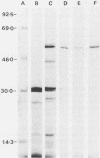
Abstract
A recombinant lambda bacteriophage has been isolated that carries DNA from Streptococcus pneumoniae and expresses a potent hemolysin that has been shown to be pneumolysin, the sulfhydryl-activated toxin of the pneumococcus. Hemolytic activity is inhibited by cholesterol and neutralized by serum against streptolysin O. The cloned gene expresses two polypeptides (Mrs, 56,000 and 53,000) in an Escherichia coli in vitro transcription-translation system, and both are precipitated by the addition of anti-alveolysin serum and anti-streptolysin O serum in the presence of Staphylococcus aureus cells. Expression of pneumolysin occurs when the gene is cloned in both possible orientations in pUC8. The DNA sequence of a 5-kilobase ClaI fragment that carries the pneumolysin gene has been determined. An open reading frame was identified that encodes a polypeptide of 471 amino acids that is hydrophobic in character and has an N-terminal amino acid sequence which is identical to that deduced from amino acid sequencing of the purified protein. The predicted amino acid sequence of the polypeptide reveals a single cysteine residue located 44 residues from the C terminus. Putative promoter and ribosome binding sites have been identified 5' to the pneumolysin coding sequence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Alouf J. E. Interaction of alveolysin A sulfhydryl-activated bacterial cytolytic toxin with thiol group reagents and cholesterol. Toxicon. 1982;20(1):239–241. doi: 10.1016/0041-0101(82)90208-2. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Alouf J. E. Selective purification by thiol-disulfide interchange chromatography of alveolysin, a sulfhydryl-activated toxin of Bacillus alvei. Toxin properties and interaction with cholesterol and liposomes. J Biol Chem. 1983 Aug 25;258(16):9968–9972. [PubMed] [Google Scholar]
- Geoffroy C., Gilles A. M., Alouf J. E. The sulfhydryl groups of the thiol-dependent cytolytic toxin from Bacillus alvei evidence for one essential sulfhydryl group. Biochem Biophys Res Commun. 1981 Apr 15;99(3):781–788. doi: 10.1016/0006-291x(81)91233-x. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Geoffroy C., Alouf J. E. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun. 1980 Jan;27(1):97–101. doi: 10.1128/iai.27.1.97-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Knight R. J., Drew G. K. The hydrophobic character of thiol-activated cytolysins. Biochem J. 1982 Dec 1;207(3):557–560. doi: 10.1042/bj2070557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Timmis K. N. Cloning and expression in Escherichia coli of the streptolysin O determinant from Streptococcus pyogenes: characterization of the cloned streptolysin O determinant and demonstration of the absence of substantial homology with determinants of other thiol-activated toxins. Infect Immun. 1984 Mar;43(3):804–810. doi: 10.1128/iai.43.3.804-810.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984 Apr;28(1):45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Paton J. C., Berry A. M., Lock R. A., Hansman D., Manning P. A. Cloning and expression in Escherichia coli of the Streptococcus pneumoniae gene encoding pneumolysin. Infect Immun. 1986 Oct;54(1):50–55. doi: 10.1128/iai.54.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J. M., Boulnois G. J., Darby V., Orr E., Wahle E., Holland I. B. Identification of gene products programmed by restriction endonuclease DNA fragments using an E. coli in vitro system. Nucleic Acids Res. 1981 Sep 25;9(18):4459–4474. doi: 10.1093/nar/9.18.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yamakawa Y., Ito A., Sato H. Theta-toxin of Clostridium perfringens. I. Purification and some properties. Biochim Biophys Acta. 1977 Oct 26;494(2):301–313. doi: 10.1016/0005-2795(77)90159-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]