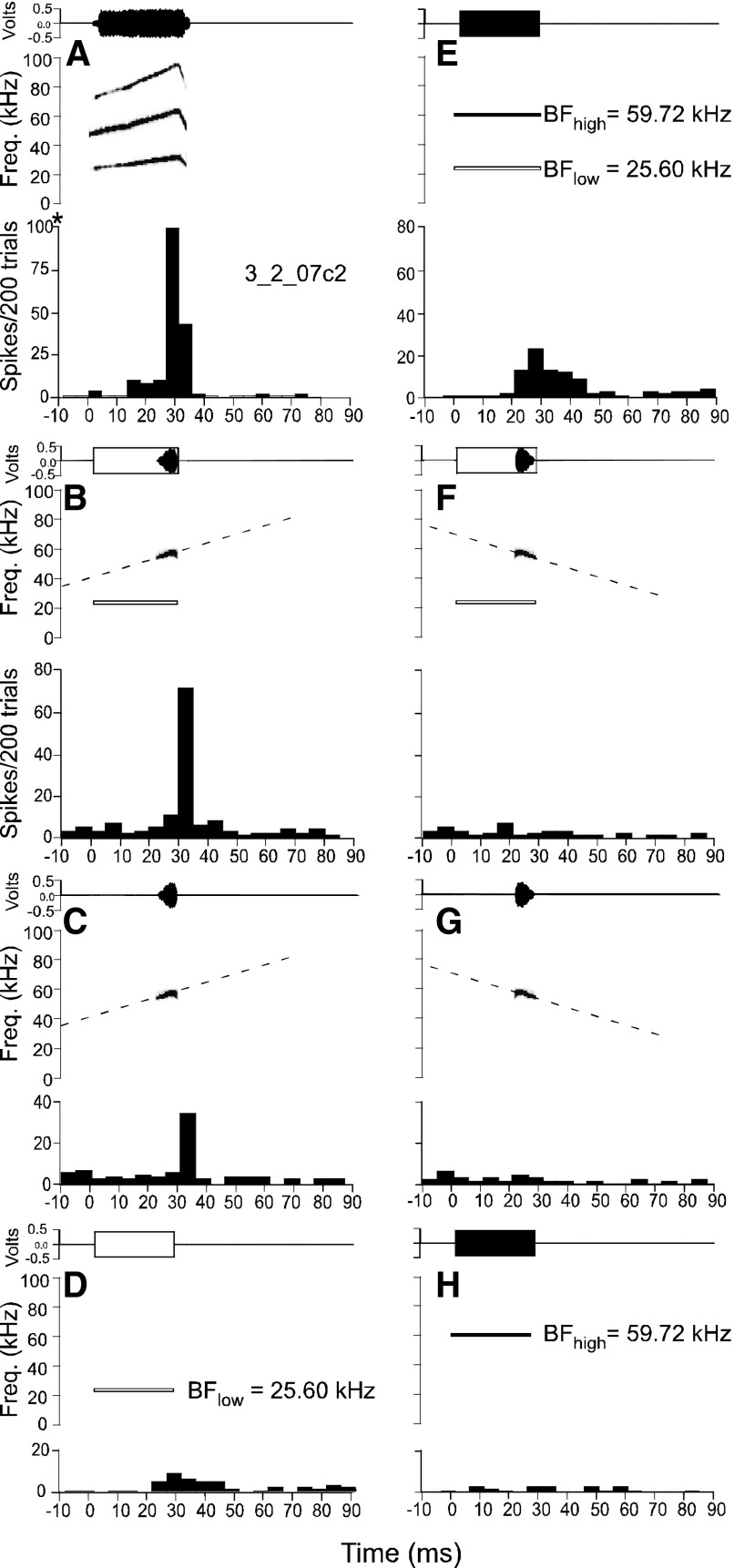
When the best calls of DSCF neurons were band-pass filtered around the BFhigh, the resultant FM call fragments elicited responses from DSCF neurons. When call fragments were paired with the BFlow, those responses often exceeded their responses to their paired BFlow and BFhigh (Fig. 14E). Figure 14, A and D, respectively, shows a DSCF neuron's response to its best call, the bent upward FM or bUFM, and to its BFlow alone. A band-pass filtered, quasi-linear fragment of the bUFM that was centered on the neuron's BFhigh elicited a response that is ∼60% less than the peak response magnitude elicited by the complete bUFM call (Fig. 14C). The addition of the BFlow more than doubled the neuron's response to the bUFM fragment (Fig. 14B).
FIG. 14.
Top panels are amplitude envelopes (top) and spectrograms (bottom) for sounds presented to awake mustached bats. Bottom panels are PSTHs (bin width = 5 ms) representing neural responses to 200 repetitions (2/s) of the sound in the corresponding top panel. All responses shown here originate from the same DSCF neuron. Amplitude envelopes and spectrograms of the CF at BFlow are shown, respectively, as unfilled, outlined rectangles and white outlined bars; amplitude envelopes of CFs at BFhigh are shown as solid, filled rectangles and bUFM. A: top: spectograms by black bars is the best call of the neuron. Bottom: PSTH the neuron's response to the bUFM call. *This axis range differs from the adjacent axis in E. Note that this panel contains the unit number. B: top: band-pass filtered bUFM call fragment paired with the BFlow (25.60 kHz). The call fragment is quasi-linear (slope = ∼0.63 kHz), centered on the BFhigh (59.19 kHz), and has a 5-kHz bandwidth (61.69–56.69 kHz). Bottom: PSTH depicting the response of the neuron to the call fragment and BFlow. C: top: band-pass filtered bUFM call fragment alone. Call fragment is parametrically identical to its description in B. Bottom: PSTH depicting the response of the neuron to the call fragment. D: top: the BFlow presented alone. Bottom: the neuron's PSTH depicting the response to the BFlow. E: top: the BFlow paired with the BFhigh. Bottom: PSTH response of the neuron to the BFlow and BFhigh. F: top: reversed band-pass filtered bUFM call fragment paired with the BFlow. All stimulus parameters are the same as in B except for call fragment direction. Bottom: response to the reversed call fragment and BFlow. G: top: reversed band-pass filtered bUFM call fragment alone. All stimulus parameters are the same as in C except for call fragment direction. Bottom: response to the reversed call fragment alone. H: top: the BFhigh presented alone. Bottom: PSTH depicting the neuron's response to the BFhigh. All stimuli presented at BA.
DSCF neuron responses to their best calls depend on spectral summation of the call elements that enter their BFlow and BFhigh excitatory response areas, but responses to calls also depend on FM direction. When the same bUFM fragment was reversed (and as such became a DFM), it elicited no response from the neuron in Fig. 14. The neuron was unresponsive to the reversed bUFM fragment whether or not it was presented alone (Fig. 14G) or paired with the BFlow (Fig. 14F). Similarly, the neuron shown in Fig. 13, A–E, that responded to the predominantly downward sHFM call had a strong downward selectivity (DSI = −0.42) and did not respond to the bUFM call (data not shown).
SINUSOIDAL FMS.
We compared one neuron's responses to its best DFM and best UFM to its response to the stretched rippled FM (RFM) call, which contains, a sinusoidal modulations, and was one of the two best calls for this neuron (Fig. 15). PSTHs show that the peak response magnitude to the neuron's best DFM is lower than that of the best UFM (DSI = 0.45). The accompanying raster diagrams show that the DFM not only elicited fewer spikes than the UFM but the responses to the DFM were less stimulus locked than those to the UFM (Fig. 15, A and B). This same UFM selective neuron showed similar stimulus locked responses to at least three cycles of the sRFM sinusoids (Fig. 15C). Based on its response latency and direction selectivity, these three stimulus-locked responses indicate the neuron's excitation by the UFM phases contained within the sRFM and, possibly, its inhibition by the DFM phases. Note that the best FM slope for this neuron (0.40 kHz/ms) was similar to the slope of the bUFM call component that enters the BFhigh range of DSCF neurons (∼0.63 kHz/ms). This neuron responded to the bUFM with equal peak response latency and magnitude as to its best UFM but was unresponsive to the sHFM call (Fig. S3). On the far right, we show the responses of a second neuron to the sRFM call. This second neuron is the same UFM selective neuron as in Fig. 14.
FIG. 15.
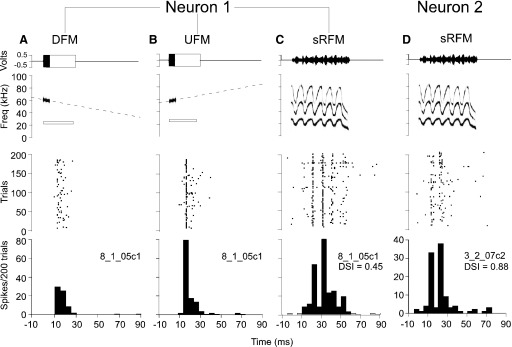
A–C represent stimuli and responses of a single DSCF neuron and D represents the responses of a second DSCF neuron. Top panels are amplitude envelopes (top) and spectrograms (bottom) for sounds presented to awake mustached bats. Middle panels are peristimulus raster plots of neural responses to 200 repetitions of the sound in the corresponding top panel. Bottom panels are PSTHs of neural responses in 5-ms-wide bins. Amplitude envelopes and spectrograms of the CF at BFlow are shown in as unfilled, outlined rectangles and white outlined bars; the amplitude envelopes of other CF stimuli are shown as filled, solid rectangles and spectograms are plotted using SIGNAL software. A: top: BFlow and paired with best DFM for the neuron. DFM was delivered at 61 dB SPL and had a 1.75-kHz bandwidth, a slope of 0.402 kHz/ms, and a central frequency of 59.48 kHz. Middle: responses of the neuron to repeated presentations of the DFM. Bottom: PSTH of the neuron's responses summed over all repetitions to the DFM. B: top: CF at BFlow paired with the best UFM for the neuron. UFM was parametrically identical to the DFM in A. Middle: responses of the neuron to repeated presentation of the UFM. Bottom: PSTH for the sum of the neuron's responses to repeated presentation of the UFM. C: top: the sRFM call. Middle: raster plot responses of the neuron to repeated presentations of the sRFM. Bottom: sum of the neuron's responses to repeated presentation of the sRFM call. D: top: the sRFM call. Middle: responses of a 2nd neuron to repeated presentations of the sRFM. Bottom: PSTH of the neuron's responses summed over all repetitions. Note that this neuron (3_2_07c2) is the same as in Fig. 14.
DISCUSSION
At least five parameters of an FM signal (slope, bandwidth, central frequency, amplitude, and direction) have been described as important determinants for neural responses in the central auditory system (Erulkar et al. 1968). The question of how FM parameters may be represented along the ascending auditory pathway and within the neocortex cannot be easily answered without thoroughly understanding the systematic changes in neural response patterns that accompany changes in FM parameters. Studying the neural responses to shifts in the value of each individual FM parameter is time consuming and single units are generally difficult to hold for long in awake animals. Awake preparations are necessary, however, for studies at the cortical level. Previous studies show that neurophysiological recordings can be obtained over many hours from single units over many hours in the mustached bat auditory cortex (Kanwal et al. 1999). Therefore, mustached bats provided an excellent model for conducting our detailed studies.
Most studies of neural responses to FMs use either linear or logarithmic FMs. One major rationale for the use of logarithmic FMs is that they more closely conform to cochleotopic organization and thus, unlike linear FMs, ensure equivalent acoustic stimulation across audible frequencies. This rationale is not applicable to our study, however, because the echo CF2 range in mustached bats represents only a narrow band of frequencies. Logarithmic FMs that span a wide frequency range are largely absent in mustached bat calls (Kanwal et al. 1994). Furthermore, linear FMs can simulate instantaneous rates of frequency change, and, potentially, the information-bearing elements within mustached bat calls.
Representation of FMs in the mustached bat A1
Our data showed that, although DSCF neurons respond well to Doppler-shifted echo CF2 frequencies (Fitzpatrick et al. 1993; Kanwal et al. 1999; Suga 1978), they may also respond equally well to and, in some cases prefer, linear FMs traversing the excitatory and inhibitory response areas in the echo CF2 frequency range. Slope, bandwidth, central frequency, and modulation direction seemed to be the four key parameters dictating neural responses to FMs in the DSCF area. Neural responses to FM slopes within a 0.04- to 4.0-kHz/ms range, on average, yielded curves that monotonically decreased with increasing FM slope. However, over half (55%) of DSCF neurons studied preferred FM slopes >0.04 kHz/ms. Furthermore, nearly one half (48%) of the neurons preferred multiple FM slopes in the range of 0.04–4.0 kHz/ms.
Because the peak response magnitudes of UFM- and DFM-selective DSCF neurons were significantly modified by shifts of the same FM parameters (slope and central frequency), we concluded that there are no overt differences in how UFM and DFM selective DSCF neurons encode FM slope, FM bandwidth, or central frequency. UFM selectivity in the mustached bat auditory system, however, is an intriguing result that was first documented within the inferior colliculus (IC) by Gordon and O'Neill (1998). This UFM selectivity seems to be unrelated to echolocation, because UFMs are not prominent within echolocation signals and do not have an apparent function (Fitzpatick et al. 1991). From a communication perspective, however, the UFM selectivity observed in the majority of DSCF neurons could enable bats to discern modulation slopes that exist within calls (most of which are either UFMs or bidirectional/sinusoidal FMs) from the DFM in the echolocation pulse. Indeed, the fact that DSCF neurons are largely selective for UFMs with slopes between 0.04 and 1 kHz/ms suggests that the 4-kHz/ms downward echolocation FM2 is perfectly orchestrated to avoid stimulating DSCF neurons.
DSCF neuron responses to FMs ranging in bandwidth between 0.44 and 7.88 kHz, on average, responded more to FMs with the greatest bandwidths than those with the least. The narrowband excitatory frequency region of the BFhigh is flanked by inhibitory side bands (Kanwal et al. 1999). This arrangement, by itself, suggests that DSCF neurons should prefer FMs that are composed of only a narrow band of frequencies that correspond to the excitatory range. The observed preference for FMs >5 kHz could be explained, however, by nonlinear interactions resulting from stimulation of excitatory and inhibitory frequencies with certain temporal delays. These interactions between the excitatory and inhibitory frequency response areas could lead to postinhibitory rebound that would expand the effective facilitatory response area of a neuron. However, more detailed studies, perhaps at the intracellular level, would be needed to confirm or deny the involvement of postinhibitory rebound as a mechanism for FM processing in the DSCF area.
Neither FM slope (n = 116) nor bandwidth (n = 162) response profiles produced bell-shaped curves for single unit activity averaged over the sample. A bell-shaped or sharp-peaked response curve is commonly obtained for single A1 neuron responses to the presentation of a series of varying frequencies, and such a unimodal response curve would indicate a neuron's tuning to one frequency. These response curves are thus indicative of a neuron's selectivity for specific parameters. In the case of DSCF neurons, frequency-amplitude response areas elicited by the presentation of CFs in the BFhigh range exhibit narrowband excitatory frequency tuning that is flanked by broad inhibitory sidebands (Kanwal et al. 1999). Likewise, sharp, single peaked response curves elicited by changes in FM slope, for instance, would imply that DSCF neurons respond to very stereotypic FMs, like the A1 neurons of the pallid bat that emits only a stereotypic FM during echolocation (Razak and Fuzessery 2006). The lack of such response curves elicited by changes in FM slope and bandwidth implies that single DSCF neurons are not designed to process stereotypic FM stimuli, because mustached bats emit a wide variety of FMs during communication.
Bell-shaped response curves were observed for the majority of DSCF neurons in response to changes in FM central frequency. These distributions were almost always obtained for both directions of modulation. In >70% of cases for both UFMs and DFMs, a neuron's best FM contained the BFhigh, but the central frequency of a neuron's best FM was not always the BFhigh. Indeed, in only one half of the best DFMs and one third of the best UFMs found in this study was the neuron's BFhigh equal to its best central frequency. In the anterior auditory field (AAF) and posterior auditory field (PAF) of the cat, it was determined that a neuron's BF is not necessarily the instantaneous frequency that elicits its response to a linear FM (Tian and Rauschecker 1994, 1998).
Bell-shaped response distributions that are elicited by changes along multiple FM parameters have proven elusive in several animal models and at different levels of the auditory system (Gordon and O'Neill 1998, 2000; Heil et al. 1992a; Mendelson et al. 1993; Nelken and Versnel 2000; Poon et al. 1991; Shamma et al. 1993). Likewise, variations in two of our parameters (slope and bandwidth) did not yield bell-shaped response curves from DSCF neurons. Most notably, one study of linear FM response characteristics in the rat IC used a procedure very similar to that in this study to independently examine IC neuron responses to shifts in FM slope, bandwidth, and amplitude parameters (Poon et al. 1991). Bell-shaped distributions along all three parameters were obtained for only 11% of neurons in the rat IC.
In the pallid bat, A1 neurons have a downward directional preference and bell-shaped response curves to FM slopes (Razak and Fuzessery 2006). The differences between FM response properties in the mustached and pallid bat A1 may result from species differences, because the pallid bat does not emit a CF signal that can be used to calculate target velocity (Razak and Fuzessery 2002). Therefore data obtained in the pallid bat high-frequency FM sweep-selective area cannot be directly compared with data obtained in the DSCF area, and the FM sweep selective area may be more comparable to the FM-FM area in mustached bats.
Responses to FMs in other species
FM RESPONSES AT CORTICAL LEVELS.
There have been many studies on the neural representations of FMs in A1 that have focused on a variety of different FM classes, including linear (Heil and Scheich 1992; Heil et al. 1992a,b; Nelken and Versnel 2000), logarithmic (Mendelson et al. 1993; Zhang et al. 2003), and sinusoidal FMs (Liang et al. 2002; Suga et al. 1983). These studies mapped representations of FMs in the primary auditory cortex (A1) by determining the FM rate (or slope) and FM direction that elicited peak responses from multiunit clusters and, in some cases, from single units. When logarithmic FMs were used in the cat, a significant minority (∼45%) of multiunit clusters showed a direction preference. More than 50% of those direction preferring clusters preferred DFMs (Mendelson et al. 1993). When linear FMs were used instead, 66% of those direction preferring clusters preferred DFMs (Heil et al. 1992a,b). Only 5% of the multiunit clusters showed no directional preference. Studies in the ferret that used logarithmic FMs showed an overall UFM preference in A1 (Nelken and Versnel 2000; Shamma et al. 1993). However, subsequent research in the ferret A1 using both linear and logarithmic FMs showed that, like in cats, the percentage of A1 neurons displaying directional preference in the ferret was also largely dependent on the class of FM, with linear FMs showing greater direction preference (Nelken and Versnel 2000).
These same studies in the cat and ferret A1 found that FM slope preferences were independent of FM class and reported that the A1 of both species contained consistent topographic representations of FM slope along the iso-frequency axis (Heil et al. 1992a,b; Mendelson et al. 1993; Nelken and Versnel 2000; Shamma et al. 1993). In cats, where a 5.5- to 35-kHz bandwidth was used to assess slope preference, FMs responded both monotonically (62%) and nonmonotonically (38%) to increasing the magnitude of linear FM slopes (Heil et al. 1992a). Only 21% of the total sample in that study responded to variations in linear FM slopes with a bell-shaped distribution response curve. A topographic organization of FM slope preference was also observed in the nonprimary auditory cortex of the cat (Tian and Rauschecker 1994, 1998) and rhesus macaque (Tian and Rauschecker 2004). In our study, a clear map of FM parameters was not obvious.
Studies across different species have led to disparate conclusions. In ferrets and cats, FM slope was systematically organized within A1 along the isofrequency axis for both linear and logarithmic FMs. Neurons in the A1 of cats are often selective for multiple linear FMs that vary in slope between 1 and 10 kHz/s, and the majority of these neurons have a downward preference (Heil et al. 1992a). The distribution of FM direction therefore seems to be dependent on the experimental paradigm and animal model (Heil et al. 1992b; Mendelson et al. 1993; Nelken and Versnel 2000).
FM RESPONSES AT SUBCORTICAL LEVELS.
We elected to study FM processing in the auditory cortex, because auditory information from regions such as the auditory nerve, cochlear nucleus (CN), inferior colliculus (IC), and medial geniculate body of the thalamus (MGB) eventually reaches the cortex. Subcortical regions in a number of mammalian species, including bats, show a preference for FMs of a particular direction and slope, and this direction preference is species dependent. In the auditory nerves of cats, 60% of neurons are insensitive to FM direction except at slopes >5 kHz/ms (Sinex and Geisler 1981). Neurophysiological studies of the CN of cats, however, have demonstrated that 72% of neurons in this region have a DFM preference that is dependent on FM slopes (Britt and Starr 1976). Sixty-one percent of rat IC neurons are selective for direction, slope, and bandwidth (Poon et al. 1991). Neurons in the MGB of the cat also display a directional preference for DFMs that is slope dependent (Purser and Whitfield 1972). Therefore, the FM direction preference observed here in A1 is already extracted at subcortical levels.
Neural mechanisms underlying FM responses
In our experiments, shifting the central frequency of an FM on either side of the BFhigh yielded bell-shaped distributions of peak response rates from DSCF neurons. The mean absolute maxima of these distributions were usually centered on the BFhigh and coincided with responses to FMs that contained frequencies that were within the neuron's excitatory frequency response area. Shapes of these distributions were largely unaffected by modulation direction. Our data suggest that the overall spectrum of the FM is important for eliciting responses from A1 neurons. A procedure involving the deletion of the first, second, third, and last quarters of the pulse and echo of a stereotyped 15 kHz/ms (60 kHz/4 ms) FM was used to test delay-dependent responses in FM-FM neurons of the little brown bat (Myotis lucifugus); those experiments demonstrated that echo-FM quarters that were essential to eliciting the best delay-dependent responses varied between FM-FM neurons (Maekawa et al. 1992). We did not adopt a systematic trimming procedure to further refine each the frequency range of best FM extracted by our standard procedure.
DIRECTION PREFERENCE.
Directional preference has been seen in DSCF neuron responses to calls in this study and in previously published work. For example, reversing a complete bUFM call has also been shown to reduce peak response magnitude in DSCF neurons but not necessarily in other A1 neurons (Kanwal 2006; Medvedev and Kanwal 2004). The directional preference for an FM does not correlate with the central frequency of the preferred FM in the DSCF area. In mustached bat DSCF neurons, asymmetric inhibitory response areas flank the excitatory response area at BFhigh (Kanwal et al. 1999). Thus an excitatory response area alone may give no indication of a neuron's preference for a DFM or a UFM. Suga (1965), Shamma et al. (1993), and, subsequently, others, have suggested that direction selectivity for an FM is less a function of excitatory frequency tuning than a result of asymmetries in inhibitory side bands (Gordon and O'Neill 1998; Razak and Fuzessery 2006; Shamma et al. 1993; Suga 1965). Detailed in vivo intracellular studies of the rat A1 have provided additional evidence for this proposed substrate of direction preference (Zhang et al. 2003).
DSCF neurons are sensitive to sinusoidal frequency modulations or SFMs (Suga et al. 1983). This sensitivity is considered to be a mechanism used by mustached bats during echolocation for targeting the wing beats of their insect prey. Our results suggest that the responses to SFM stimuli, and slope in fact, depend on the selectivity for a particular FM direction and slope as seen in Fig. 15. Thus a large response to an SFM may emerge from the presence of successive upward and/or downward FMs (depending on the neuron's DSI) within an SFM. Temporal summation of these responses to successive FMs may also occur because of temporal interactions between two or more FMs within an SFM.
SLOPE AND DURATION.
Two neural mechanisms have been shown to underlie preference for FM slope in bats. One mechanism depends on high- or low-frequency inhibition that arrives later than the initial excitation caused by the FM's onset (Razak and Fuzessery 2006). A second mechanism depends on duration tuning for component CFs (Fuzessery et al. 2006; Gordon and O'Neill 1998). Although experiments in both the mustached and pallid bats confirm the presence of both slope preference mechanisms in the IC, experiments in the pallid bat confirm only the presence of the former mechanism in A1. This study is the first to investigate the responses of A1 neurons in the mustached bat to FMs presented in their principle excitatory frequency band. Although we did not explore slope preference mechanisms per se, the fact that DSCF neurons are mostly onset neurons is evidence against duration tuning to component CFs. Although the pallid bat does not echolocate using a CF signal (Razak and Fuzessery 2002), the mechanisms of FM slope preference in that mammalian species could give insight into the mechanisms of FM slope preference in the A1 of mustached bats and other mammals. Future studies in the mustached bat or another species of CF-FM bat, such as Rhinolophus rouxi, may also show how similar the FM slope tuning mechanisms are between FM and CF-FM bats, and between other mammals as well.
Correlating FM selectivity with call responses
We compared the responses of some single DSCF neurons to their respective best calls and FMs. Because it was time prohibitive to combine the procedures for finding the best call and FM for every neuron, we have only a few such examples. Nevertheless, certain FM response characteristics were clearly apparent in the call selectivity of DSCF neurons. When calls were filtered such that the only remaining spectral elements traversed the BFhigh range, DSCF neurons responded to these frequency modulated “call fragments” with a greater peak response magnitude than they did to CFs (Fig. 13). This result was consistent with the finding that single DSCF neurons respond to and may prefer FMs over CFs in the BFhigh range. In addition, the responses of the neuron in Fig. 14 show that FM direction preference plays a major role in the call selectivity of DSCF neurons. Indeed, it was previously reported that reversing the best call of a DSCF neuron will often drastically reduce its peak response magnitude (Kanwal 1999, 2006). Last, DSCF neuron responses to linear FMs may underlie their responses to the sRFM and other behaviorally relevant SFM sounds.
There have been relatively few studies of auditory cortical processing of FMs that directly link FM selectivity with selectivity for social calls. Systematic studies of FM processing in the AAF, PAF, and A1 in cats (Heil et al. 1992a,b; Mendelson et al. 1993; Tian and Rauschecker 1994, 1998) and A1 in ferrets (Kowalski et al. 1995; Nelken and Versnel 2000; Shamma et al. 1993) included no studies of either call selectivity or call responsiveness. Studies in the lateral belt of the rhesus macaque (Tian and Rauschecker 2004) did not directly compare the responses of neurons to synthetic FMs with calls, despite the fact that recordings were made in a call-responsive subregion (anterior-lateral belt). Studies of FM processing in the A1 of squirrel monkeys (Bieser 1998) and marmosets (Cheung et al. 2005; Wang et al. 1995) used natural calls as stimuli but focused only on a highly restricted call set (specifically, sinusoidal FM “twitter” calls).
Much of the knowledge of call selectivity (Hurley and Pollak 2005; Klug et al. 2002; Pillat and Schuller 1998; Portfors 2004) and FM selectivity (Casseday et al. 1997; Gordon and O'Neill 1998, 2000; Suga 1968) in the IC has emerged from neurophysiological studies of various bat species. To our knowledge, no study of auditory processing in the IC has directly compared selectivity for calls to selectivity for FMs in the same neurons, but results of bat IC studies do predict many of the FM and call response characteristics of DSCF neurons. Like the DSCF area, the mustached bat IC contains neurons that are combination-sensitive to spectral elements of calls and echolocation signals (Portfors 2004). Neurons in the mustached bat IC are also predominantly UFM selective and are responsive to multiple FM slopes of ≤12 kHz/ms (Gordon and O'Neill 1998). Almost none of the FM responsive neurons in the mustached bat IC is unresponsive to CFs (O'Neill 1985). In the Mexican free tailed bat, IC neurons responded selectively only to some calls that traversed their excitatory response regions but not to other calls that did the same (Klug et al. 2002). Removal of GABA-ergic and glycinergic inhibition in these same IC neurons in the Mexican free tailed bat resulted in neural responses to most calls that traversed their excitatory response regions. Similar effects have been shown for FMs in the IC of the big brown bat (Lu et al. 1998). Thus inhibition shapes both frequency tuning and slope selectivity in the IC, much as it does in the AI of the pallid bat (Razak and Fuzessery 2006) and, presumably, the mustached bat. IC (or avian auditory midbrain) neuron responses to calls and FMs in other species such as cats (McAnally and Calford 1989), rats (Lee et al. 2002; Poon et al. 1991), and songbirds (Woolley and Casseday 2005) similarly reflect cortical (or avian auditory forebrain) responses to calls and FMs.
An underlying reason that call processing is not often integrated into FM studies may be that it is difficult to make assumptions about key auditory features without prior knowledge of the animal's species-specific vocalizations. This integration becomes especially difficult when studying species that do not regularly emit highly stereotyped vocalizations, as bats do for echolocation, in addition to their communication sounds (Young 1998). The technique of reverse correlation, in which a neuron's responses to random noise enables an auditory neuron to define its own ideal feature set has recently enabled studies of stimulus preference in auditory neurons to be performed without a priori knowledge of the acoustic structure of calls (deCharms et al. 1998; Fritz et al. 2003). Reverse correlation estimates the optimal response of the neuron as a function of frequency and time [a spectro-temporal receptive field (STRF)] and can be used to determine which calls may maximally drive a given neuron. This approach, however, assumes the absence of nonlinear interactions (combination-sensitivity) between stimulus components in both the time and spectral domains. Because calls are multidimensional acoustic signals (Kanwal et al. 1994), systematic variations in call parameters can result in unpredictable interactions in the frequency, time, and amplitude domains. These interactions are difficult to study because it is virtually impossible to independently alter only one parameter without influencing others. Given the propensity of nonlinearity within the cortex of mustached bats and the previous work on DSCF neurons, our studies do not stress the usage of either STRFs or of reverse correlation to determine the best FM for a neuron.
Despite their narrowband excitatory and facilitatory frequency tuning, previous studies indicate that DSCF neurons respond well to many calls that contain FMs (Kanwal 1999, 2006). Our results suggest that the ability of DSCF neurons to respond to species-specific calls may emerge from their tuning to linear FMs. These FMs likely constitute some of the acoustic features that make one call distinct from another. The neural mechanisms related to combination-sensitivity, tuning to FM slopes, bandwidth, and central frequency as well as directionality seem to underlie the observed call selectivity, although the response to its best FM alone cannot be used to predict a neuron's overall call selectivity. We propose that a tuning to or selectivity for a specific set of FM parameters plays a critical role in the extraction of key acoustic features that distinguish one call from another. Additional experiments, however, are needed to completely define the multidimensional stimulus space of a DSCF neuron and possibly explain its selectivity for calls.
GRANTS
This work was supported in part by a National Research Service Award to S. D. Washington and National Institute for Deafness and Other Communication Disorders Grant DC-02054 to J. S. Kanwal.
Supplementary Material
Acknowledgments
The Ministry of Agriculture, Land and Marine Resources in Trinidad kindly permitted us to export mustached bats, and F. Muradali assisted with collection and exportation procedures. We thank the thesis review committee of S. D. Washington for comments on this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Atencio et al. 2007.Atencio CA, Blake DT, Strata F, Cheung SW, Merzenich MM, Schreiner CE. Frequency-modulation encoding in the primary auditory cortex of the awake owl monkey. J Neurophysiol 98: 2182–2195, 2007. [DOI] [PubMed] [Google Scholar]
- Bieser 1998.Bieser A Processing of twitter-call fundamental frequencies in insula and auditory cortex of squirrel monkeys. Exp Brain Res 122: 139–148, 1998. [DOI] [PubMed] [Google Scholar]
- Boinski and Mitchell 1995.Boinski S, Mitchell CL. Wild squirrel monkey (Saimiri sciureus) “caregiver” calls: contexts and acoutic structure. Am J Primatol 35: 129–137, 1995. [DOI] [PubMed] [Google Scholar]
- Britt and Starr 1976.Britt R, Starr A. Synaptic events and discharge patterns of cochlear nucleus cells. II. Frequency-modulated tones. J Neurophysiol 39: 179–194, 1976. [DOI] [PubMed] [Google Scholar]
- Brudzynski 2005.Brudzynski SM Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet 35: 85–92, 2005. [DOI] [PubMed] [Google Scholar]
- Casseday et al. 1997.Casseday JH, Covey E, Grothe B. Neural selectivity and tuning for sinusoidal frequency modulations in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol 77: 1595–1605, 1997. [DOI] [PubMed] [Google Scholar]
- Cheung et al. 2005.Cheung SW, Nagarajan SS, Schreiner CE, Bedenbaugh PH, Wong A. Plasticity in primary auditory cortex of monkeys with altered vocal production. J Neurosci 25: 2490–2503, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement et al. 2006.Clement MJ, Gupta P, Dietz N, Kanwal JS. Audiovocal communication and social behavior in mustached bats. In: Behavior and Neurodynamics for Auditory Communication, edited by Kanwal JS, Ehret G. New York: Cambridge, 2006, p. 57–84.
- deCharms et al. 1998.deCharms RC, Blake DT, Merzenich MM. Optimizing sound features for cortical neurons. Science 280: 1439–1443, 1998. [DOI] [PubMed] [Google Scholar]
- DiMattina and Wang 2006.DiMattina C, Wang X. Virtual vocalization stimuli for investigating neural representations of species-specific vocalizations. J Neurophysiol 95: 1244–1262, 2006. [DOI] [PubMed] [Google Scholar]
- Erulkar et al. 1968.Erulkar SD, Nelson PG, Bryan JS. Experimental and theoretical approaches to neural processing in the central auditory pathway. Contrib Sens Physiol 3: 149–189, 1968. [PubMed] [Google Scholar]
- Fishman et al. 2000.Fishman YI, Reser DH, Arezzo JC, Steinschneider M. Complex tone processing in primary auditory cortex of the awake monkey. II. Pitch versus critical band representation. J Acoust Soc Am 108: 247–262, 2000. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick et al. 1993.Fitzpatrick DC, Kanwal JS, Butman JA, Suga N. Combination-sensitive neurons in the primary auditory cortex of the mustached bat. J Neurosci 13: 931–940, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick et al. 1991.Fitzpatrick DC, Suga N, Misawa H. Are the initial frequency-modulated components of the mustached bat's biosonar pulses important for ranging? J Neurophysiol 66: 1951–1964, 1991. [DOI] [PubMed] [Google Scholar]
- Fritz et al. 2003.Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci 6: 1216–1223, 2003. [DOI] [PubMed] [Google Scholar]
- Fuzessery and Feng 1983.Fuzessery ZM, Feng AS. Mating call selectivity in the thalamus and midbrain of the Leopard frog (Rana p. pipiens): single and multiunit analyses. J Comp Physiol [A] 150: 333–344, 1983. [Google Scholar]
- Fuzessery et al. 2006.Fuzessery ZM, Richardson MD, Coburn MS. Neural mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the inferior colliculus of the pallid bat. J Neurophysiol 96: 1320–1336, 2006. [DOI] [PubMed] [Google Scholar]
- Godey et al. 2005.Godey B, Atencio CA, Bonham BH, Schreiner CE, Cheung SW. Functional organization of squirrel monkey primary auditory cortex: responses to frequency-modulation sweeps. J Neurophysiol 94: 1299–1311, 2005. [DOI] [PubMed] [Google Scholar]
- Gordon and O'Neill 2000.Gordon M, O'Neill WE. An extralemniscal component of the mustached bat inferior colliculus selective for direction and rate of linear frequency modulations. J Comp Neurol 426: 165–181, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon and O'Neill 1998.Gordon M, O'Neill WE. Temporal processing across frequency channels by FM selective auditory neurons can account for FM rate selectivity. Hear Res 122: 97–108, 1998. [DOI] [PubMed] [Google Scholar]
- Hauser 1991.Hauser MD Sources of acoustic variation in rhesus macaque vocalization. Ethology 89: 29–46, 1991. [Google Scholar]
- Heil et al. 1992a.Heil P, Rajan R, Irvine DR. Sensitivity of neurons in cat primary auditory cortex to tones and frequency-modulated stimuli. I. Effects of variation of stimulus parameters. Hear Res 63: 108–134, 1992a. [DOI] [PubMed] [Google Scholar]
- Heil et al. 1992b.Heil P, Rajan R, Irvine DR. Sensitivity of neurons in cat primary auditory cortex to tones and frequency-modulated stimuli. II. Organization of response properties along the ‘isofrequency’ dimension. Hear Res 63: 135–156, 1992b. [DOI] [PubMed] [Google Scholar]
- Heil and Scheich 1992.Heil P, Scheich H. Spatial representation of frequency-modulated signals in the tonotopically organized auditory cortex analogue of the chick. J Comp Neurol 322: 548–565, 1992. [DOI] [PubMed] [Google Scholar]
- Hurley and Pollak 2005.Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. J Comp Physiol [A] Neuroethol Sens Neural Behav Physiol 191: 535–546, 2005. [DOI] [PubMed] [Google Scholar]
- Imig et al. 1977.Imig TJ, Ruggero MA, Kitzes LM, Javel E, Brugge JF. Organization of auditory cortex in the owl monkey (Aotus trivirgatus). J Comp Neurol 171: 111–128, 1977. [DOI] [PubMed] [Google Scholar]
- Kanwal 1999.Kanwal JS Processing species-specific calls by combination-sensitive neurons in an echolocating bat. In: The Design of Animal Communication, edited by Hauser MD, Konishi M. Cambridge, MA: MIT Press, 1999, p. 135–157.
- Kanwal 2006.Kanwal JS A distributed cortical representation of social communication calls. In: Behavior and Neurodynamics for Auditory Communication, edited by Kanwal JS, Ehret G. New York: Cambridge, 2006, p. 156–188.
- Kanwal et al. 1999.Kanwal JS, Fitzpatrick DC, Suga N. Facilitatory and inhibitory frequency tuning of combination-sensitive neurons in the primary auditory cortex of mustached bats. J Neurophysiol 82: 2327–2345, 1999. [DOI] [PubMed] [Google Scholar]
- Kanwal et al. 1994.Kanwal JS, Matsumura S, Ohlemiller K, Suga N. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J Acoust Soc Am 96: 1229–1254, 1994. [DOI] [PubMed] [Google Scholar]
- Kanwal et al. 2000.Kanwal JS, Gordon M, Peng J, Heinz-Esser KH. Neural responses to species-specific sounds in the frontal cortex of the mustached bat. J Neuroreport 11: 367–372, 2000. [DOI] [PubMed] [Google Scholar]
- Kanwal and Rauschecker 2007.Kanwal JS, Rauschecker JP. Auditory cortex of bats and primates: managing species-specific calls for social communication. Front Biosci 12: 4621–4640, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug et al. 2002.Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol 88: 1941–1954, 2002. [DOI] [PubMed] [Google Scholar]
- Kowalski et al. 1995.Kowalski N, Versnel H, Shamma SA. Comparison of responses in the anterior and primary auditory fields of the ferret cortex. J Neurophysiol 73: 1513–1523, 1995. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2002.Lee HJ, Wallani T, Mendelson JR. Temporal processing speed in the inferior colliculus of young and aged rats. Hear Res 174: 64–74, 2002. [DOI] [PubMed] [Google Scholar]
- Liang et al. 2002.Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol 87: 2237–2261, 2002. [DOI] [PubMed] [Google Scholar]
- Liberman et al. 1967.Liberman AM, Cooper FS, Shankweiller DP, Studdert-Kennedy M. Perception of the speech-code. Psychol Rev 74: 431, 1967. [DOI] [PubMed] [Google Scholar]
- Lu et al. 1998.Lu Y, Jen PH, Wu M. GABAergic disinhibition affects responses of bat inferior collicular neurons to temporally patterned sound pulses. J Neurophysiol 79: 2303–2315, 1998. [DOI] [PubMed] [Google Scholar]
- Ma et al. 2006.Ma J, Kobayasi K, Zhang S, Metzner W. Vocal communication in adult greater horseshoe bats, Rhinolophus ferrumequinum. J Comp Physiol [A] Neuroethol Sens Neural Behav Physiol 192: 535–550, 2006. [DOI] [PubMed] [Google Scholar]
- Maekawa et al. 1992.Maekawa M, Wong D, Paschal WG. Spectral selectivity of FM-FM neurons in the auditory cortex of the echolocating bat, Myotis lucifugus. J Comp Physiol [A] 171: 513–522, 1992. [DOI] [PubMed] [Google Scholar]
- Manabe et al. 1978.Manabe T, Suga N, Ostwald J. Aural representation in the Doppler-shifted-CF processing area of the auditory cortex of the mustache bat. Science 200: 339–342, 1978. [DOI] [PubMed] [Google Scholar]
- Margoliash 1983.Margoliash D Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci 3: 1039–1057, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler and Pickert 1984.Marler P, Pickert R. Species-universal microstructure in the learned song of the swamp sparrow (Melospiza georgiana). Anim Behav 32: 673–689, 1984. [Google Scholar]
- McAnally and Calford 1989.McAnally KI, Calford MB. Spectral hyperacuity in the cat: neural response to frequency modulated tone pairs. Hear Res 41: 237–248, 1989. [DOI] [PubMed] [Google Scholar]
- Medvedev and Kanwal 2004.Medvedev AV, Kanwal JS. Local field potentials and spiking activity in the primary auditory cortex in response to social calls. J Neurophysiol 92: 52–65, 2004. [DOI] [PubMed] [Google Scholar]
- Mendelson et al. 1993.Mendelson JR, Schreiner CE, Sutter ML, Grasse KL. Functional topography of cat primary auditory cortex: responses to frequency-modulated sweeps. Exp Brain Res 94: 65–87, 1993. [DOI] [PubMed] [Google Scholar]
- Merzenich et al. 1975.Merzenich MM, Knight PL, Roth GL. Representation of cochlea within primary auditory cortex in the cat. J Neurophysiol 38: 231–249, 1975. [DOI] [PubMed] [Google Scholar]
- Mudry and Capranica 1987.Mudry KM, Capranica RR. Correlation between auditory evoked responses in the thalamus and species-specific call characteristics. I. Rana catesbeiana (Anura: Ranidae). J Comp Physiol [A] 160: 477–489, 1987. [DOI] [PubMed] [Google Scholar]
- Nelken et al. 2003.Nelken I, Fishbach A, Las L, Ulanovsky N, Farkas D. Primary auditory cortex of cats: feature detection or something else? Biol Cybern 89: 397–406, 2003. [DOI] [PubMed] [Google Scholar]
- Nelken and Versnel 2000.Nelken I, Versnel H. Responses to linear and logarithmic frequency-modulated sweeps in ferret primary auditory cortex. Eur J Neurosci 12: 549–562, 2000. [DOI] [PubMed] [Google Scholar]
- Newman and Wollberg 1973.Newman JD, Wollberg Z. Responses of single neurons in the auditory cortex of squirrel monkeys to variants of a single call type. Exp Neurol 40: 821–824, 1973. [DOI] [PubMed] [Google Scholar]
- Ohlemiller et al. 1994.Ohlemiller K, Kanwal JS, Butman JA, Suga N. Stimulus design for auditory neuroethology: synthesis and manipulation of complex communication sounds. Auditory Neurosci 1: 19–37, 1994. [Google Scholar]
- O'Neill 1985.O'Neill WE Responses to pure tones and linear FM components of the CF-FM biosonar signal by single units in the inferior colliculus of the mustached bat. J Comp Physiol [A] 157: 797–815, 1985. [DOI] [PubMed] [Google Scholar]
- Payne and McVay 1971.Payne RS, McVay S. Songs of humpback whales. Science 173: 585–597, 1971. [DOI] [PubMed] [Google Scholar]
- Pillat and Schuller 1998.Pillat J, Schuller G. Audiovocal behavior of Doppler-shift compensation in the horseshoe bat survives bilateral lesion of the paralemniscal tegmental area. Exp Brain Res 119: 17–26, 1998. [DOI] [PubMed] [Google Scholar]
- Poon et al. 1991.Poon PW, Chen X, Hwang JC. Basic determinants for FM responses in the inferior colliculus of rats. Exp Brain Res 83: 598–606, 1991. [DOI] [PubMed] [Google Scholar]
- Portfors 2004.Portfors CV Combination sensitivity and processing of communication calls in the inferior colliculus of the Moustached Bat Pteronotus parnellii. An Acad Bras Cienc 76: 253–257, 2004. [DOI] [PubMed] [Google Scholar]
- Purser and Whitfield 1972.Purser D, Whitfield IC. Thalamo-cortical connexions and tonotopicity in the cat medial geniculate body. J Physiol 222: 161P–162P, 1972. [PubMed] [Google Scholar]
- Razak and Fuzessery 2002.Razak KA, Fuzessery ZM. Functional organization of the pallid bat auditory cortex: emphasis on binaural organization. J Neurophysiol 87: 72–86, 2002. [DOI] [PubMed] [Google Scholar]
- Razak and Fuzessery 2006.Razak KA, Fuzessery ZM. Neural mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex of the pallid bat. J Neurophysiol 96: 1303–1319, 2006. [DOI] [PubMed] [Google Scholar]
- Reale and Imig 1980.Reale RA, Imig TJ. Tonotopic organization in auditory cortex of the cat. J Comp Neurol 192: 265–291, 1980. [DOI] [PubMed] [Google Scholar]
- Shamma et al. 1993.Shamma SA, Fleshman JW, Wiser PR, Versnel H. Organization of response areas in ferret primary auditory cortex. J Neurophysiol 69: 367–383, 1993. [DOI] [PubMed] [Google Scholar]
- Sinex and Geisler 1981.Sinex DG, Geisler CD. Auditory-nerve fiber responses to frequency-modulated tones. Hear Res 4: 127–148, 1981. [DOI] [PubMed] [Google Scholar]
- Suga 1965.Suga N Responses of cortical auditory neurones to frequency modulated sounds in echo-locating bats. Nature 206: 890–891, 1965. [DOI] [PubMed] [Google Scholar]
- Suga 1968.Suga N Analysis of frequency-modulated and complex sounds by single auditory neurones of bats. J Physiol 198: 51–80, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga 1978.Suga N Specialization of the auditory system for reception and processing of species-specific sounds. Federation Proc 37: 2342–2354, 1978. [PubMed] [Google Scholar]
- Suga and Manabe 1982.Suga N, Manabe T. Neural basis of amplitude-spectrum representation in auditory cortex of the mustached bat. J Neurophysiol 47: 225–255, 1982. [DOI] [PubMed] [Google Scholar]
- Suga et al. 1983.Suga N, Niwa H, Taniguchi I. Representation of biosonar information in the auditory cortex of the mustached bat, with emphasis on representation of target velocity information. In: Advances in Vertebrate Neuroethology, edited by Ewert JP, Capranica RR, Ingle DJ. New York: Plenum Press, 1983, p. 829–870.
- Tian and Rauschecker 1994.Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the cat's anterior auditory field. J Neurophysiol 71: 1959–1975, 1994. [DOI] [PubMed] [Google Scholar]
- Tian and Rauschecker 1998.Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the cat's posterior auditory field. J Neurophysiol 79: 2629–2642, 1998. [DOI] [PubMed] [Google Scholar]
- Tian and Rauschecker 2004.Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol 92: 2993–3013, 2004. [DOI] [PubMed] [Google Scholar]
- Wang et al. 1995.Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol 74: 2685–2706, 1995. [DOI] [PubMed] [Google Scholar]
- Washington and Kanwal 2004.Washington SD, Kanwal JS. Linear FM synthesis: test stimuli for rapid analysis of auditory neurodynamics. Hoya Technical Res Bull Article 1D-20041208; 1–4, 2004.
- Winter and Funkenstein 1973.Winter P, Funkenstein HH. The effect of species-specific vocalization on the discharge of auditory cortical cells in the awake squirrel monkey (Saimiri sciureus). Exp Brain Res 18: 489–504, 1973. [DOI] [PubMed] [Google Scholar]
- Woolley and Casseday 2005.Woolley SM, Casseday JH. Processing of modulated sounds in the zebra finch auditory midbrain: responses to noise, frequency sweeps, and sinusoidal amplitude modulations. J Neurophysiol 94: 1143–1157, 2005. [DOI] [PubMed] [Google Scholar]
- Woolsey and Walzl 1942.Woolsey CN, Walzl EM. Topical projection of nerve fibers from local regions of the cochlea in the cerebral cortex of the cat. Bull Johns Hopkins Hosp 71: 315–344, 1942. [Google Scholar]
- Xiao and Suga 2002.Xiao Z, Suga N. Reorganization of the cochleotopic map in the bat's auditory system by inhibition. Proc Natl Acad Sci USA 99: 15743–15748, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young 1998.Young ED What's the best sound? Science 280: 1402–1403, 1998. [DOI] [PubMed] [Google Scholar]
- Zatorre et al. 1994.Zatorre RJ, Evans AC, Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14: 1908–1919, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. 2003.Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature 424: 201–205, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.