Abstract
Calcium imaging of bulk-loaded fluorescent indicators can be used to record the spiking activity of hundreds of neurons. Recent advances promise imaging technologies that are faster, more efficient, and applicable to awake animals, thereby moving imaging capabilities closer to the traditional strengths of multi-electrode arrays. This article reviews these technical achievements and discusses how they can help us achieve the goal of understanding neural circuits.
INTRODUCTION
Activity in a single neuron results from the flow of charged ions and is inherently electrical by nature. Electrophysiology, the measurement of these electrical currents, provides great insights to neural systems. In simpler neural circuits such as the stomatogastric ganglion in crustaceans, multiple intracellular electrodes can be used to record membrane potentials from all of the identified component cells in the network. From these recordings, knowledge of the various synaptic connections, ionic currents, and activity patterns can then be combined to generate a basic understanding of the circuit operating principles. However, scaling this approach to more complex neural systems is limited by the number of recordings possible, as constrained by the patience of the experimenter and the available table space for micromanipulators.
One method to circumvent this limitation is multi-electrode arrays (Buzsáki 2004), which use an array of closely spaced electrodes to record spiking activity from many neurons at once. When applied to animal models, multi-electrode arrays have been useful in obtaining insights about the firing patterns of neural circuits that underlie different types of behaviors. Recently another technique, calcium imaging of tissues bulk-loaded with membrane-permeable fluorescent indicators (Stosiek et al. 2003), has emerged which also enables spiking activity to be recorded from multiple neurons. This technique was received with much excitement because imaging is compatible with other optical methods and can be used on transgenic animal models that express fluorescent markers. In the last year, significant progress (Dombeck et al. 2007; Duemani Reddy et al. 2008; Holekamp et al. 2008) has been made in improving the optical and experimental setup (Fig. 1), such that the capability of population calcium imaging is approaching some of the traditional strengths of multi-electrode arrays. Here I will discuss these technical advances in the context of using population calcium imaging as a tool to understand neural circuits.
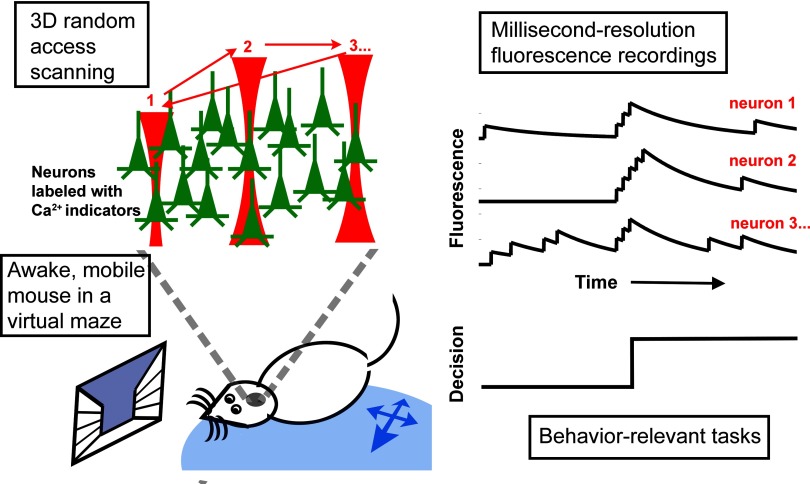
FIG. 1.
An ideal population calcium imaging experiment for studying neural circuits. This diagram illustrates a calcium imaging experiment made possible by recent advances: recording spiking activity from neuronal networks in an awake mouse with single-cell spatial and millisecond temporal resolutions. Left: two enabling technical advances. First, a 3-dimensional (3-D) random access method is used to steer the laser beam efficiently to points of interests. By eliminating the slow mechanical scanning mirrors, calcium imaging can now be done at selected locations with millisecond resolution. Second, a head-fixed, trained mouse is comfortable while its feet are grounded on a free-floating ball. When incorporated into a microscope, the “mouse-on-a-ball” setup permits imaging in awake, mobile mice with minimal movement artifacts. Data from such experiments (right) will provide insights for understanding neural circuits including ones that underlie decision-making tasks.
Calcium imaging of neuronal populations
Currently, the most widely used method for population calcium imaging was introduced by Arthur Konnerth's laboratory (Stosiek et al. 2003). In their approach, acetoxymethyl (AM)-ester calcium indicator dyes (popular choices are calcium green-1 AM and Oregon green 488-1 AM) are ejected into the tissue sample when an outward pressure is applied. The AM-ester dyes are membrane permeable; they enter the cells and remain inside the cell after an intracellular esterase cleaves the ester. Using this bulk-labeling method, Stosiek et al. (2003) were able to stably label hundreds of cortical cells within a ∼400-μm-diameter region for many hours. Kerr et al. (2005) subsequently showed that fluorescence changes recorded from bulk-labeled neurons are direct results of calcium influx from action potentials. To demonstrate this tight relationship, they patched bulk-labeled pyramidal neurons in a cell-attached configuration and were able to show that 97% of single action potentials can be reliably detected by using fluorescence. With past studies in which calcium indicators have been introduced by other methods (e.g., whole cell patching, bath incubation, etc.), this result confirms that fluorescence reports suprathreshold neuronal activity. Thus with many labeled cells, stable fluorescence, and direct correlation with action potentials, population calcium imaging fulfills the basic requirements to be a useful technique for monitoring the spiking activity of neuronal populations.
Imaging of the bulk-labeled tissue is usually done with two-photon microscopy (Denk et al. 1990; Zipfel et al. 2003). Here a femtosecond pulsed laser provides infrared photons that are focused onto the fluorescent sample. When two infrared photons are absorbed nearly simultaneously, their combined energy is enough to excite a fluorophore and produce a fluorescence photon. The uncertainty principle tells us that “nearly simultaneous” is <1 femtosecond, so two-photon absorption is a rare event except at the focus of the objective lens, where there are the most number of infrared photons. Knowing that the fluorescence must come from the focal volume is important, because then all detected fluorescence photons, scattered or not, can contribute to a useful signal. This is the key reason why in scattering tissues such as the brain, two-photon microscopy has been the de facto instrument of choice.
However, despite its unique capability for deep-tissue imaging, most two-photon microscopes are relatively slow and bulky. Attempts to create miniature two-photon microscopes have compromised on spatial resolution or field of view, which limits their practical utility for small animal studies. Furthermore, to generate an image, the laser beam is raster-scanned by a pair of mechanical galvanometers that typically oscillate at <2 kHz. Scanning in the z direction is usually done by moving the microscope objective, which is even slower. Imaging at this speed will miss the millisecond-scale events in the brain, for example the rising and falling of single action potentials.
Imaging from a new perspective
How can we scan faster while maintaining the deep-tissue advantage of two-photon microscopy? Duemani Reddy et al. (2008) offer an efficient solution for scanning a three-dimensional (3-D) volume: send the laser beam only to the interesting regions, such as the soma or the dendrites, and waste no time on other regions. To implement this idea, they chose not to rely on mechanical galvanometers and motorized microscope objectives, which are slow because mirrors and objective lenses are relatively heavy. Instead, the laser beam is steered using acoustic-optical deflectors (AODs). Re-positioning can be fast (∼10 μs, depending on size of AOD), allowing the beam to be moved to multiple spots quickly and not necessarily in sequential order. An exciting development in this work is that by steering with a series of four AODs driven by custom waveforms, Duemani Reddy et al. are now able to randomly access spots in a 3-D volume, whereas previous implementations were limited to two dimensions.
The AOD method is tantalizing because although it may not be faster than galvanometers, it is more efficient. Scanning only the points of interest allows more pixel dwell time to collect fluorescence and improve signal-to-noise. To test their system, Duemani Reddy et al. imaged sub-micron fluorescent beads to measure the point spread function, a key parameter of spatial resolution for a microscope. While the resolution was not flattering (∼0.5 × 2.3 μm at focus, which is ∼3 times the expected size in an optimal two-photon microscope) (see Zipfel et al. 2003), they found only slightly worse resolution when scanning the z axis with AODs and were able to resolve dendritic spines. Fast functional imaging was demonstrated in acute brain slices. On a single calcium indicator-filled CA1 pyramidal neuron, they initially determined several dendritic locations from an image stack of the neuron, then return to those points of interest to measure calcium transients. The current implementation has some remaining technical problems. For example, the z range is only 50 μm, and the pulse tends to broaden to picosecond duration. Both of these shortcomings can be improved with better or additional optical elements. The real question is whether the added optical complexity can be justified by the new capability; I believe the answer is a resounding yes, especially for certain applications such as uncaging or imaging membrane potentials.
To tackle the same problem on imaging speed, Holekamp et al. came up with a different solution (Holekamp et al. 2008). They knew that wide-field illumination can excite dyes across the entire field of view, and fluorescence can be collected with a fast CCD detector. This is the most basic form of microscopy, but it is not suited to tissue imaging because it does not provide optical sections, so cells at different axial locations will overlap. To overcome this problem, Holekamp et al. separated the excitation and detection paths. For excitation, instead of a microscope objective, they used illumination optics that include a cylindrical lens to generate a light sheet that excites only a thin layer of cells. Fluorescence is then collected by an orthogonally placed CCD detector that is coupled in movement with the illumination optics. Finally, the microscope was set up at a 45° angle such that both excitation and detection paths enter from the same surface, enabling the system to be used for preparations such as cranial windows.
This method, termed objective-coupled planar illumination microscopy, provides optical sections with a specific thickness contingent on the light sheet, which is ∼5 μm thick. For population calcium imaging, this thickness matches with the typical size of a neuronal cell body, so one section would contain no more than a monolayer of cells. The CCD detector captures images at hundreds of frames per second, so when the technique was demonstrated in the mouse accessory olfactory system, population calcium transients were clean and distinct. One concern for this technique is that in scattering tissues, the light sheet illumination may not be uniform. In fact, Holekamp et al. pointed out the consequences of this nonuniformity: imaging depth is limited to ∼150 μm and deep-lying cells tend to be imaged at reduced spatial resolution. For neural systems with certain geometries, such as the epithelium-like cell layer demonstrated here, this approach improves imaging speed 100-fold without losing the sectioning capability to observe single cells.
Bridging the gap with behavioral studies
A significant improvement in in vivo experimental preparation was presented in a convincing paper by Dombeck et al. (2007). Many in vivo two-photon microscopy studies have been performed on anesthetized animals because microscopes are not portable and miniaturization compromises the spatial resolution or field of view. To extend imaging to awake animals, Dombeck et al. took inspiration from insect experiments where one part of the body is fixed, for example by a tiny glob of glue, but the rest of the insect is unrestrained. In their scheme, a mouse is head-fixed to the microscope but its feet are grounded on an air-supported floating styrofoam ball. The ball acts as a spherical treadmill where the mouse can rest or run. Most importantly, awake mice seem at ease in this head-fixed, ball-supporting setup after only a short adaptation session.
Using this “mouse-on-a-ball” setup, Dombeck et al. were able to monitor the movement of the mouse while doing population calcium imaging through a cranial window. The system was remarkably stable such that they observed lateral displacements of <5 μm and minimal axial displacement. Moreover, spatial resolution was higher than miniature two-photon microscopes because tabletop optics were used. As a result, this work opens up exciting, new lines of experiments where behavior of awake, mobile mice can be studied in conjunction with population calcium imaging of neural circuits. For instance, one could imagine a virtual reality display set up around the mouse to simulate a maze to evoke neuronal activity responsible for decision-making and spatial learning.
Comparison with multi-electrode arrays
The combination of these technical advances is bringing population calcium imaging to almost the same level as the traditional strengths of multi-electrode arrays (Buzsáki 2004). The temporal resolution of imaging, demonstrated by using AODs on selected areas or by using objective-coupled planar illumination, is in the millisecond time scale. Behavior studies, which have been the forte of multi-electrode array experiments, can now be simulated by a virtual reality, mobile mouse setup. In addition, a distinct advantage of optical imaging is its compatibility with transgenic models that express fluorescent markers for genetically identifying cells. However, repeated population calcium imaging sessions, the equivalent to chronic electrode recordings, has not yet been demonstrated. Moreover, AM-ester calcium indicator dyes label predominantly the cell bodies, so measured fluorescence changes reflect calcium influx in the soma, but not in the neurites.
An interesting comparison can be made between population calcium imaging and two-dimensional planar electrode arrays (Hutzler et al. 2006). In planar arrays, neuronal cultures or organotypic slices are grown on a grid of microfabricated transistors, which permits recording from multiple cells. The spatial resolution, defined by the number of elements in the array, can be reduced to single-cell sizes for a millimeter-wide field. Also possible (but not demonstrated by Hutzler et al. 2006) is the ability to use the same recording transistors for stimulation, allowing exquisite control at single-cell level. This dual ability to stimulate and record can be a powerful tool for dissecting neural circuits by enabling perturbation of one element at a time. Single-cell stimulation is not yet possible in population calcium imaging, although there is hope with the rapid progress of light-gated ion channels.
Lost in the translation: going from electrons to calcium
With these new imaging tools, are we any closer to understanding neural circuits? Missing in many imaging-only experiments is complementary knowledge of the circuit, including the intrinsic properties of each neuron type and the synaptic connections between them. By going from recording membrane potential to imaging calcium, only the suprathreshold activities are measured. With additional analysis, it is possible to recover characteristics such as the firing rate from fluorescence traces with a very good signal-to-noise ratio (Yaksi and Friedrich 2006). Nonetheless it is not yet possible to use fluorescence to distinguish between such neuronal firing mechanisms as plateau potentials and postinhibitory rebound, which have been shown to be essential for understanding simpler, invertebrate neural circuits.
Part of the difficulty in going from firing patterns to circuit operation is also the lack of connectivity information. One can imagine the possibility of partially recovering some information on neuronal firing mechanisms from fluorescence data if we know how each neuron is connected to another. Detailed connectivity information includes synapse locations and dendritic branching patterns, which can currently be obtained from several neurons by 3-D neuron tracing or from fixed tissues by electron microscopy. But if the detailed connectivity is unknown, as is the case for neural circuits with more than tens of cells in live tissues, then it is an intractable problem to figure out how the neural circuit computes to generate the changing firing patterns that we observe.
New optical tools come to the rescue
Major technical advances have made population calcium imaging an excellent tool for looking at a neural circuit's firing pattern, which is likely to be computationally relevant. This type of imaging is also one of the very few ways to observe a circuit and all of its components in action at single-cell resolution. On the other hand, to truly understand neural circuits, more information about subthreshold activity and connectivity is needed. This challenge highlights, again, one key advantage of population calcium imaging, which is its compatibility with other optical techniques. Emerging methods, such as two-photon uncaging for mapping functional connectivity (Matsuzaki et al. 2008) or development of better voltage-sensitive fluorescent dyes (Kuhn et al. 2008), are promising a future where we can measure multiple aspects of a neural circuit at the same time.
GRANTS
A. C. Kwan is supported by the Nanobiotechnology Center, an STC Program of the National Science Foundation under Agreement ECS-9876771, and National Institutes of Health Grant 9-P41-EB001976.
Acknowledgments
Thanks to R. Harris-Warrick, R. Williams, and W. Zipfel for comments and to M. Williams for editorial assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Buzsáki 2004.Buzsáki G Large-scale recording of neuronal ensembles. Nat Neurosci 7: 446–451, 2004. [DOI] [PubMed] [Google Scholar]
- Denk et al. 1990.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science 248: 73–76, 1990. [DOI] [PubMed] [Google Scholar]
- Dombeck et al. 2007.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake mobile mice. Neuron 56: 43–57, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duemani Reddy et al. 2008.Duemani Reddy G, Kelleher K, Fink R, Saggau P. Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity. Nat Neurosci 11: 713–720, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp et al. 2008.Holekamp TF, Turaga D, Holy TE. Fast three-dimensional fluorescence imaging of activity in neural populations by objective-coupled planar illumination microscopy. Neuron 57: 661–672, 2008. [DOI] [PubMed] [Google Scholar]
- Hutzler et al. 2006.Hutzler M, Lambacher A, Eversmann B, Jenkner M, Thewes R, Fromherz P. High-resolution multitransistor array recording of electrical field potentials in cultured brain slices. J Neurophysiol 96: 1638–1645, 2006. [DOI] [PubMed] [Google Scholar]
- Kerr et al. 2005.Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci USA 102: 14063–14068, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn et al. 2008.Kuhn B, Denk W, Bruno RM. In vivo two-photon voltage-sensitive dye imaging reveals top-down control of cortical layers 1 and 2 during wakefulness. Proc Natl Acad Sci USA 105: 7588–7593, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki et al. 2008.Matsuzaki M, Ellis-Davies GC, Kasai H. Three-dimensional mapping of unitary synaptic connections by two-photon macro photolysis of caged glutamate. J Neurophysiol 99: 1535–1544, 2008. [DOI] [PubMed] [Google Scholar]
- Stosiek et al. 2003.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: 7319–7324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksi and Friedrich 2006.Yaksi E, Friedrich RW. Reconstruction of firing rate changes across neuronal populations by temporally deconvolved Ca2+ imaging. Nat Methods 3: 377–383, 2006. [DOI] [PubMed] [Google Scholar]
- Zipfel et al. 2003.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21: 1369–1377, 2003. [DOI] [PubMed] [Google Scholar]