Abstract
Acute alcohol consumption causes deficits in motor coordination and gait, suggesting an involvement of cerebellar circuits, which play a role in the fine adjustment of movements and in motor learning. It has previously been shown that ethanol modulates inhibitory transmission in the cerebellum and affects synaptic transmission and plasticity at excitatory climbing fiber (CF) to Purkinje cell synapses. However, it has not been examined thus far how acute ethanol application affects long-term depression (LTD) and long-term potentiation (LTP) at excitatory parallel fiber (PF) to Purkinje cell synapses, which are assumed to mediate forms of cerebellar motor learning. To examine ethanol effects on PF synaptic transmission and plasticity, we performed whole cell patch-clamp recordings from Purkinje cells in rat cerebellar slices. We found that ethanol (50 mM) selectively blocked PF–LTD induction, whereas it did not change the amplitude of excitatory postsynaptic currents at PF synapses. In contrast, ethanol application reduced voltage-gated calcium currents and type 1 metabotropic glutamate receptor (mGluR1)–dependent responses in Purkinje cells, both of which are involved in PF–LTD induction. The selectivity of these effects is emphasized by the observation that ethanol did not impair PF–LTP and that PF–LTP could readily be induced in the presence of the group I mGluR antagonist AIDA or the mGluR1a antagonist LY367385. Taken together, these findings identify calcium currents and mGluR1-dependent signaling pathways as potential ethanol targets and suggest that an ethanol-induced blockade of PF–LTD could contribute to the motor coordination deficits resulting from alcohol consumption.
INTRODUCTION
Acute and chronic alcohol consumption can result in impaired cerebellar function and, consequently, in motor coordination deficits and ataxias (Brust 2002; Servais et al. 2005). Ethanol is known to enhance γ-aminobutyric acid (GABA)–mediated transmission and to impair glutamatergic transmission (for review, see Lovinger 1997; Woodward 1999). Moreover, ethanol affects voltage-gated calcium channels (for review, see Walter and Messing 1999). It thus seems likely that ethanol can impair cerebellar function by interfering with synaptic transmission properties. It has indeed been shown that ethanol application depresses Purkinje cell activity (Chu 1983; Urrutia and Gruol 1992) and that this effect is partially mediated by an increase in GABAergic input (Kelm et al. 2007; Mameli et al. 2008; Servais et al. 2005; for review, see Botta et al. 2007b). Ethanol also enhances GABAergic inhibition of granule cells, which has been attributed to increases in Golgi cell excitability (Botta et al. 2007a; Carta et al. 2004) and enhanced extrasynaptic GABAA receptor activity (Hanchar et al. 2005), respectively. The inhibitory effects of ethanol on glutamatergic transmission are most obvious for N-methyl-d-aspartate (NMDA) receptors (Lovinger et al. 1989). These receptors are expressed in Purkinje cells during development; only recently, functional NMDA receptors have been described at climbing fiber (CF) synapses onto mature Purkinje cells (Piochon et al. 2007; Renzi et al. 2007). Ethanol indeed inhibits the late phase of CF-evoked complex spikes, but this down-regulation is due to an inhibition of mGluR1 receptors (Carta et al. 2006), which can mediate slow, metabotropic currents (mGluR1–excitatory postsynaptic currents [EPSCs]) on CF activation (Dzubay and Otis 2002; Yuan et al. 2007). Early complex spike components and CF–EPSCs remained unaffected by ethanol application (Carta et al. 2006), suggesting that ethanol does not modulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor function in Purkinje cells. Ethanol effects on parallel fiber (PF) synaptic transmission have not been reported.
In addition to modulating synaptic transmission through diverse pre- and postsynaptic mechanisms, ethanol has been shown to affect forms of synaptic plasticity and learning. Ethanol has been described to impair hippocampal long-term potentiation (LTP) and long-term depression (LTD) (Izumi et al. 2005; Morrisett and Swartzwelder 1993) as well as striatal LTP (Yin et al. 2007). In the cerebellum, LTD and LTP at PF synapses onto Purkinje cells are likely involved in forms of motor learning (Jörntell and Hansel 2006). Impaired PF–LTD has been described in mice that were prenatally exposed to ethanol to mimic fetal alcohol syndrome (Servais et al. 2007), but acute ethanol effects on PF synaptic plasticity have not yet been described. However, ethanol blocks the induction of CF–LTD (Carta et al. 2006), which could result in indirect consequences for PF synaptic plasticity because CF coactivation leads to PF–LTD induction, whereas PF stimulation alone induces PF–LTP (Coesmans et al. 2004).
Here, we examined the effects of ethanol application on PF–LTD and PF–LTP. We demonstrate that ethanol (50 mM) selectively blocks PF–LTD, but not PF–LTP induction. We also show that ethanol has no significant effect on AMPA receptor-mediated PF–EPSCs, but rather on voltage-gated calcium currents and on slow, metabotropic responses that are triggered by mGluR1 activation.
METHODS
The experiments were performed at two sites: the Department of Neuroscience at the Erasmus University Medical Center in Rotterdam (Figs. 1A, 2, 3, and 6) and the Department of Neurosciences at the University of New Mexico Health Sciences Center in Albuquerque (Figs. 1, B and C, 4, and 5). The methods used are described separately in the following text. All animal procedures described were approved by a Dutch Ethical Committee for animal experiments and the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center, respectively.
FIG. 1.
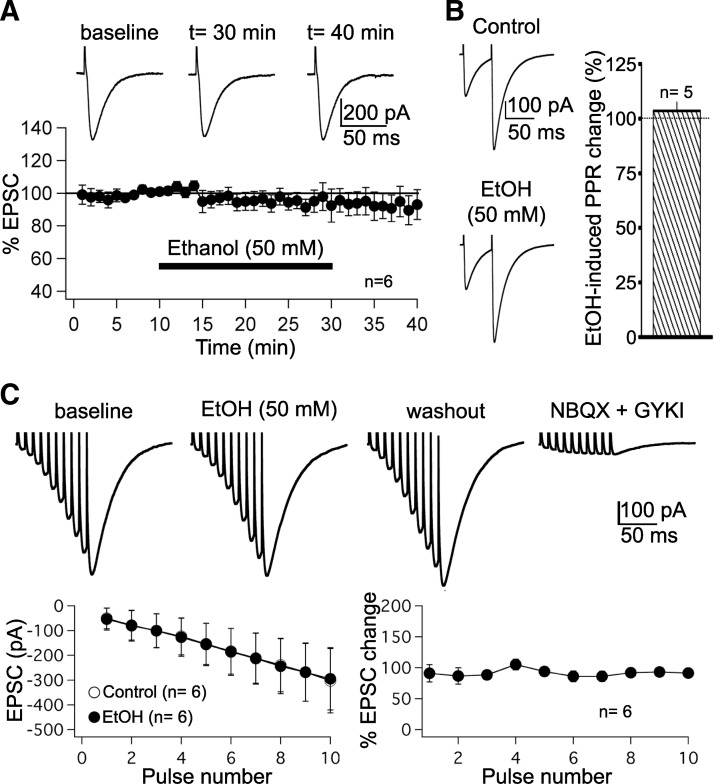
Ethanol does not impair parallel fiber (PF) synaptic transmission. A: PF–excitatory postsynaptic currents (EPSCs) were monitored during a 10-min baseline period, after which ethanol (50 mM) was bath-applied for 20 min. PF responses were subsequently monitored during a 10-min washout period. Top: typical traces recorded before, during, and after ethanol bath application. Bottom: time graph showing PF–EPSC amplitudes (n = 6) in the presence of ethanol (bar). Each data point represents the average of 3 successive test responses evoked at 0.05 Hz. Data were normalized to a baseline recorded during the last 5 min before ethanol washin. B: paired-pulse facilitation (PPF) is unaffected by ethanol (n = 5). Left: PF–EPSC pairs (50-ms interval) before (top) and after ethanol application (bottom). Right: averaged changes in the PPF ratio (n = 5). C: ethanol (50 mM) does not affect PF–EPSC trains evoked by 10 stimuli applied at 100 Hz (n = 6). All recordings were performed in the presence of SR95531 (10 μM) and LY367385 (100 μM) to block γ-aminobutyric acid type A (GABAA) receptors and type 1 metabotropic glutamate receptor (mGluR1) receptors, respectively. Ethanol bath application did not change EPSC trains (top and bottom left). EPSCs remained unaffected independent of their position in the train (bottom right). Top right: PF–EPSCs were blocked in the presence of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonists NBQX (10 μM) and GYKI 53655 (50 μM). Error bars are means ±SE.
FIG. 2.
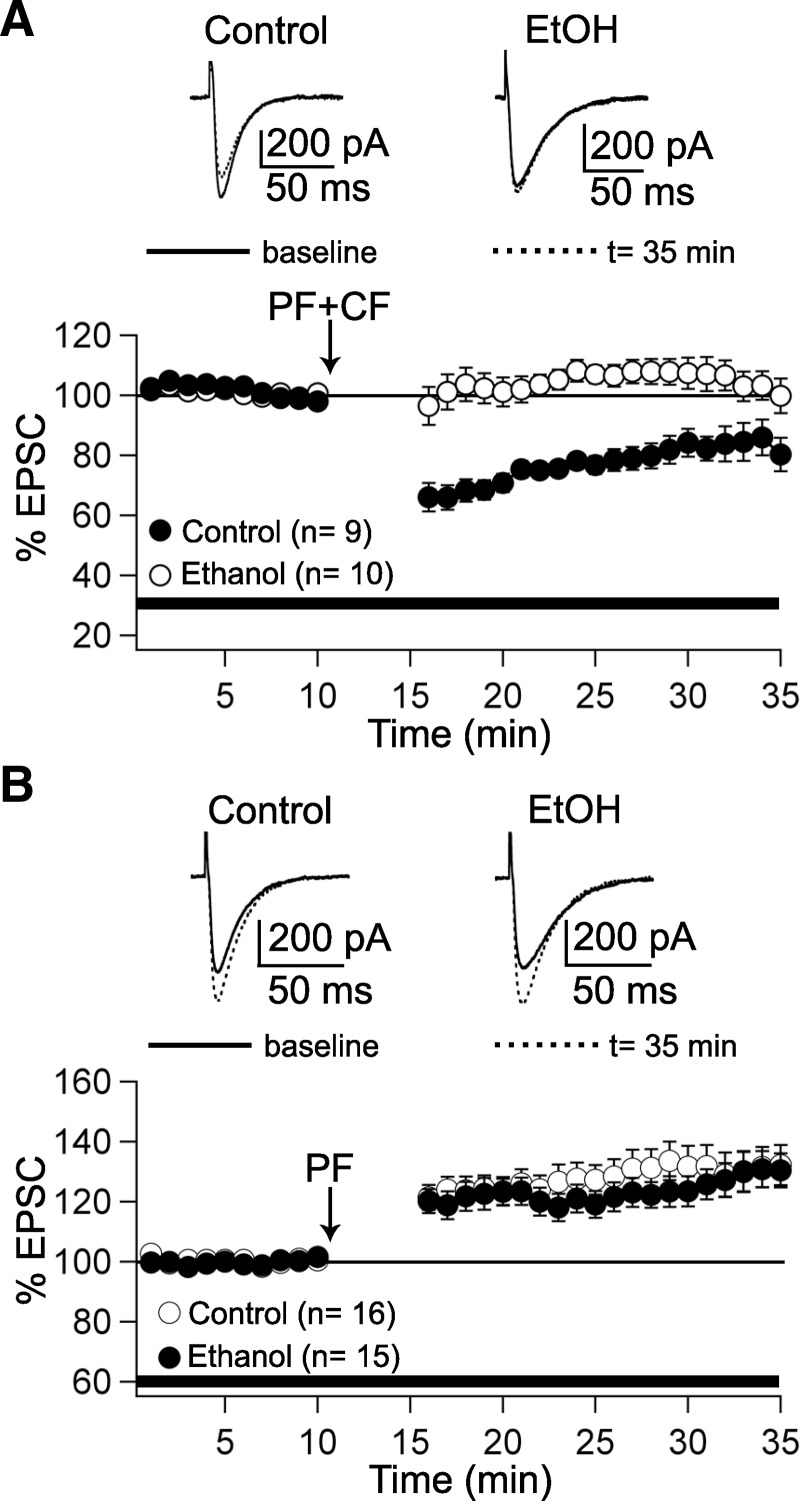
Ethanol selectively blocks the induction of PF–long-term depression (LTD). A: under control conditions, PF–LTD is induced by paired PF + climbing fiber (CF) stimulation at 1 Hz for 5 min (closed circles; n = 9). In the presence of ethanol (50 mM) in the bath, LTD induction is blocked (open circles; n = 10). Top traces show PF–EPSCs before and after tetanization (t = 35 min). B: PF–long-term potentiation (LTP) can be induced under control conditions (open circles; n = 16) and in the presence of ethanol (closed circles; n = 15). Top traces show PF–EPSCs before and after tetanization (t = 35 min). The arrows indicate the time point of tetanization. The bars indicate the presence of ethanol in the bath. Data were normalized to a baseline recorded during the last 5 min before tetanization. Error bars are means ±SE.
FIG. 3.
Ethanol affects voltage-gated calcium currents. A, top: calcium currents activated by voltage steps from −80 to −10 mV in the presence of 50 mM ethanol (left) and 20 mM ethanol (right). Bottom: time course of changes in calcium current amplitudes in the presence of 50 mM (closed circles; n = 9) and 20 mM ethanol (open circles; n = 6), respectively. B, top: calcium currents (voltage steps were applied to command voltages between −70 and +70 mV) under control conditions and in the presence of 50 mM ethanol (middle). Bottom: current–voltage (I–V) curves recorded under control conditions (closed circles; n = 11) and in the presence of 50 mM ethanol (open circles; n = 11). Error bars are means ±SE.
FIG. 6.
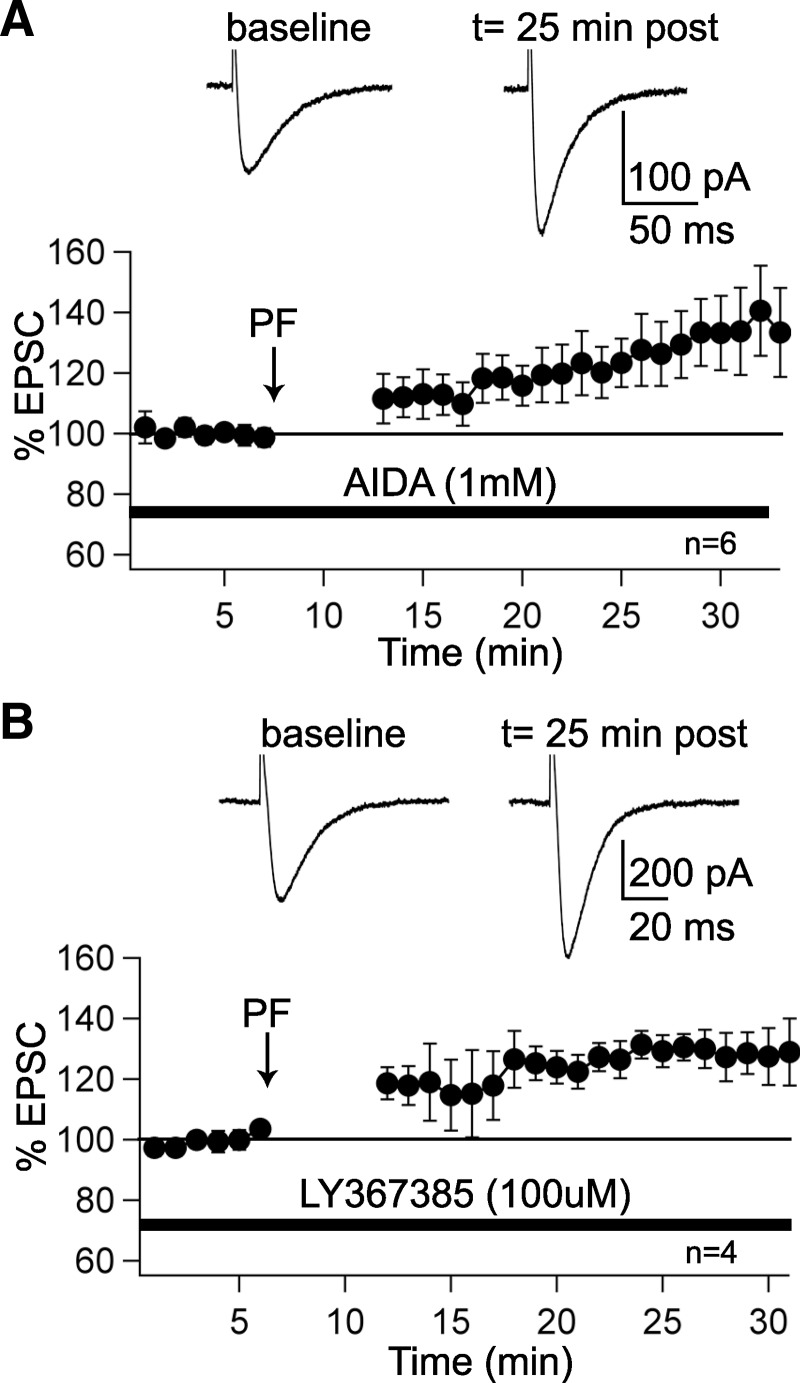
PF–LTP induction is mGluR1 independent. A: LTP can be induced when the group I mGluR antagonist AIDA (1 mM) is present in the bath (n = 6). B: LTP is also induced in the presence of the mGluR1a antagonist LY367385 (100 μM) in the bath (n = 4). In A and B, traces show PF–EPSCs before and 25 min after tetanization (arrows). The bars indicate the presence of AIDA and LY367385, respectively, in the bath. Data were normalized to a baseline recorded during the last 5 min before tetanization. Error bars are means ±SE.
FIG. 4.
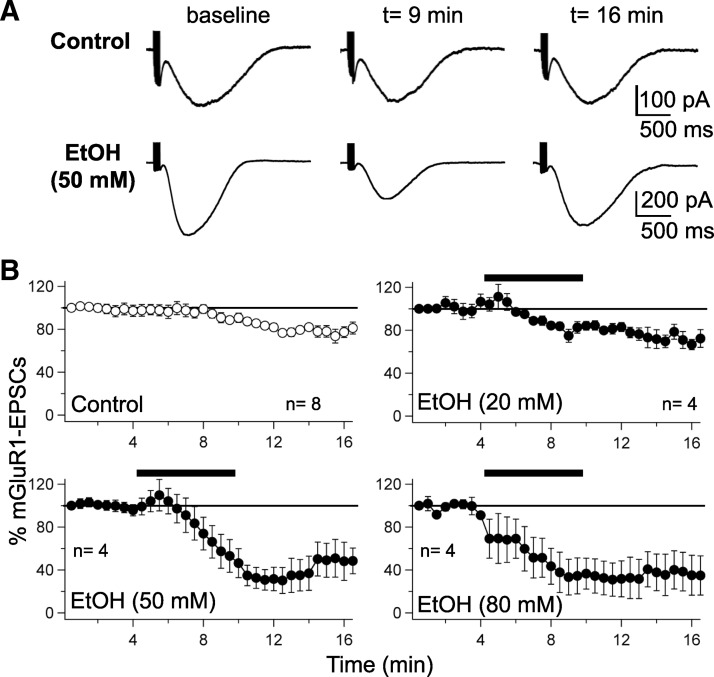
Ethanol down-regulates slow, mGluR1-activated currents. A, top: mGluR1–EPSC traces under control conditions, during the baseline, at 9 and 16 min of whole cell recording. Bottom: mGluR1–EPSCs before, during, and after ethanol (50 mM) application. The time points correspond to those selected for the control recordings (top traces). B: time course of normalized mGluR1–EPSC amplitudes under control conditions (n = 8) and in the presence of 20 mM (n = 4), 50 mM (n = 4), and 80 mM ethanol (n = 4). The bars represent ethanol bath application. Error bars are ±SE.
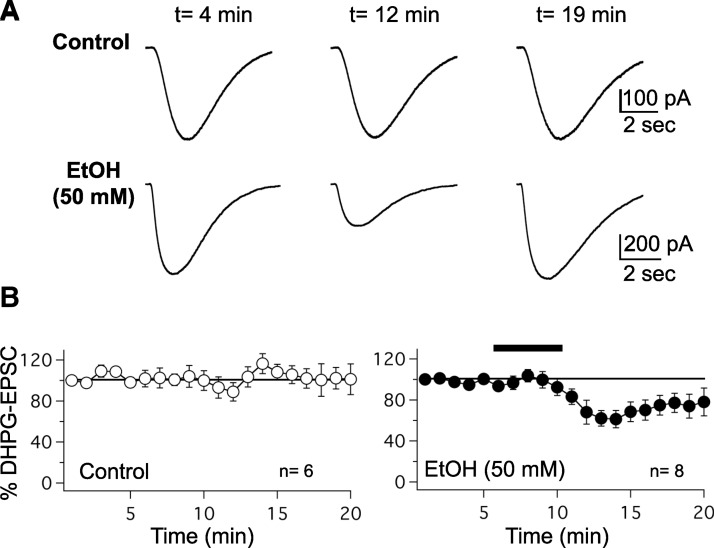
FIG. 5.
Ethanol down-regulates slow (RS)-3,5-dihydroxyphenylglycine (DHPG)–evoked currents. A, top: DHPG (100 μM) puff application evokes slow metabotropic EPSCs, which are shown under control conditions, at 4, 12, and 19 min of whole cell recording. Bottom: DHPG–EPSCs before, during, and after ethanol (50 mM) application. The time points correspond to those selected for the control recordings (top traces). B: time course of normalized DHPG–EPSC amplitudes under control conditions (n = 6), and in the presence of 50 mM ethanol (n = 8). The bar represents ethanol bath application. Error bars are ±SE.
Whole cell patch-clamp recordings of PF–EPSCs
Sagittal slices of the cerebellar vermis (200–250 μm) were prepared from postnatal day 18 (P18) to P30 Sprague–Dawley rats. Slices were kept in artificial cerebrospinal fluid (ACSF) containing the following (in mM): 124 NaCl, 5 KCl, 1.25 Na2HPO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, and 10 d-glucose, bubbled with 95% O2-5% CO2. The perfusion ACSF was supplemented with 100 μM picrotoxin to block GABAA receptors. Whole cell patch-clamp recordings were performed at room temperature using an EPC-10 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). The recording electrodes were filled with a solution containing the following (in mM): 9 KCl, 10 KOH, 120 K-gluconate, 3.48 MgCl2, 10 HEPES, 4 NaCl, 4 Na2ATP, 0.4 Na3GTP, and 17.5 sucrose (pH adjusted to 7.25). All drugs were purchased from Sigma (St. Louis, MO), except for (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA) and (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385) (both from Tocris, Bristol, UK). Currents were filtered at 3 kHz, digitized at 8 kHz, and acquired using PULSE software. In voltage-clamp mode, holding potentials in the range of −60 to −75 mV were chosen to prevent spontaneous spike activity. For extracellular stimulation, standard patch pipettes were used that were filled with external saline. CFs were stimulated in the granule cell layer and PFs in the molecular layer. Test responses were activated at a frequency of 0.05 Hz using approximately 3-μA pulses that were applied for 500 μs (LTP) or 700 μs (LTD). In all experiments, cells were switched to current-clamp mode for tetanization. Recordings were excluded from the study if the series or input resistance varied by >15% over the course of the experiment. All values are shown as percentages of baseline ± SE. For statistical analysis, we used the paired Student's t-test and the Mann–Whitney U test, when appropriate. EPSC recordings shown in Fig. 1, B and C were performed on slices prepared as subsequently described for mGluR1–EPSCs. For the paired-pulse experiments, the ACSF contained 10 μM 2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl) pyridazinium bromide (SR95531). For the PF–EPSC trains evoked by 10 stimuli at 100 Hz, the ACSF contained 10 μM SR95531 hydrobromide and 100 μM LY367385. These studies were performed using the same potassium gluconate–based internal solution as described in the following text for the recording of mGluR1–EPSCs.
Whole cell patch-clamp recordings of voltage-gated calcium currents
Slices were prepared from P5–P6 Sprague–Dawley rats as described earlier. The ACSF was supplemented with tetrodotoxin (TTX, 1 μM) to block voltage-gated Na+ currents. The patch pipettes were filled with a solution containing the following (in mM): 100 CsMeSO4, 20 tetraethylammonium chloride (TEA), 2 MgCl2, 10 EGTA, 4 Na2ATP, 0.4 Na3GTP, 10 Na-phosphocreatine, 5 N-(2,6-dimethylphenyl carbamoylmethyl)triethylammonium bromide (QX-314), and 10 HEPES. Calcium currents were evoked by voltage steps from a holding potential of −80 mV to command voltages between −70 and +70 mV. All drugs were purchased from Sigma (St. Louis, MO), except for TTX (Tocris, Bristol, UK).
Whole cell patch-clamp recordings of mGluR1–EPSCs
Parasagittal slices of the vermis (200 μm) were prepared from P18–P22 Sprague–Dawley rats. The ACSF contained the following (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 glucose equilibrated with 95% O2-5% CO2. All these chemicals were from Sigma (St. Louis, MO). Slices were placed in a recording chamber and superfused at 31–32°C with ACSF supplemented with the following blockers of ionotropic receptors: 10 μM SR95531 hydrobromide, 10 μM 2,3-dihydroxy-6-nitro-7-sulfamoylbenzol[f]quinoxaline (NBQX), and 50 μM GYKI 53655 (all from Tocris, Ellisville, MO). Whole cell patch-clamp recordings were performed from visualized Purkinje cells under differential interference contrast/infrared microscopy with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Recordings were discarded when series or input resistance values changed by >20%. The mGluR1–EPSCs were recorded using an internal saline containing the following (in mM): 125 K-gluconate, 10 MgCl2, 0.1 CaCl2, 1 EGTA, 10 HEPES, 4 Na2ATP, and 2 QX-314 (pH adjusted to 7.3). QX-314 was obtained from Tocris. In the course of these experiments, we also used ethanol (190 proof; Sigma) and the mGluR1a antagonist LY367385 (see earlier text; obtained from Tocris). Extracellular stimulation was performed using a concentric bipolar electrode at intensities in the range of 0.04–2 mA. Data were acquired and analyzed with pClamp 9.2 (Molecular Devices). Statistical analysis was performed with GraphPad Prizm 4. For statistical analysis, we used the two-way ANOVA test, followed by the Bonferroni post hoc test, and the paired Student's t-test, when appropriate.
RESULTS
To examine whether ethanol impairs PF synaptic transmission, we performed whole cell patch-clamp recordings from Purkinje cells in rat cerebellar slices. Test responses to PF stimulation were monitored in voltage-clamp mode. Bath application of ethanol (50 mM) had no significant effect on the amplitude of PF–EPSCs (94.5 ± 5.7% of baseline ± SE; t = 25–30 min; n = 6; P > 0.05; paired Student's t-test; Fig. 1A). Likewise, the paired-pulse facilitation (PPF) ratio was unaffected by bath-application of 50 mM ethanol (103.5 ± 3.8%; t = 10 min after EtOH washin; n = 5; P > 0.05; paired Student's t-test; Fig. 1B), suggesting that transmitter release was not impaired. Ethanol (50 mM) also did not affect EPSC trains evoked by 100-Hz PF burst stimulation (n = 6; Fig. 1C; this type of stimulation was used to evoke mGluR1–EPSCs as well, as subsequently described). Application of 10 stimuli at 100 Hz resulted in an increase in the EPSC amplitude over the course of stimulation (Fig. 1C, top). The amplitudes of these EPSCs were unaffected by ethanol (P > 0.05; Fig. 1C, bottom left). This absence of ethanol effects was observed for all 10 EPSCs within a train (Fig. 1C, bottom right). To ensure that these EPSC trains were mediated by AMPA receptor activation, recordings were performed in the presence of the GABAA receptor antagonist SR95531 (10 μM) and the mGluR1 receptor antagonist LY367385 (100 μM) in the bath, respectively. PF–EPSCs were blocked when the competitive AMPA/kainate receptor antagonist NBQX (10 μM) and the noncompetitive AMPA receptor antagonist GYKI 53655 (50 μM) were applied in the bath (Fig. 1C, top right), confirming that these PF responses are non-NMDA receptor-mediated. These findings show that non-NMDA receptor-mediated PF synaptic transmission is insensitive to ethanol (50 mM). A concentration of 50 mM ethanol produces marked motor function alterations in nontolerant individuals (for comparison, the legal blood alcohol level in the United States is <0.08 g/dl = 17.4 mM, and in most countries of the European Union the legal concentration is <0.05 g/dl = 10.6 mM).
The observation that ethanol does not affect AMPA receptor function at PF synapses does not exclude the possibility that PF–LTD can be ethanol impaired. We therefore applied the PF–LTD induction protocol when ethanol (50 mM) was present in the bath throughout the recordings. Test responses were monitored in voltage-clamp mode before and after tetanization. Under control conditions, PF–LTD was observed after paired PF + CF stimulation at 1 Hz for 5 min in current-clamp mode (83.3 ± 5.3%, t = 30–35 min; n = 9; P < 0.05; paired Student's t-test; Fig. 2A). In contrast, LTD induction was blocked in the presence of ethanol in the bath (103.9 ± 4.9%; t = 30–35 min; n = 10; P > 0.05; paired Student's t-test; Fig. 2A). This difference in the efficacy to induce LTD reached statistical significance (Mann–Whitney U test; P < 0.05). These data show that ethanol can impair PF–LTD induction. To examine whether ethanol application also affects PF–LTP induction, we first measured LTP under control conditions. PF stimulation alone at 1 Hz for 5 min evoked a potentiation of PF–EPSCs (130.9 ± 6.8%; t = 30–35 min; n = 16; P < 0.01; paired Student's t-test; Fig. 2B). When ethanol (50 mM) was present in the bath, LTP was unaffected (128.8 ± 5.7%; t = 30–35 min; n = 15; P < 0.01; paired Student's t-test; Fig. 2B). Thus EtOH did not significantly affect the LTP induction probability (Mann–Whitney U test; P > 0.05). These data suggest that ethanol specifically impairs LTD, but not LTP induction.
We next attempted to determine a molecular ethanol target, whose interference with ethanol could lead to a selective inhibition of LTD, but not LTP, induction. Obvious candidate targets are AMPA receptors, but these are unlikely affected because basic PF synaptic transmission, which is AMPA receptor mediated, was not down-regulated by ethanol (Fig. 1). We have previously shown that both LTD and LTP induction are calcium dependent, but that a larger calcium transient is required for LTD than for LTP induction (Coesmans et al. 2004). A major source of calcium influx is provided by voltage-gated calcium channels. We therefore performed voltage-clamp recordings of calcium currents to examine whether these are impaired by ethanol in cerebellar Purkinje cells. To obtain sufficient voltage control, we performed these recordings in P5–P6 Purkinje cells. (It is acknowledged that future work should establish whether voltage-gated calcium channels in more mature Purkinje cells have a similar sensitivity to ethanol.) Voltage-gated Na+ currents were blocked by adding TTX (1 μM) to the bath solution and by adding QX-314 (5 mM) to the pipette saline. We used a cesium-based internal saline (100 mM CsMeSO4) and added TEA (20 mM) to block a wide range of K+ conductances. Under these conditions, currents activated by depolarizing voltage steps from a holding potential of −80 mV are mediated by voltage-dependent calcium currents. Bath-application of ethanol (50 mM) reduced the amplitudes of these currents (for voltage steps to −10 mV: 81.9 ± 3.3%; t = 10–14 min; n = 9; P < 0.01; Fig. 3A). This effect was seen at voltage steps that would maximally activate calcium currents (steps to −10, 0, +10, and +20 mV; Fig. 3B). Bath-application of 20 mM ethanol did not impair voltage-dependent calcium currents (96.6 ± 1.8%, n = 6; P > 0.05; Fig. 3), suggesting that a moderately high ethanol concentration is needed to block calcium conductances.
It was recently shown that ethanol reduces mGluR1–EPSCs evoked by CF stimulation and blocks CF–LTD (Carta et al. 2006). To examine whether PF-evoked mGluR1–EPSCs are affected as well, we triggered these events by trains of 10 PF stimuli delivered at 100 Hz every 30 s (Fig. 4). Fast inhibitory and excitatory transmission was blocked by adding the GABAA receptor antagonist SR95531 (10 μM) to the bath as well as the AMPA/kainate receptor antagonist NBQX (10 μM) and the AMPA receptor antagonist GYKI 53655 (50 μM). NBQX and GYKI 53655 were used in combination to ensure complete blockade of AMPA/kainate receptors during high-frequency stimulation. The stimulus intensity was adjusted to produce mGluR1–EPSCs whose amplitude reached 40–50% of the maximum. These mGluR1–EPSCs are triggered by mGluR1a receptors, in that they could be blocked by bath-application of the mGluR1a antagonist LY367386 (n = 2; data not shown). Under control conditions, the mGluR1–EPSCs gradually decreased over time (78.7 ± 3.5%; t = 12.5 min; n = 8; P < 0.01; Fig. 4, A and B), in agreement with previous reports (Tempia et al. 1998). At a low concentration of 20 mM, ethanol did not significantly affect mGluR1–EPSCs compared with the control condition (80.2 ± 3.2%; t = 12.5 min; n = 4; two-way ANOVA followed by Bonferroni post hoc test; P > 0.05; Fig. 4B). In contrast, at concentrations of 50 and 80 mM, mGluR1–EPSCs were strongly attenuated compared with those recorded under control conditions (50 mM: 30.2 ± 12.0%; n = 4; P < 0.01; 80 mM: 32.6 ± 19.2%; n = 4; P < 0.05; Fig. 4B; two-way ANOVA followed by Bonferroni's post hoc test). It should be noted that in these recordings, the reduction of mGluR1–EPSC amplitudes persisted after ethanol washout. This observation points toward a lasting physiological impact on mGluR1-mediated signaling. These data suggest that ethanol reduces mGluR1–EPSCs and identify signaling pathways dependent on PF activation of mGluR1 as potential ethanol targets.
Slow EPSCs mediated by mGluR1 receptors can also be evoked by puff application of the group I mGluR agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) to Purkinje cell dendrites. To examine whether these DHPG–EPSCs are affected by ethanol, we located the glass puffing pipette containing DHPG (100 μM; in ACSF) in the distal part of the dendritic arbor of Purkinje cells. These recordings were performed in the presence of the AMPA/kainate receptor antagonist NBQX (10 μM) and the AMPA receptor antagonist GYKI 53655 (50 μM), the GABAA receptor antagonist SR95531 (10 μM), and the Na+ channel blocker TTX (500 nM). Puff application of DHPG resulted in slow EPSCs (Fig. 5A), whose amplitude was reduced in the presence of 50 mM ethanol (73.4 ± 7.5%; n = 8; t = 10–15 min; P < 0.01; control: 100.4 ± 8.0%; n = 6; P > 0.05; Fig. 5B). These results show that not only synaptically evoked mGluR1–EPSCs but also slow EPSCs evoked by DHPG puff application are reduced in the presence of ethanol.
As reported earlier, ethanol inhibits mGluR1-activated EPSCs (Figs. 4 and 5), and impairs LTD, but not LTP, induction (Fig. 2). A prediction derived from these observations is that LTP induction is not mGluR1 dependent. Indeed, LTP could be induced by PF stimulation at 1 Hz for 5 min when the group I mGluR antagonist AIDA (1 mM) was applied in the bath (130.0 ± 9.9%; t = 25–30 min; n = 6; P < 0.05; Fig. 6A). In control experiments, AIDA (1 mM) did not change PF–EPSC amplitudes (97.3 ± 5.1%; n = 3; data not shown). Likewise, LTP could be induced in the presence of the mGluR1a antagonist LY367385 (100 μM) in the bath (128.8 ± 4.9%; t = 25–30 min; n = 4; P < 0.05; Fig. 6B). In control experiments, LY367385 (100 μM) did not alter PF–EPSC amplitudes (96.3 ± 3.6%; n = 3; data not shown). These findings demonstrate that, in contrast to LTD, PF–LTP induction is mGluR1 independent and thus provide an explanation as to why LTP was not impaired in the presence of 50 mM ethanol.
DISCUSSION
The main finding of this study is that ethanol selectively impairs PF–LTD, but not PF–LTP, induction. Moreover, we show that ethanol acutely inhibits voltage-gated calcium currents as well as slow mGluR1–EPSCs, which can be triggered by synaptic activation or by puff application of the group I mGluR agonist DHPG. Previous studies have demonstrated ethanol effects on mGluR agonist-evoked spike patterns and calcium transients (Netzeband and Gruol 1995; Netzeband et al. 2002). Moreover, ethanol has been shown to reduce CF-evoked mGluR1–EPSCs (Carta et al. 2006). These studies added mGluR1 receptor-dependent signaling pathways to the list of molecular ethanol targets. However, it has not been examined thus far how acute ethanol application affects metabotropic signaling and plasticity at PF synapses onto Purkinje cells. The observed ethanol-mediated reduction of mGluR1–EPSCs could explain why ethanol impairs LTD induction: mGluR1 activation has been shown to be required for PF–LTD induction (Aiba et al. 1994; Conquet et al. 1994; Linden et al. 1991), and signaling events downstream of mGluR1 activation, including production of inositol-1,4,5-trisphosphate (IP3), IP3-mediated calcium signaling (Miyata et al. 2000), and protein kinase C (PKC) activation (De Zeeuw et al. 1998) are crucially involved in PF–LTD induction as well (for review, see Hansel and Bear 2008). The induction requirements for PF–LTP are less well characterized, but it has been shown that LTP induction depends on dendritic calcium transients, which are smaller than those required for LTD induction (Coesmans et al. 2004). Moreover, PF–LTP induction depends on the activation of protein phosphatases 1, 2A, and 2B (Belmeguenai and Hansel 2005). Here, we demonstrate that mGluR1 receptors are not involved in LTP induction, which might explain why LTP is not ethanol sensitive. Likewise, the higher calcium threshold for LTD induction (Coesmans et al. 2004) explains why the reduction of voltage-gated calcium current amplitudes by ethanol affects LTD more strongly than it affects LTP.
In this study, we took advantage of the fact that in Purkinje cells slow metabotropic currents provide an electrophysiological measure that allows us to monitor mGluR1 function. These currents are activated by glutamate binding to mGluR1 receptors (or by mGluR agonists, such as DHPG), but are mediated by C-type transient receptor potential canonical (TRPC) cation channels. Both TRPC1 (Kim et al. 2003) and TRPC3 channels (Hartmann et al. 2008) have been suggested to mediate mGluR1–EPSCs (but see Canepari et al. 2004). Next to the activation of the phospholipase Cβ/1,2-diacylglycerol/IP3 pathway, the activation of these nonspecific cation channels provides a second G protein-coupled signaling mechanism triggered by group I mGluR receptors. The two Gαq-dependent signaling limbs can be functionally coupled by the adaptor protein Homer, which physically links IP3 receptors to TRPC1 channels, providing a cellular basis for capacitative calcium influx after depletion of intracellular calcium stores (Yuan et al. 2003; but see Hartmann et al. 2008). Whether such a crosslink between IP3 receptors and TRPC1 channels occurs in Purkinje cells remains to be determined. It is conceivable that ethanol targets TRPC channels (or another protein that is attached to the mGluR1/TRPC complex), rather than mGluR1 itself. The consequences for LTD induction might well be the same because slow mGluR1-activated potentials can enhance dendritic calcium transients (Yuan et al. 2007) and, by doing so, might facilitate LTD induction (Coesmans et al. 2004). It certainly is conceivable that ethanol affects a signaling factor downstream of mGluR1 receptor activation. This is suggested by the finding that the function of recombinant mGluR1 receptors expressed in Xenopus oocytes is unaffected by ethanol (Minami et al. 1998). Nevertheless, it is possible that in Purkinje cells the crucial ethanol-sensitive signal required for LTD induction is a sufficient activation of the mGluR1/Gαq/PLC signaling cascade. In this scenario, the slow TRPC-mediated current would merely provide a noncausal correlate of mGluR1 activation.
Ethanol is known to affect a large number of neuronal signaling processes, including GABAergic and glutamatergic transmission as well as voltage-gated ion channels (for review, see Lovinger 1997; Walter and Messing 1999; Woodward 1999). It is therefore unlikely that mGluR1 receptors and voltage-gated calcium channels are the only ethanol targets that are relevant for LTD induction and cerebellar circuit function. Other candidate targets include (among others) GABAergic transmission (Carta et al. 2004; Hanchar et al. 2005; Kelm et al. 2007; Mameli et al. 2008); NMDA receptors, which are involved in a presynaptic form of PF–LTP (Qiu and Knöpfel 2007) and were recently described in mature Purkinje cells (Piochon et al. 2007; Renzi et al. 2007); and the γ-isoform of PKC, which controls voltage-gated calcium currents (Servais et al. 2007). All of these signaling factors can potentially adjust the amplitude of dendritic calcium transients, which are crucially involved in the regulation of bidirectional plasticity at PF synapses (Coesmans et al. 2004; Jörntell and Hansel 2006). This multitude of potential ethanol targets makes it likely that ethanol impairs LTD by affecting other signaling factors in addition to mGluR1 receptors and voltage-gated calcium channels.
Despite these cautionary notes on the limited specificity of ethanol effects, mGluR1-dependent signaling pathways provide an ethanol target that fits our current understanding of cerebellar synaptic plasticity and the control of motor coordination. In mGluR1 mutant mice, PF–LTD, motor coordination, and motor learning are impaired (Aiba et al. 1994; Conquet et al. 1994). Moreover, in particular forms of paraneoplastic cerebellar ataxia (PCA), patients show severe motor coordination deficits that have been attributed to the generation of autoantibodies against mGluR1 receptors (Sillevis Smitt et al. 2000). Application of mGluR1 autoantibodies purified from the serum of patients with Hodgkin's disease (which can develop PCA) to mouse cerebellar slices causes a blockade of PF–LTD as well as a reduction in simple spike firing (Coesmans et al. 2002). These observations suggest that mGluR1-triggered signaling cascades are crucial for cerebellar circuit function and motor coordination, thus resembling the role of mGluR1 receptors in trace eyelid conditioning, which is a form of associative learning mediated by the hippocampus (Gil-Sanz et al. 2007).
It has recently been demonstrated that in fetal alcohol syndrome (FAS), which is characterized by ataxia and motor learning deficits, prenatal ethanol exposure results in a reduction of voltage-gated calcium currents because of reduced expression levels of PKCγ (Servais et al. 2007). Moreover, in slices prepared from mice that were prenatally exposed to ethanol, application of a PF–LTD protocol rather resulted in LTP, thus resembling our observations in slices acutely exposed to ethanol. Although it is difficult to compare chronic and acute effects of ethanol exposure, it is apparent that ethanol impairs signaling cascades required for LTD induction (mGluR1 receptors and voltage-dependent calcium currents) and, consequently, affects bidirectional PF plasticity. Alterations in synaptic plasticity mechanisms could certainly cause motor coordination deficits. This assumption is the basis of Albus-Ito-style models of cerebellar function, which describe PF–LTD as a key correlate of cerebellar motor learning. However, this view has been challenged (Welsh et al. 2005). In vivo studies indeed show that motor coordination deficits are frequently associated not only with alterations in synaptic plasticity, but also in spike firing properties. FAS mice, for example, show enhanced firing rates of Purkinje cells in vivo (Servais et al. 2007), which may or may not be a direct consequence of a higher probability for LTP induction, but on their own could affect motor coordination. Interestingly, chronic ethanol exposure in adult mice also results in motor coordination impairment, but rather causes reduced spike firing rates, which are not associated with an impaired ability to learn or to recall a motor coordination task (Servais et al. 2005). Chronic alcohol exposure could well trigger compensatory mechanisms restoring cerebellar plasticity and learning, but these observations regardless suggest that alterations in spike firing rates alone do not inevitably result in motor learning deficits.
In the present in vitro study, we characterized ethanol effects on mGluR1 signaling, voltage-dependent calcium currents, and synaptic plasticity. We show that at an ethanol concentration of 50 mM, mGluR1–EPSCs and calcium currents are reduced and PF–LTD is affected. This ethanol concentration produces marked ataxia and is well within a range relevant for the study of acute effects of alcohol consumption. However, it needs to be pointed out that ethanol starts to impair motor coordination at lower doses (the legal blood alcohol threshold in the United States is 0.08 g/dl = 17.4 mM). It therefore seems likely that other factors affecting cerebellar function contribute to intoxication (for instance, alterations in GABAergic transmission), but that nevertheless ethanol effects on synaptic plasticity play a key role in the motor coordination and motor learning deficits that are typical for acute alcohol consumption.
GRANTS
This work was supported by Netherlands Organization for Scientific Research (NWO) Grant NWO-VENI to A. Belmeguenai; NWO-ZonMW and De Stichting ter Bevordering van Volkskracht en de Stichting Volksbond grants to C. I. De Zeeuw; a Reinout Pfeiffer Foundation grant to J. T. Weber and C. I. De Zeeuw; National Institute on Alcohol Abuse and Alcoholism Grant AA-14973 to C. F. Valenzuela; and NWO-VIDI Grant to C. Hansel.
Acknowledgments
We thank members of the Valenzuela and Hansel laboratories for helpful comments on the manuscript.
Present addresses: J. T. Weber, School of Pharmacy, Memorial University of Newfoundland, St. John's, Newfoundland, Canada; M. Carta, Bordeaux Neuroscience Institute, University of Bordeaux, Bordeaux, France.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aiba et al. 1994.Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79: 377–388, 1994. [PubMed] [Google Scholar]
- Belmeguenai and Hansel 2005.Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci 25: 10768–10772, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta et al. 2007a.Botta P, Mameli M, Floyd KL, Radcliffe RA, Valenzuela CF. Ethanol sensitivity of GABAergic currents in cerebellar granule neurons is not increased by a single amino acid change (R100Q) in the α6 GABAA receptor subunit. J Pharm Exp Ther 323: 684–691, 2007a. [DOI] [PubMed] [Google Scholar]
- Botta et al. 2007b.Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol 41: 187–199, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust 2002.Brust JC Neurologic complications of substance abuse. J Acquir Immune Defic Syndr Hum Retrovirol 31: S29–S34, 2002. [DOI] [PubMed] [Google Scholar]
- Canepari et al. 2004.Canepari M, Auger C, Ogden D. Ca2+ ion permeability and single-channel properties of the metabotropic slow EPSC of rat Purkinje neurons. J Neurosci 24: 3563–3573, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta et al. 2004.Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci 24: 3746–3751, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta et al. 2006.Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber–Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci 26: 1906–1912, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu 1983.Chu NS Effects of ethanol on rat cerebellar Purkinje cells. Int J Neurosci 21: 265–277, 1983. [DOI] [PubMed] [Google Scholar]
- Coesmans et al. 2002.Coesmans M, Sillevis Smitt PA, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, van Alphen AM, Luo C, van der Geest JN, Kros JM, Gaillard CA, Frens MA, De Zeeuw CI. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol 53: 325–336, 2002. [DOI] [PubMed] [Google Scholar]
- Coesmans et al. 2004.Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44: 691–700, 2004. [DOI] [PubMed] [Google Scholar]
- Conquet et al. 1994.Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372: 237–243, 1994. [DOI] [PubMed] [Google Scholar]
- De Zeeuw et al. 1998.De Zeeuw CI, Hansel C, Bian F, Koekkoek SKE, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20: 495–508, 1998. [DOI] [PubMed] [Google Scholar]
- Dzubay and Otis 2002.Dzubay JA, Otis TS. Climbing fiber activation of metabotropic glutamate receptors on cerebellar Purkinje neurons. Neuron 36: 1159–1167, 2002. [DOI] [PubMed] [Google Scholar]
- Gil-Sanz et al. 2007.Gil-Sanz C, Delgado-Garcia JM, Fairen A, Gruart A. Involvement of the mGluR1 receptor in hippocampal synaptic plasticity and associative learning in behaving mice. Cereb Cortex 18: 1653–1663, 2007. [DOI] [PubMed] [Google Scholar]
- Hanchar et al. 2005.Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci 8: 339–345, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel and Bear 2008.Hansel C, Bear MF. LTD—synaptic depression and memory storage. In: Learning and Memory: A Comprehensive Reference, Molecular Mechanisms of Memory, edited by Byrne JH. Oxford, UK: Elsevier/Academic, 2008, vol. 4, p. 327–366. [Google Scholar]
- Hartmann et al. 2008.Hartmann J, Dragivic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59: 392–398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi et al. 2005.Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136: 509–517, 2005. [DOI] [PubMed] [Google Scholar]
- Jörntell and Hansel 2006.Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber–Purkinje cell synapses. Neuron 52: 227–238, 2006. [DOI] [PubMed] [Google Scholar]
- Kelm et al. 2007.Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous γ-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther 323: 356–364, 2007. [DOI] [PubMed] [Google Scholar]
- Kim et al. 2003.Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426: 285–291, 2003. [DOI] [PubMed] [Google Scholar]
- Linden et al. 1991.Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron 7: 81–89, 1991. [DOI] [PubMed] [Google Scholar]
- Lovinger 1997.Lovinger DM Alcohols and neurotransmitter gated ion channels: past, present and furture. Naunyn-Schmiedeberg's Arch Pharmacol 356: 267–282, 1997. [DOI] [PubMed] [Google Scholar]
- Lovinger et al. 1989.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724, 1989. [DOI] [PubMed] [Google Scholar]
- Mameli et al. 1998.Mameli M, Botta P, Zamudio PA, Zucca P, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther (August 28, 2008). doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed]
- Minami et al. 1998.Minami K, Gereau RW, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther 53: 148–156, 1998. [DOI] [PubMed] [Google Scholar]
- Miyata et al. 2000.Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28: 233–244, 2000. [DOI] [PubMed] [Google Scholar]
- Morrisett and Swartzwelder 1993.Morrisett RA, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci 13: 2264–2272, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzeband and Gruol 1995.Netzeband JG, Gruol DL. Modulatory effects of acute ethanol on metabotropic glutamate responses in cultured Purkinje neurons. Brain Res 688: 105–113, 1995. [DOI] [PubMed] [Google Scholar]
- Netzeband et al. 2002.Netzeband JG, Schneeloch JR, Trotter C, Caguioa-Aquino JN, Gruol DL. Chronic ethanol treatment and withdrawal alter ACPD-evoked calcium signals in developing Purkinje neurons. Alcoholism 26: 386–393, 2002. [PubMed] [Google Scholar]
- Piochon et al. 2007.Piochon C, Irinopoulou T, Brusciano D, Bailly Y, Mariani J, Levenes C. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J Neurosci 27: 10797–10809, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu and Knöpfel 2007.Qiu D, Knöpfel T. An NMDA receptor/nitric oxide cascade in presynaptic parallel fiber–Purkinje neuron long-term potentiation. J Neurosci 27: 3408–3415, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzi et al. 2007.Renzi M, Farrant M, Cull-Candy SG. Climbing-fibre activation of NMDA receptors in Purkinje cells of adult mice. J Physiol 585: 91–101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais et al. 2005.Servais L, Bearzatto B, Delvaux V, Noel E, Leach R, Brasseur M, Schiffmann SN, Cheron G. Effect of chronic ethanol ingestion on Purkinje and Golgi cell firing in vivo and on motor coordination in mice. Brain Res 1055: 171–179, 2005. [DOI] [PubMed] [Google Scholar]
- Servais et al. 2007.Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci USA 104: 9858–9863, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillevis Smitt et al. 2000.Sillevis Smitt PA, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, Henzen-Logmans S, Vecht C, De Zeeuw CI, Sekiyama N, Nakanishi S, Shigemoto R. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 342: 21–27, 2000. [DOI] [PubMed] [Google Scholar]
- Tempia et al. 1998.Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol 80: 520–528, 1998. [DOI] [PubMed] [Google Scholar]
- Urrutia and Gruol 1992.Urrutia A, Gruol DL. Acute alcohol alters the excitability of cerebellar Purkinje neurons and hippocampal neurons in culture. Brain Res 569: 26–37, 1992. [DOI] [PubMed] [Google Scholar]
- Walter and Messing 1999.Walter HJ, Messing RO. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem Int 35: 95–101, 1999. [DOI] [PubMed] [Google Scholar]
- Welsh et al. 2005.Welsh JP, Yamaguchi H, Zeng XH, Kojo M, Nakada Y, Takagi A, Sugimori M, Llinás RR. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc Natl Acad Sci USA 102: 17166–17171, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward 1999.Woodward JJ Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem Int 35: 107–113, 1999. [PubMed] [Google Scholar]
- Yin et al. 2007.Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci 25: 3226–3232, 2007. [DOI] [PubMed] [Google Scholar]
- Yuan et al. 2003.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley P. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114: 777–789, 2003. [DOI] [PubMed] [Google Scholar]
- Yuan et al. 2007.Yuan Q, Qiu DL, Weber JT, Hansel C, Knöpfel T. Climbing fiber-triggered metabotropic slow potentials enhance dendritic calcium transients and simple spike firing in cerebellar Purkinje cells. Mol Cell Neurosci 35: 596–603, 2007. [DOI] [PubMed] [Google Scholar]