Abstract
Because the response of cancer cells to chemotherapeutic agents depends upon the supporting extracellular matrix (ECM), the response in vivo may not be reproduced in 2-dimensional cell culture. The dose-response to curcumin and two derivatives by bladder cancer cells grown on both normal (SISgel) and cancer-derived ECM (Matrigel) and on plastic were contrasted. Cells grown on Matrigel were resistant to curcumins, but cells growing on SISgel, which mimic cancer cells suppressed by normal ECM, were nearly as sensitive as cells grown on plastic. SV40-immortalized urothelial cells, which are models for premalignant cells, were the most sensitive, but even aggressive cell lines were nearly as sensitive when grown on SISgel as on plastic. Curcumin response depends highly on the supporting ECM, and cells grown on plastic poorly models cells growing on natural ECM. Curcumin could prove an effective chemopreventive for bladder cancer recurrence when administered intravesically post-therapy.
Keywords: Bladder cancer, curcumin, chemoprevention
Introduction
Bladder cancer is the fifth most common cancer with 60,000 new cases per year in the US and 336,000 worldwide, resulting in 13,000 deaths per year in the US and 132,000 worldwide (1;11;20). The clinical problem with bladder cancer is the high recurrence rate, and although 75-85% of bladder cancers are initially superficial and noninvasive, as many as 70% of these patients will experience a recurrence within 5 years, and of those 20-30% will experience progression to invasive disease (1). The survival statistics of invasive cancer are 50% at 2 years, but the 5-year survival with metastatic bladder cancer is only 6% (1). Although immunotherapy with attenuated tuberculosis bacteria (BCG) shows efficacy, it nonetheless has appreciable toxicity and adverse reactions are common (1). Other, less toxic alternatives for preventing recurrences would be desirable.
The spice curcumin has significant antiviral, anti-inflammatory, and anti-cancer activities (17). Curcumin has been shown to induce apoptosis in bladder cancer cells cultured on plastic (19) and to prevent implantation of bladder cancer cells in vivo when administered intravesically (16). In a small clinical trial of oral curcumin, doses of 8 g per day showed no toxicity, and improvement in 1 of 2 patients was noted in spite of not finding detectable levels of curcumin or metabolites in urine (3). However, most agents are less active against cells in tissues than against cancer cells growing on plastic (7;12;18), and the degree to which results obtained with cells cultured on plastic can be extrapolated clinically is not clear. In vivo, cancer cells must escape the regulatory controls of normal matrix, and the evidence is strong that cancer cells suppressed by normal matrix are responsible for recurrences and delayed metastasis (6). In order to determine whether curcumin would be effective against cancer cells growing on an extracellular matrix and to develop a more realistic pre-clinical model of curcumin’s anticancer activity, we investigated the dose-response of curcumin and two derivatives against several bladder cell lines growing on both a cancer-modulated extracellular matrix as well as a normal extracellular matrix. We tested two cell lines that are non-tumorigenic (HUC and UROtsa) as well as four cancer cell lines that vary in the degree of aggressiveness from papillomas (RT4) to cells that are derived from undifferentiated and invasive carcinomas (J82, TCC-Sup). Growth on Matrigel, a cancer-modulated basement membrane preparation, provided a tumormimetic model in which the cells recapitulated the phenotype reported in the patient from which the tumor cells were derived (8). Cells from invasive tumors invaded the Matrigel whereas those derived from papillary tumors grew papillary structures (8). In contrast, the malignant phenotype was suppressed when the cells were grown on a gel produced from small intestine submucosa (SIS), a normal extracellular matrix (8). The findings of this study suggest that intravesical curcumin might be an effective therapy in prevention of bladder cancer recurrence.
Materials and Methods
Cell Culture
HUC, RT4, TCC-SUP, 5637, and J82 cells were obtained from American Type Culture Collection, Bethesda MD, USA. UROtsa cells were obtained from Dr. Donald Sens (15). The HUC cell line was immortalized with SV-40 and is non-tumorigenic, RT4 cells were derived from a papilloma, 5637 cells were from an invasive grade II tumor, J82 cells were from an anaplastic tumor, and TCC-Sup cells were from a metastatic tumor. All tissue culture media and supplements were from Invitrogen (Rockville, MD). Matrigel was obtained from Becton-Dickinson (Bedford, MA). SIS gel was obtained from Cook Biotech (W. Lafayette, IN) and prepared by mixing 5 ml of SISgel, 5 ml of 0.025 N HCl, and 1.25 ml 10x PBS, followed by adjustment of pH to 7.4 with 1 M NaOH. The three-dimensional cultures were conducted according to protocols published previously (8;13). Briefly, 50 μl of ice cold Matrigel or pH-adjusted SISgel was added to Costar 3610 white, clear bottom 96-well plates (Corning, Corning, NY) and the liquid was allowed to gel at 37°C. Cells were trypsinized with 1 ml 0.25% trypsin/1 mM EDTA, and 30,000 cells/50 μl were added to each well of the 96 well plate. For cultures of cells on plastic, 2 × 106 cells were added to each T-75 flask.
Assay for Curcumin Activity
One day after seeding, cells were treated with various concentrations of curcumin, 4MC, or 4HC for 48 h in medium. Synthetic curcumin and analogues were provided by Professor Zhong-Li Liu of the National Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, China. The purities of Curcumin, 4HC and 4MC were 99.7%, 99.3% and 99%, respectively. A marker of cell proliferation using the substrate 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) cleaved to fluorescein by non-specific cellular esterases was used to assess the response to curcumin (9). Briefly, media was removed from the cells and 25μM CFDA-AM (Molecular Probes, Eugene, OR) in PBS was added for 2 hours at 37°C. Plates were then read by a BIOCHEMI digital darkroom (UV Products, Upland, CA) using 304 nm excitation, 430 nm broadband emission filters.
DAPI stain
The media was removed from the wells and cells were fixed with 50 μl of 2.5 % glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) for 30 minutes at room temperature. The cells were then rinsed with PBS, incubated with 50 ng/ml DAPI in PBS for 30 minutes at room temperature, and rinsed again with PBS. Cells were analyzed by fluorescence microscopy using a Nikon Eclipse inverted microscope TE2000-U. Images were collected using Lucia camera software. Apoptotic cells were identified as densely stained granular nuclear bodies (5).
Results
Chemosensitivity of bladder cell lines cultured on plastic, Matrigel, and SISgel
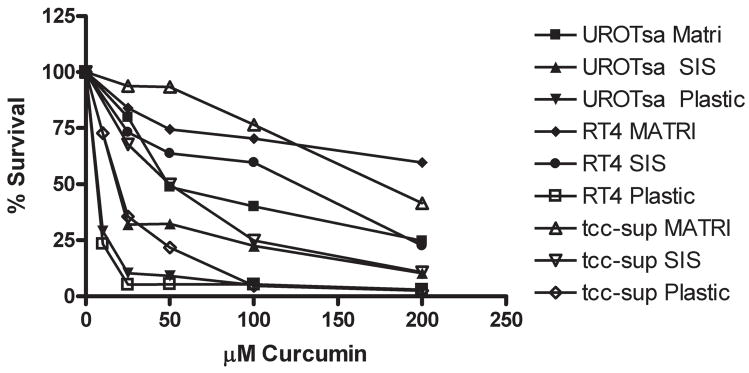
An example of the dose-response data showing sensitivity of urothelial cells to curcumin induced cytoxicity is shown in Figure 1, complete IC50 data are shown in Table I. As shown in Table I, treatment of cells on plastic with curcumin resulted in an IC50 of 4-21 μM, demonstrating that the sensitivity of all of the cell lines was roughly equivalent. All of the six urothelial cell lines tested were most sensitive to treatment with curcumin, 4MC, and 4HC when they were cultured on plastic. All cell lines were less sensitive when cultured on SISgel; treatment of cells cultured on SISgel with curcumin resulted in an IC50 between 10-87 μM. When cells were cultured on SISgel or plastic, as the aggressiveness of the tumor cells increased, they usually became more resistant to killing by curcumin and the analogs. Cells grown on Matrigel exhibited the highest resistance to curcumin, with IC50 values of 25 to > 400 μM. The efficacy of curcumin was independent of aggressiveness for cells cultured on Matrigel. In comparing the efficacy of the curcumin derivatives, 4MC was much less active. The 4HC derivative was roughly as active as curcumin, but was significantly more effective than curcumin in killing HUC, RT4, 5637, and J82 cells cultured on Matrigel.
Figure 1. Effect of growth environment on curcumin-induced cytotoxicity.
UROtsa, RT4, or TCC-Sup cells were cultured on plastic, SISgel, or Matrigel and treated with variable doses of curcumin for 48 hours then CFDA-AM was added and fluorescence was measured after a 2 hour incubation. Data are plotted as percent control of the CFDA-AM conversion to fluorescein of untreated cells.
Table I.
Effects of curcumin, 4MC, and 4HC on the viability of urothelial cells cultured on Matrigel, SISgel, or plastic
| IC50 (μM) | |||
|---|---|---|---|
| Curcumin | MATRI | SIS | PLASTIC |
| UROTSA | 64.2 ± 1.1 | 10.3 ± 1.4 | 3.9 ± 1.2 |
| HUC | >400 | 24.6 ± 1.1 | 7.8 ± 1.1 |
| RT4 | 243.4 ± 1.1 | 87.0 ± 1.1 | 3.5 ± 1.3 |
| 5637 | 234.4 ± 1.1 | 38.2 ± 1.1 | 10.5 ± 1 |
| J82 | 115.5 ± 1 | 22.9 ± 1.3 | 20.5 ± 1.1 |
| TCCSup | 171.6 ± 1 | 46.0 ± 1.1 | 18.3 ± 1.1 |
| 4MC | MATRI | SIS | PLASTIC |
| UROTSA | 237.1 ± 1 | 9.1 ± 1.4 | 1.2 ± 2.6 |
| HUC | >400 | 7.9 ± 1.4 | 7.3 ± 1 |
| RT4 | >400 | 134.4 ± 1.1 | 20.8 ± 1.2 |
| 5637 | >400 | 41.5 ± 1.1 | 10.5 ± 1.8 |
| J82 | >400 | 30.4 ± 1.2 | 21.2 ± 1.7 |
| TCCSup | >400 | 85.7 ± 1.1 | 15.3 ± 1.2 |
| 4HC | MATRI | SIS | PLASTIC |
| UROTSA | 84.3 ± 1.1 | 8.3 ± 1.4 | 6.9 ± 1.1 |
| HUC | 171.2 ± 1.1 | 2.8 ± 2 | 1.6 ± 1.4 |
| RT4 | 102.5 ± 1 | 63.6 ± 1.1 | 10.6 ± 1 |
| 5637 | 106.8 ± 1.1 | 29.1 ± 1.1 | 7.6 ± 1 |
| J82 | 155.3 ± 1 | 22.4 ± 1.2 | 12.8 ± 1.1 |
| TCCSup | 164.7 ± 1.1 | 46.5 ± 1 | 21.4 ± 1.2 |
Curcumin induces apoptosis of bladder cell lines
To determine if curcumin and its derivatives killed cells by apoptosis in all matrices, J82 cells cultured on SISgel were treated with curcumin, 4MC, and 4HC. Only the cells that were treated with curcumin, 4MC, or 4HC exhibited features typically observed in apoptotic cells (Figure 2 b-d), such as nuclear fragmentation, while no signs of apoptosis were detected in the untreated control cells (2 a).
Figure 2. Nuclear fragmentation is observed when J82 cells are cultured on SISgel and treated with curcumin, 4MC, or 4 HC.
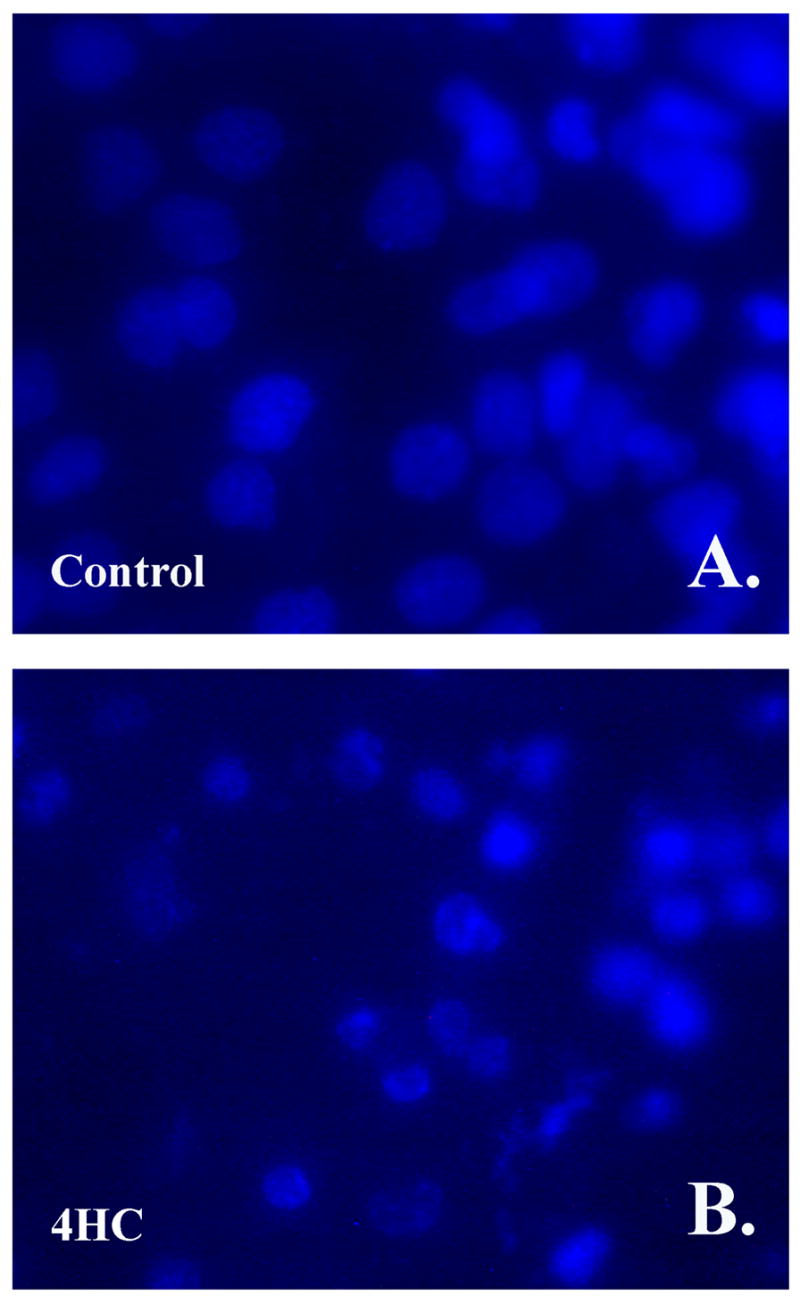
J82 cells were cultured on SISgel and were left untreated for Control (A), or were treated with 100 μM of 4HC for 48 hours (B), then the cells were fixed and the nucleus was stained with DAPI as described in Materials and Methods. Similar results were observed for 4MC and curcumin (data not shown).
Discussion
The high rate of recurrence of bladder cancer may be attributed to the several factors, including failure to detect all of the lesions with cystoscopy (4), or the possibility that the microenvironment may suppress cancer cells in the normal appearing regions of the bladder, where the scar microenvironment can promote them to emerge at a later time (6). Also, earlier findings of abnormal biomarkers in histopathologically normal urothelium that was located up to 10 cm away from tumor (14) suggests that the phenotypic suppression seen in vitro (8) occurs in vivo as well. In that study, over 30% of the cases showed abnormalities in EGFR and Her2/neu expression, and 20% of cases showed abnormal ploidy. The main therapy for prevention of recurrence is BCG, but the toxicity is high, and many patients are accordingly lost to follow-up or are forced to abandon the therapy (1;10).
Our findings here suggest that curcumin may provide an alternative or at least a supplement for prevention of recurrence. Although curcumin was effective against bladder cancer cells of all grades and immortalized bladder cells growing on plastic, it was not particularly effective against the aggressive lines growing on Matrigel, where they express their full malignant phenotype. Curcumin therefore is unlikely to be effective as a chemotherapeutic agent. However, curcumin was quite effective against suppressed cancer cells and immortalized urothelial cells growing on normal extracellular matrix. This suggests it would be effective in a chemotherapeutic mode against precisely those cells that evade BCG. That it was effective against SV40-immortalized urothelial cells is particularly encouraging because such cells likely represent a model for premalignant cells.
Curcumin is generally considered to be non-toxic (3;17). It induces apoptosis in target cells (19), which we confirmed occurs as well in cells growing on either Matrigel or SISgel. Of the forms tested, curcumin and 4HC were slightly more effective than the MC derivative. Curcumin is poorly absorbed and none appears in urine even after large oral doses (3) and will therefore likely need to be administered intravesically. In a mouse model, intravesically administered curcumin showed little evidence of toxicity to the normal bladder but was effective in preventing implantation of tumor cells (16). We therefore suggest that curcumin administered intravesically could be tested as a chemopreventive for bladder cancer recurrence following transurethral resection or BCG therapy. Because bladder cancer is the most expensive cancer to manage from diagnosis to death of the patient from any cause (2), alternatives to current approaches are needed.
Acknowledgments
The UROtsa cells were a generous gift of Dr. Donald Sens. This work was supported in part by grants from the NIH (DK 069808) (to REH), Stellar Pharmaceuticals (to REH), and by a Jasren Foundation Grant (#JF-05-2 BR) (to ZH).
Reference List
- 1.Amling CL. Diagnosis and management of superficial bladder cancer. Curr Probl Cancer. 2001;25:219–278. doi: 10.1067/mcn.2001.117539. [DOI] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Research. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 4.Grossman HB, Soloway M, Messing E, Katz G, Stein B, Kassabian V, Shen Y. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295:299–305. doi: 10.1001/jama.295.3.299. [DOI] [PubMed] [Google Scholar]
- 5.Han Z, Li G, Bremner TA, Lange TS, Zhang G, Jemmerson R, Wyche JH, Hendrickson EA. A cytosolic factor is required for mitochondrial cytochrome c efflux during apoptosis. Cell Death Differ. 1998;5:469–479. doi: 10.1038/sj.cdd.4400367. [DOI] [PubMed] [Google Scholar]
- 6.Hockel M, Dornhofer N. The hydra phenomenon of cancer: why tumors recur locally after microscopically complete resection. Cancer Research. 2005;65:2997–3002. doi: 10.1158/0008-5472.CAN-04-3868. [DOI] [PubMed] [Google Scholar]
- 7.Hurst RE, Kamat CD, Kyker KD, Green DE, Ihnat MA. A novel multidrug resistance phenotype of bladder tumor cells grown on Matrigel or SIS gel. Cancer Letters. 2005;217:171–180. doi: 10.1016/j.canlet.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Hurst RE, Kyker KD, Bonner RB, Bowditch RG, Hemstreet GP. Matrix-Dependent Plasticity of the Malignant Phenotype of Bladder Cancer Cells. Anticancer Research. 2003;23:3119–3128. [PMC free article] [PubMed] [Google Scholar]
- 9.Ihnat MA, Lariviere JP, Warren AJ, La Ronde N, Blaxall JR, Pierre KM, Turpie BW, Hamilton JW. Suppression of P-glycoprotein expression and multidrug resistance by DNA cross-linking agents. Clinical Cancer Research. 1997;3:1339–1346. [PubMed] [Google Scholar]
- 10.Jakse G, Hall R, Bono A, Holtl W, Carpentier P, Spaander JP, van der Meijden AP, Sylvester R. Intravesical BCG in patients with carcinoma in situ of the urinary bladder: long-term results of EORTC GU Group phase II protocol 30861. European Urology. 2001;40:144–150. doi: 10.1159/000049765. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA: A Cancer Journal for Clinicians. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H, Man S, Graham CH, Kapitain SJ, Teicher BA, Kerbel RS. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3294–3298. doi: 10.1073/pnas.90.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyker KD, Culkin DJ, Hurst RE. A model for 3-dimensional growth of bladder cancers to study mechanisms of phenotypic expression. Urologic Oncology. 2003;21:255–261. doi: 10.1016/s1078-1439(02)00279-x. [DOI] [PubMed] [Google Scholar]
- 14.Rao JY, Hemstreet GP, Hurst RE, Bonner RB, Jones PL, Min KW, Fradet Y. Alterations in phenotypic biochemical markers in bladder epithelium during tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8287–8291. doi: 10.1073/pnas.90.17.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi MR, Masters JR, Park S, Todd JH, Garrett SH, Sens MA, Somji S, Nath J, Sens DA. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect. 2001;109:801–808. doi: 10.1289/ehp.01109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sindhwani P, Hampton JA, Baig MM, Keck R, Selman SH. Curcumin prevents intravesical tumor implantation of the MBT-2 tumor cell line in C3H mice. Journal of Urology. 2001;166:1498–1501. [PubMed] [Google Scholar]
- 17.Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 18.Teicher BA, Maehara Y, Kakeji Y, Ara G, Keyes SR, Wong J, Herbst R. Reversal of in vivo drug resistance by the transforming growth factor-β inhibitor decorin. International Journal of Cancer. 1997;71:49–58. doi: 10.1002/(sici)1097-0215(19970328)71:1<49::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Tong QS, Zheng LD, Lu P, Jiang FC, Chen FM, Zeng FQ, Wang L, Dong JH. Apoptosis-inducing effects of curcumin derivatives in human bladder cancer cells. Anticancer Drugs. 2006;17:279–287. doi: 10.1097/00001813-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]