Abstract
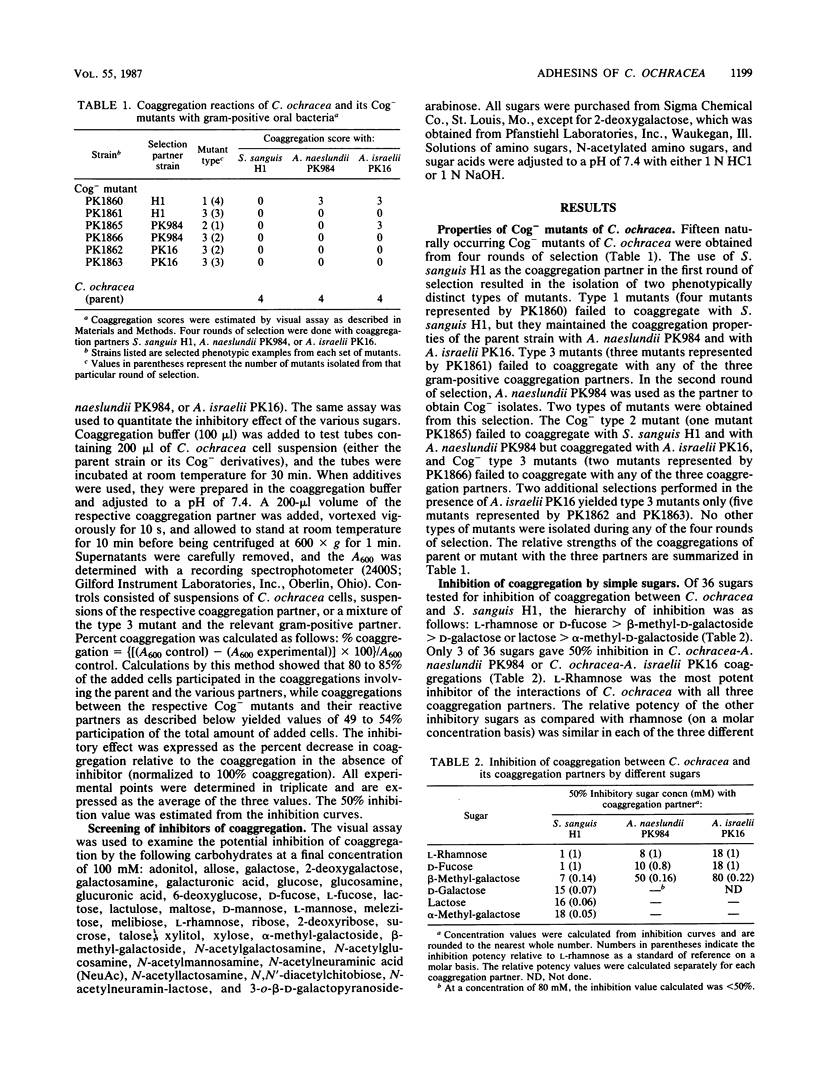
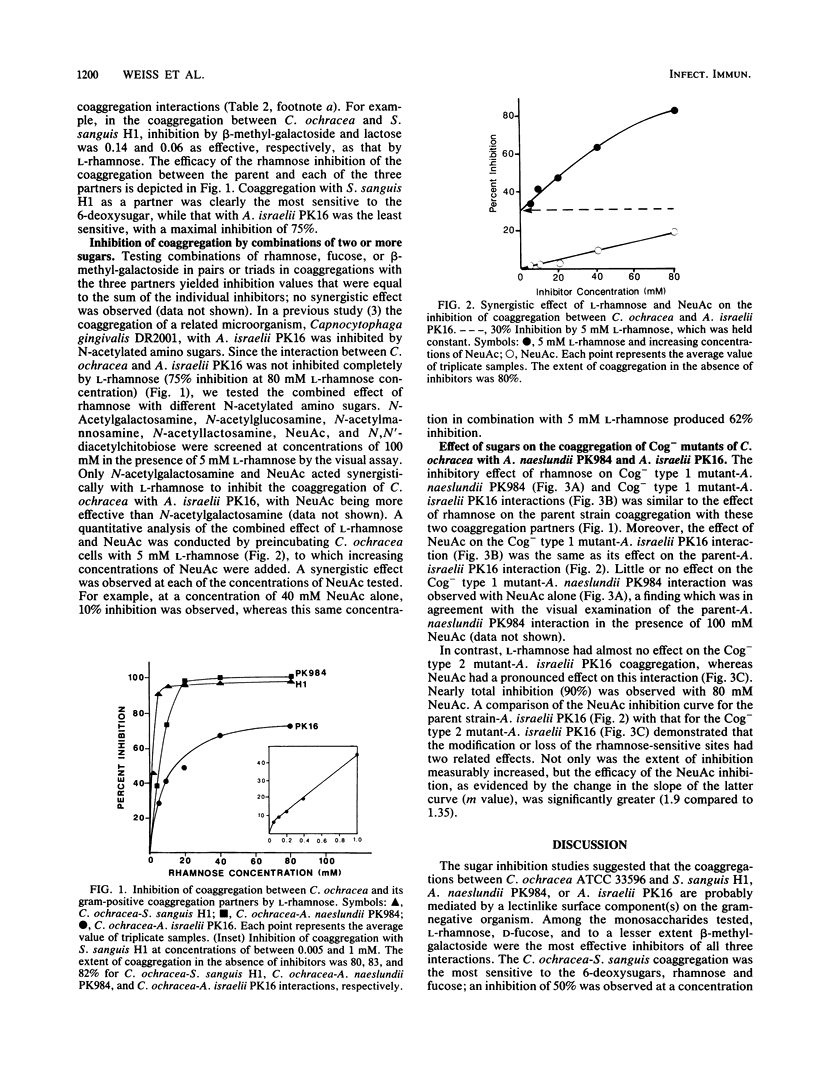
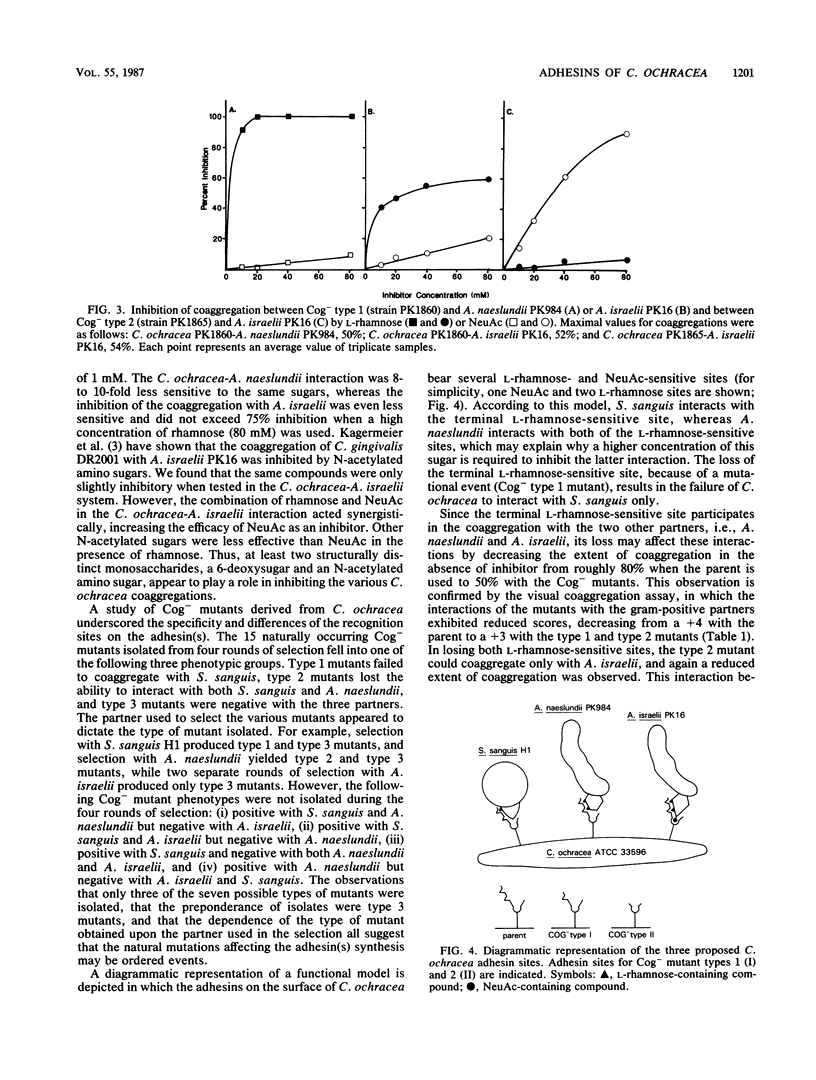
The interactions between Capnocytophaga ochracea ATCC 33596 and Streptococcus sanguis H1, Actinomyces naeslundii PK984, or Actinomyces israelii PK16 are dependent on specific recognitions between heat-sensitive adhesins on C. ochracea and heat-stable structures (probably carbohydrate-containing receptors) on the surfaces of these gram-positive coaggregation partners. The coaggregation of C. ochracea with each of these three organisms was inhibited by L-rhamnose and D-fucose and to a lesser extent by beta-methyl-galactoside. The reaction with S. sanguis was the most sensitive, while the coaggregation with A. israelii was the least sensitive and was only partially inhibited by each of the sugars that were considered to be effective inhibitors. A more effective inhibition of the coaggregation between C. ochracea and A. israelii was achieved by adding a combination of the 6-deoxysugars and N-acetylneuraminic acid. To further characterize the coaggregations, naturally occurring coaggregation-defective (Cog-) mutants of C. ochracea were obtained from several different selections. Three phenotypically distinct groups of mutants were were isolated. Type 1 mutants failed to coaggregate with S. sanguis only. Type 2 mutants lost ability to interact with both S. sanguis and A. naeslundii. Type 3 mutants failed to coaggregate with all three coaggregation partners. Characterization of the Cog- mutants by sugar inhibition studies made it possible to distinguish three classes of adhesin activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Kagermeier A. S., London J., Kolenbrander P. E. Evidence for the participation of N-acetylated amino sugars in the coaggregation between Cytophaga species strain DR2001 and Actinomyces israelii PK16. Infect Immun. 1984 May;44(2):299–305. doi: 10.1128/iai.44.2.299-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Celesk R. A. Coaggregation of human oral Cytophaga species and Actinomyces israelii. Infect Immun. 1983 Jun;40(3):1178–1185. doi: 10.1128/iai.40.3.1178-1185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Isolation and characterization of coaggregation-defective mutants of Actinomyces viscosus, Actinomyces naeslundii, and Streptococcus sanguis. Infect Immun. 1982 Sep;37(3):1200–1208. doi: 10.1128/iai.37.3.1200-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Prevalence of viridans streptococci exhibiting lactose-inhibitable coaggregation with oral actinomycetes. Infect Immun. 1983 Aug;41(2):449–452. doi: 10.1128/iai.41.2.449-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



