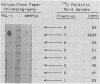
Abstract
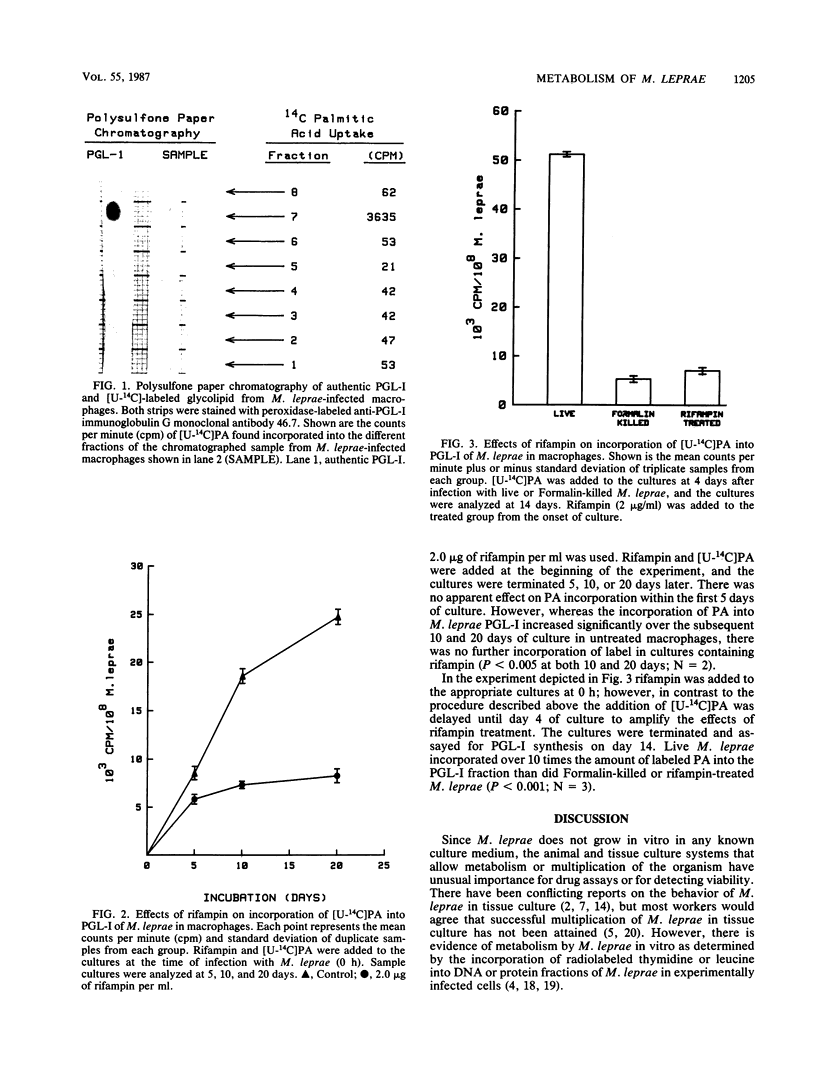
The incorporation of 14C-labeled palmitic acid ( [U-14C]PA) into the phenolic glycolipid-I (PGL-I) fraction of Mycobacterium leprae was studied in a murine macrophage system in vitro. Peritoneal macrophages from Swiss Webster mice were infected with fresh viable or Formalin-killed M. leprae harvested from infected footpads of nu/nu mice, and [U-14C]PA was added to the culture medium. Labeled glycolipid synthesized by live M. leprae was fractionated on a Florisil-silicic acid column and identified as PGL-I by using thin-layer chromatography and localization on a polysulfone membrane with an anti-PGL-I monoclonal antibody. Increased incorporation of [U-14C]PA into the PGL-I fraction was observed in macrophages infected with only live M. leprae. Treatment of the infected macrophages with rifampin caused a significant reduction in the incorporation of palmitic acid into PGL-I. These preliminary studies suggest that PGL-I synthesis can be used to quantitate the metabolism of M. leprae in macrophages in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arioli V., Pallanza R., Furesz S., Carniti G. Rifampicin: a new rifamycin. I. Bacteriological studies. Arzneimittelforschung. 1967 May;17(5):523–529. [PubMed] [Google Scholar]
- Brennan P. J., Barrow W. W. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1980 Dec;48(4):382–387. [PubMed] [Google Scholar]
- Chang Y. T., Neikirk R. L. Mycobacterium lepraemurium and Mycobacterium leprae in cultures of mouse peritoneal macrophages (preliminary results). Int J Lepr. 1965 Jul-Sep;33(3 Suppl):586–603. [PubMed] [Google Scholar]
- Cho S. N., Hunter S. W., Gelber R. H., Rea T. H., Brennan P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J Infect Dis. 1986 Mar;153(3):560–569. doi: 10.1093/infdis/153.3.560. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Cline M. J. Incorporation of tritiated thymidine by leprosy bacilli in cultures of human lepromatous macrophages. J Infect Dis. 1972 Apr;125(4):416–419. doi: 10.1093/infdis/125.4.416. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Garbutt E. W. Studies on M. lepraemurium and M. leprae in tissue culture. Int J Lepr. 1965 Jul-Sep;33(3 Suppl):578–585. [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Hunter S. W., Stewart C., Brennan P. J. Purification of phenolic glycolipid I from armadillo and human sources. Int J Lepr Other Mycobact Dis. 1985 Sep;53(3):484–486. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. The role of activated macrophages in specific and nonspecific cytostasis of tumor cells. J Immunol. 1974 Aug;113(2):507–516. [PubMed] [Google Scholar]
- Levy L. Studies of the mouse foot pad technique for cultivation of Mycobacterium leprae. 3. Doubling time during logarithmic multiplication. Lepr Rev. 1976 Jun;47(2):103–106. doi: 10.5935/0305-7518.19760019. [DOI] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Prasad H. K., Hastings R. C. Alternate radiolabeled markers for detecting metabolic activity of Mycobacterium leprae residing in murine macrophages. J Clin Microbiol. 1985 May;21(5):861–864. doi: 10.1128/jcm.21.5.861-864.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish M., Nath I. The uptake of 3H-thymidine in Mycobacterium leprae inoculated mouse macrophage cultures as a rapid indicator of bacillary viability. Factors influencing the specificity of the in vitro assay. Int J Lepr Other Mycobact Dis. 1981 Jun;49(2):187–193. [PubMed] [Google Scholar]
- Sharp A. K., Banerjee D. K. Attempts at cultivation of Mycobacterium leprae in macrophages from susceptible animal hosts. Int J Lepr Other Mycobact Dis. 1984 Jun;52(2):189–197. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Young D. B., Fohn M. J., Buchanan T. M. Use of a polysulfone membrane support for immunochemical analysis of a glycolipid from Mycobacterium leprae. J Immunol Methods. 1985 May 23;79(2):205–211. doi: 10.1016/0022-1759(85)90100-0. [DOI] [PubMed] [Google Scholar]
- Young D. B., Harnisch J. P., Knight J., Buchanan T. M. Detection of phenolic glycolipid I in sera from patients with lepromatous leprosy. J Infect Dis. 1985 Nov;152(5):1078–1081. doi: 10.1093/infdis/152.5.1078. [DOI] [PubMed] [Google Scholar]