Abstract
Many birds and mammals show changes in the hypothalamo-pituitary-gonadal (HPG) axis in response to social or sexual interactions between breeding partners. While alterations in GnRH neuronal activity play an important role in stimulating these changes, it remains unclear if acute behaviorally-induced alterations in GnRH release are accompanied by parallel changes in GnRH synthesis. To investigate this relationship, we examined changes in the activity of GnRH neurons in the brains of male ring doves following brief periods of courtship interactions with females. Such interactions have been previously shown to increase plasma LH in courting male doves at 24 h, but not at 1 h, after pairing with females. In the first study, males allowed to court females for 2 h had 60% more cells that showed immunocytochemical labeling for GnRH-I in the preoptic area (POA) of the hypothalamus than did control males that remained isolated from females. To determine whether an increase in GnRH gene expression preceded this increase in GnRH immunoreactivity in the POA, changes in the number of cells with detectable GnRH-I mRNA in the POA were measured by in situ hybridization following a 1 h period of courtship interactions with females. In this second study, courting males exhibited 40% more cells with GnRH-I in this region than did isolated control males. GnRH-immunoreactive neurons in two other diencephalic regions failed to show these courtship-induced changes. Plasma LH was not elevated after 1 or 2 h of courtship. These results demonstrate that the release of GnRH-I in the POA that is presumably responsible for courtship-induced pituitary and gonadal activation is accompanied by a rapid increase in GnRH synthesis that occurs before plasma LH levels increase. We suggest that this increase in GnRH synthesis is necessary to support the extended period of HPG axis activation that is seen in this species during the 5–10 day period of courtship and nest building activity.
Social and environmental cues act via the hypothalamo-pituitary-gonadal (HPG) axis to regulate the timing of reproduction vertebrate reproduction and to modulate reproductive activity within a breeding season (Ball and Hahn, 1997; Rissman, 1997; Fernald, 2002). Changes in the activity of GnRH neurons in the hypothalamus are likely to play a critical role in mediating the effects of social and sexual stimuli on gonadal activity, but a detailed characterization of the nature and temporal characteristics of these changes have only been investigated in a handful of species. Male cichlids (Haplochromis burtoni) have been reported to show increased GnRH mRNA expression and increased size of GnRH cell bodies in the preoptic area within seven days of being introduced to an all-female group (White et al, 2002). Female ferrets show increased GnRH release from the hypothalamus within the first 15 min of coital stimulation (Lambert et al., 1992) even though the number of cells that express GnRH mRNA either remain unchanged or decline when sampled at frequent intervals during the first 24 h after mating stimulation (Bakker et al., 1999). Similarly, male mice and hamsters show a detectable rise in plasma LH levels within 15–30 min of exposure to pheromones from female conspecifics without corresponding changes in GnRH mRNA expression (Richardson et al., 2004; Gore et al., 2000). In contrast, exposure to male mouse bedding reportedly increased GnRH mRNA in male mice at 90 minutes after the onset of exposure (Gore et al., 2000).
Despite the critical role that social cues play in avian reproduction, there are no published data on the effects of behavioral interactions between male and their female breeding partners on GnRH release and GnRH synthesis in birds. To help clarify these relationships, the present study characterized the effects that courtship interactions with females exert on GnRH-I synthesis in male ring doves (Streptopelia risoria). The ring dove is an attractive species to study in this context because previous work has demonstrated social facilitation of HPG axis activity in both sexes during the courtship phase of the breeding cycle (Cheng 1974, 1979, 2005; Feder et al., 1977; Lehrman et al., 1961; O’Connell et al., 1981). In male doves, concentrations of plasma androgen begin to rise at 4 h and reach peak levels by 72 h after pairing with a female (Feder et al., 1977), while plasma LH is increased after 24 h, but not after 1 h of pairing (Silver et al., 1980). Because these cues stimulate increased secretion of both gonadotropin and gonadal steroids, it is likely that they act at the highest level of the HPG axis to influence the secretion of gonadotropin releasing hormone (GnRH) and/or gonadotropin inhibiting hormone (GnIH; Tsutsui et al., 2006) from axon terminals in the hypothalamus. Previous studies in male doves have shown that courtship interactions increase the number of mast cells in the medial habenula that exhibit GnRH-like immunoreactivity (GnRH-ir) (Silverman et al., 2002). However, these cells do not release GnRH into the median eminence capillaries and are therefore unlikely to play any direct role in mediating courtship-induced changes in HPG activity.
The preoptic area (POA) is a likely site for mediating courtship-induced changes in GnRH and the HPG axis because it contains the highest density of GnRH cell bodies in the dove brain (Silver et al., 1992), and because GnRH neurons in this region project to the median eminence in birds (Mikami, 1986). Although three GnRH isoforms are expressed in avian brain, GnRH-I is the only form that is expressed by POA neurons (Ball and Hahn, 1997; Bentley et al., 2004). To characterize courtship-induced changes, the number of neuronal cell bodies in the POA and nearby regions that express GnRH-I immunoreactivity and GnRH-I mRNA were measured in the present studies by immunocytochemistry and in situ hybridization, respectively, in sexually active males exposed to females for a brief period of courtship and in control males that remained isolated during this period.
Methods
Animals
Adult male ring doves were drawn from the colony maintained at the University of Wisconsin-Milwaukee Animal Care Facility. Doves were housed individually in visual isolation cages (33cm × 33cm × 45cm) and maintained under a constant photoperiod (14 h light: 10 hr dark; lights on at 0730 h) and temperature (20–22°C). Seed mixture (consisting of kafir, milo, wheat, hulled oats, buckwheat, and white proso millet), water, and grit were available ad libitum unless specified in the experimental design. All of the procedures involving animals conformed to NIH standards and were approved by the Animal Care and Use Committee at the University of Wisconsin-Milwaukee.
Transcardial perfusion
Following the experimental treatment period, the doves were given a lethal dose of Chloropent (sodium pentobarbital 4.4mg/mL, chloral hydrate 21.1mg/mL) anesthesia (1mL/bird, i.m.). Doves were then perfused transcardially with heparinized saline (0.87% NaCl) in 10mM sodium phosphate buffer followed by 4% paraformaldehyde in 0.2M sodium phosphate buffer (pH 7.4). Brains were immediately removed from the cranium and post-fixed overnight in 4% paraformaldehyde. Following the fixation period, the brains were transferred to 30% sucrose until sinking occurred. The brains were then sectioned coronally at a thickness of 60µm using a cryostat (Leica CM 1900 Bannockburn, IL). Sections were stored at −20°C in cryoprotectant solution until they were processed for immunocytochemistry.
GnRH immunocytochemistry
The anti-mammalian GnRH antibody (HU-60; gift of Dr. Henryk Urbanski) was used at a final concentration of 1:5,000. This antibody recognizes intact forms of GnRH (Urbanski et al., 1990) and has been used with success in previous studies to map GnRH pathways in avian brain (Hahn and Ball, 1995; MacDougall-Shackleton et al., 2001; Maney et al., 2007). In addition to recognizing chicken GnRH-I, this antibody cross-reacts with chicken GnRH-II (Urbanski, personal communication) and could conceivably cross-react with the lamprey GnRH-III, an isoform that has hypophysiotropic actions in songbirds (Bentley et al., 2004). However, chicken GnRH-I (GnRH-I) is the only GnRH peptide found in neuronal perikarya in the brain regions that we examined (see Ball and Hahn 1997 for review).
Sixty micron free-floating sections were rinsed in 25mM Tris-buffered saline (TBS), incubated in 0.3% hydrogen peroxide for 30 minutes, and blocked for 1 h in blocking serum composed of 7.5% normal goat serum, milk buffer (1 g. nonfat powdered milk/20 mL TBS), and 0.25% Triton-X-100. The sections were then transferred to the primary antibody and allowed to incubate for 48 h at 4°C. Following this incubation period, the sections were rinsed in TBS and then immersed in secondary antibody for one hour before they were incubated in avidin-biotin complex (Elite ABC kit; Vector Laboratories, Burlingame, CA) for 1 h. Immunoperoxidase staining was attained by reacting sections with diaminobenzidine and glucose oxidase (DAB substrate kit, Vector Laboratories, Burlingame, CA) for 3 minutes. Sections were then mounted on slides and cover slipped. The specificity of immunostaining was confirmed by pre-absorbing the antibody with chicken GnRH-I (Peninsula Laboratories, San Carlos, CA ) at a final concentration of 1µM.
Quantification of GnRH-immunoreactive (GnRH-ir) neurons
GnRH-ir cells were quantified both by cell number and cell size using the Karten and Hodos (1967) stereotaxic atlas of the pigeon for anatomical reference. Anatomical nomenclature and abbreviations conformed to Karten and Hodos (1967) except as noted. In each section, GnRH-ir neuronal cell bodies were counted by an observer blind to group assignment at 100x magnification using a Nikon Eclipse 400 microscope (Nikon Inc., Melville, NY). Cells counts were made in three brain areas: 1) the preoptic area (POA) corresponding to Karten and Hodos atlas plates A9.0-A8.5, 2) the medial preoptic nucleus (POM) and anterior portion of the paraventricular nucleus of the hypothalamus (PVN) as defined by Kuenzel and Masson (1988; Karten and Hodos atlas plates A8.5-A8.0) and 3) a region posterior to the POA and POM that includes the lateral and medial septum (SL/SM) and bed nucleus of the pallial commissure (nCPa; Kuenzel and Masson, 1988) corresponding to Karten and Hodos atlas plates A8.0-A7.25.
The area that we refer to as the POM in this study reflects recent revisions in the neuroanatomical nomenclature for this nucleus in the ring dove brain (Belle et al., 2005). The dove POM is now considered to correspond to the anterior/medial and caudal/lateral components of an area identified by Karten and Hodos (1967) as the anterior part of the nucleus periventricularis magnocellularis (PVM). This area is distinct from the area originally designated as the POM by Karten and Hodos (1967).
For analysis of cell body area, a subset of GnRH-ir cells (50 cells/brain) in each of the three brain regions were photographed using a Nikon Coolpix 990 digital camera mounted on the microscope and analyzed at 400x using NIH Image J software.
Hybridization probes
Medial basal hypothalamic tissue was quickly dissected from the brain of a male dove that had been housed in visual isolation from other birds under 14L: 10D photoperiod. Following decapitation, a tissue block containing the medial basal hypothalamus and preoptic area was quickly microdissected. After extraction in Trizol™, RNA (Invitrogen Corp., Carlsbad, CA), RNA was converted to cDNA using an Omniscript RT kit (Qiagen Inc., Valencia, CA). Degenerate primers based on the chicken GnRH-I sequence (Dunn et. al., 1993) were used to amplify a fragment spanning exon II, III, and IV. The PCR product (accession number EU699771) from hypothalamic cDNA, 264 bp in length, was cloned into a pCR4-TOPO vector using an Invitrogen cloning kit (Invitrogen Corp., Carlsbad, CA). The plasmid was then sequenced to determine the orientation of the cloned fragment. Sense (T7) and antisense (T3) digoxygenin (DIG)-labeled riboprobes were generated from this clone for use in the in situ hybridization assays using the Roche DIG RNA Labeling Kit (Roche Applied Science, Indianapolis, IN).
GnRH-I in situ hybridization
Coronal brain cryosections (20µm) were brain regions collected described above, mounted on slides, and stored at −80°C until they were processed for in situ hybridization. On day 1 of the procedure, brain sections were fixed in 4% paraformaldehyde for 30 minutes, treated with a solution containing 0.25% acetic anhydride in 0.1M triethanolamine (pH 8.0), followed by a rinse in 2X SSC. The sections were then incubated in prehybridization buffer (10 ml formamide, 5 ml 20X SSC, 300µl salmon sperm DNA (10 mg/ml), 300µl yeast tRDNA (10 mg/ml), 400µl 50X Denhardt’s , 4 ml 50% dextran sulfate) for two hours at 50°C followed by a 24 h incubation with DIG-labeled riboprobe at 45°C
On day 2 of the assay, the sections were rinsed in post-hybridization buffer (25 ml formamide, 12.5 ml 20X SSC, 0.5 ml 10% Tween, 12 ml dH2O) before being washed in SCC at increasing concentrations. The sections were then treated with RNase (4mg/mL) in 5mM NaCl, 10 mM Tris-HCl, 1mM EDTA for 30 minutes at 37°C. Finally, the sections were incubated with the anti-DIG antibody at 1:2000 dilution at 4°C for 24 h, followed by detection with NBT/BCIP for 4–6h (both from Roche Applied Science, Indianapolis, IN).
Quantification of GnRH-I mRNA expressing cells
In each brain section, stained cell bodies that expressed GnRH-I mRNA were counted by an observer blind to group assignment at 100x magnification using a Nikon Eclipse 400 microscope. Since preliminary immunocytochemical data indicated that there were distinct populations of cells located in the POA and POM, these two areas were examined for GnRH-I mRNA expressing cells. Cell counts were not conducted in the SL/SM and nCPa regions of the basal forebrain because GnRH mRNA-expressing cells in these areas were low in density and diffusely distributed. The analyzed areas correspond to coronal planes A9.25-A8.50 and A8.50-A8.0, respectively (Karten and Hodos, 1967).
Plasma LH assays
Plasma LH levels were measured by homologous chicken LH radioimmunoassay (Sharp et al., 1987). Two males in the courtship group in Experiment 1 were included for plasma LH analyses but excluded for GnRH immunocytochemical analyses because of brain perfusion and fixation problems. In addition, one courtship group male and one isolated control male in Experiment 1 were included for GnRH immunocytochemical measurements but excluded from plasma LH measurements because of problems of sample hemolysis during blood collection. Plasma samples from birds in both experiments were run in quadruplicate within a single assay. The intra-assay coefficient of variation was 6.2%.
Experimental Procedure
Experiment 1: Effect of courtship on GnRH-ir cells in the dove hypothalamus and preoptic area
After they were housed in visual isolation from conspecifics for three weeks, twelve adult male doves were randomly assigned to the courtship (experimental) or isolate (control) treatment condition (n=6/group). Immediately prior to the treatment period, six female doves, nest bowls, and nesting material were moved into cages adjacent to those that housed the six courtship group males. At the start of the treatment period, opaque partitions that were separating the two cage compartments were removed, which allowed the male dove physical access to the side of the cage that housed the female dove, nest bowl, and nesting material. Males and females were allowed to interact for 2 h and their interactions were video-taped for later analysis. During playback, the frequencies of aggressive courtship displays (bow-coos and hop-charges) by males were counted, and the total duration of male wing-flipping behavior (a component of nest soliciting) was recorded.
The six control males were housed in a separate room to avoid auditory stimulation from the courting doves. This room did not house breeding pairs but was populated by other non-breeding doves housed in visual isolation from conspecifics. The 2 h treatment period began with the removal of the partition that separated the cage where the dove was housed and the adjacent cage, which was empty. As in the courtship treatment group, the behavior of control males was videotaped for later analysis.
Following the treatment period, the male doves in both groups were removed from their cages and given a lethal dose of Chloropent anesthesia (1 ml/bird s.c.). Blood samples were taken immediately from the brachial vein for measurements of plasma luteinizing hormone (LH) concentration. Brains were perfused by transcardial catheter with heparinized saline followed by 4% paraformaldehyde as described above.
Experiment 2: Effect of courtship on GnRH-I mRNA-expressing cells in the dove hypothalamus and preoptic area
Eleven male doves were assigned to groups and housed as described in Experiment 1. The six treatment doves were exposed to female doves and videotaped as described in Experiment 1, except that the exposure period was limited to 1 h. The remaining control males were videotaped during a 1 h exposure to an empty cage, as described in Experiment 1. Following the exposure period, the male doves were removed from the cages and euthanized by rapid decapitation. Trunk blood was then collected for measurements of plasma LH levels and brains were rapidly removed from the skull and snap frozen on powdered dry ice. The fresh frozen brains were then sectioned at 20µm , mounted on slides, and stored at −80°C until they were processed by in situ hybridization to quantify the number of cells expressing GnRH-I mRNA.
Statistical analysis
All statistical analyses were performed using Sigma Stat software (Jandel Scientific, San Rafael, CA) using a two-tailed significance level of p<0.05. All cell number and cell size data are expressed as mean ± SE. The Student’s t-test was used for analysis of treatment group differences if sample sizes for comparison were 6 or greater and if no heterogeneity of variance was detected by Levene’s Median Test (Brown and Forsythe, 1974). If n≥6 and the equal variance test failed, or if n<6, then the non-parametric Mann Whitney Rank Sum test was used. In the courtship treatment group, the Pearson product moment correlation was used to determine the relationship between the frequency or duration of various courtship behaviors and the numbers of GnRH-ir and GnRH mRNA-expressing cells/section in different dove brain regions.
Results
Effect of courtship on GnRH-ir cells in the dove forebrain
Several regions of the dove basal forebrain contained clusters of GnRH-ir cell bodies when visualized by immunocytochemistry (Fig. 1). The highest density of GnRH-ir cells was found in the POA. A region corresponding to the medial preoptic and paraventricular nuclei of the hypothalamus (POM/PVN) also contained a discrete population of GnRH-ir cells. Posterior and dorsal to these areas, GnRH-ir cells were found diffusely distributed throughout the medial and lateral septum (SM, SL) and the bed nucleus of the pallial commissure (nCPa).
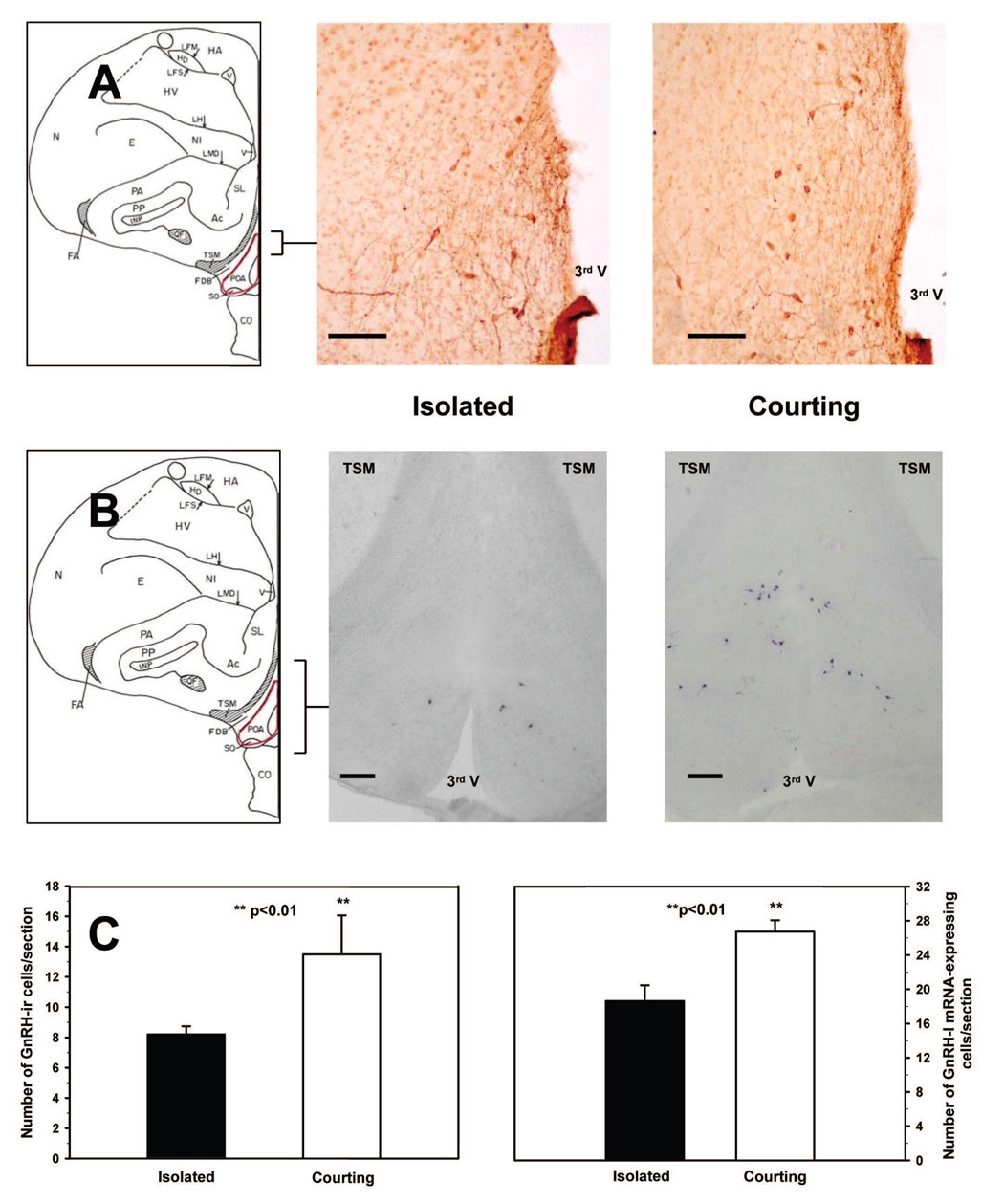
Fig. 1.
Representative depictions of GnRH-ir cell bodies in the male dove brain preoptic area (POA), the medial preoptic nucleus and paraventricular hypothalamic nucleus (POM/PVN) region, and an area that includes the lateral septum, medial septum, and bed nucleus of the pallial commissure (SL/SM/nCPa). Areas shown correspond to coronal planes A9.25-A8.50 (POA), A8.50-A8.0 (POM/PVN), and A8.0-A7.25 (SL/SM/nCPa) in the Karten and Hodos (1967) pigeon brain atlas. Scale bar = 100 µm.
As shown in Figure 3, courting males had approximately 60% more GnRH-ir cells in the POA than isolated control males (Mann Whitney Rank Sum T(6,6)=23.00, p<.01). However, courting and isolated males did not differ in GnRH-ir cell numbers in the POM/PVN (courting: 14.76 ± 1.37; control: 14.32 ± 1.42, p>0.60) or the SM/SL/nCPa (courting: 6.76 ± 1.30; control: 7.21 ± 0.78, p>0.90) In contrast to the treatment group differences in GnRH-ir cell numbers in the POA, courting and isolated males did not differin mean diameter of GnRH-ir cells in any of the three regions examined (p>.20 for all comparisons; Table 1).
Fig. 3.
(A) GnRH-ir cell bodies in the preoptic area (POA) of an isolated male and a courting male dove after a 2 h exposure period. Scale bar = 100 µm. (B) GnRH-I mRNA-expressing cells in the POA of an isolated and a courting male dove after a 1h exposure period. Scale bar = 200 µm. (C) Mean number of GnRH-ir and GnRH-I mRNA expressing cells per section + SE in the POA of isolated control males and males given 1 h (GnRH-I mRNA) or 2 h (GnRH-ir) of courtship interactions with a female.
Table 1.
Average GnRH-ir cell body area in three brain regions of courting males and isolated control males in Experiment 1. See text for abbreviations. Values are mean pixels ± SE
| Treatment Group | n | POA | POM/PVN | SM/SL |
|---|---|---|---|---|
| Isolated | 6 | 1517.7 ± 49.2 | 1244.8 ± 76.2 | 1087.4 ± 29.6 |
| Courting | 6 | 1401.8 ± 64.7 | 1174.9 ± 43.1 | 1053.2 ± 57.4 |
As expected, all males that were paired with females showed vigorous courtship activity during the 2 h exposure period, while isolated control males exhibited little or no courtship behavior (Table 2). Bow-coo frequencies and wing-flipping durations were not significantly correlated with GnRH-ir cell numbers in the POA of courting males (p>0.28 for both comparisons). However, hop-charge frequencies and GnRH-ir cell numbers in the POA showed some tendency to be negatively correlated, although the strength of the relationship only approached statistical significance (r=−0.77, p=0.07).
Table 2.
Plasma LH levels, average cumulative frequency of aggressive courtship behavior (bow-coos and hop-charges), and average cumulative duration of nest solicitation behavior (wing-flipping) in males paired with females (courting males) and control males that remained in isolation cages (isolated males) during a 1 h (Experiment 2) or 2 h (Experiment 1) exposure period. Values are mean ± SE.
| Exp. | Treatment Group | n | Plasma LH (ng/ml) | Bow-Coo Frequency | Hop-Charge Frequency | Wing-Flipping Duration (sec) |
|---|---|---|---|---|---|---|
| 1 | Courting | 7a | 1.83 ± 0.11 | 121.3 ± 14.2 | 42.7 ± 18.3 | 2443 ± 581.3 |
| Isolated | 5 | 1.82 ± 0.12 | 0 | 0 | 0 | |
| 2 | Courting | 5 | 1.86 ± 0.17 | 62.2 ± 23.1 | 34.2 ± 12.4 | 1889.8 ± 355.2 |
| Isolated | 5 | 1.84 ± 0.11 | 0 | 0 | 0 |
n=7 for LH measurements; n=6 for behavioral measurements
Effect of courtship on GnRH-I mRNA-expressing cells in the dove forebrain
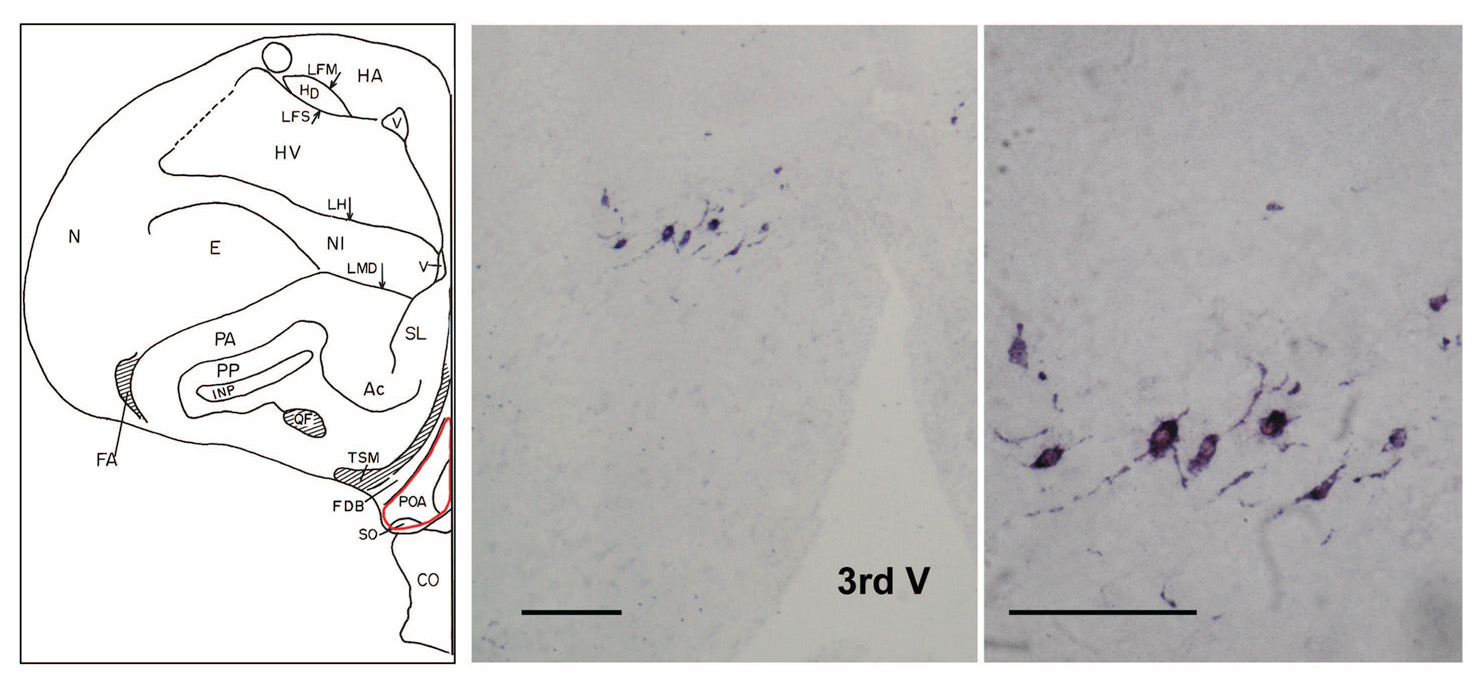
In a preliminary study, hybridization signals were observed when using the digoxygenin-labeled antisense riboprobe in the dove hypothalamus (Figure 2), but not when using the sense riboprobe. Accordingly, all brain sections assayed in Experiment 2 were exposed only to antisense riboprobe. In a pattern that paralleled the results obtained when GnRH-ir cell numbers were analyzed after 2 h of exposure to females, courting males allowed 1 h of exposure to females had significantly higher numbers of GnRH-I mRNA-containing cells in the POA than did isolated males (T(5,5)=17.00, p<.05; see Figure 3). In contrast, numbers of GnRH-I mRNA-containing cellsin the POM/PVN did not differ across treatment groups (courting: 9.66 ± 0.21; control: 10.82 ± 5.02, p>.80).
Fig. 2.
In situ hybridization staining of GnRH-I mRNA with antisense DIG-labeled riboprobe in the POA of a courting male dove. No hybridization signal was detected when tissue sections were incubated with the sense riboprobe (not shown). Scale bar = 100 µm.
As in Experiment 1, striking differences in courtship activity were observed between courting males and isolated males during the test period (Table 2). However, the number of GnRH-I mRNA-containing cells in the POA of courted males was not significantly correlated with bow-cooing frequencies, hop-charging frequencies, or wing-flipping durations (p> 0.68 for all comparisons).
Plasma LH measurements
Plasma LH levels of courting males did not differ from those of isolated males when assessed after exposure periods of 1 h (Experiment 2) or 2 h (Experiment 1) (p> 0.90 for all comparisons; Table 2).
Discussion
Using immunocytochemistry and in situ hybridization, we detected clusters of GnRH-I producing cells in the POA and in the POM/PVN of male ring doves and more diffusely distributed cells in areas surrounding the SL/SM and nCPa. This pattern is consistent with that reported in a previous immunocytochemical study that examined the anatomical distribution of GnRH-ir cells in the ring dove brain using a different GnRH antiserum (Silver, et. al., 1992). As previously indicated, the POA was a principal focus of investigation in this study because it contains the highest density of GnRH cells in the basal forebrain of doves (Silver et al., 1992) and because these cells have been shown to project to the avian median eminence to control pituitary gonadotropin secretion (Mikami, 1986). The fact that stimuli generated by courtship interactions caused substantial changes in the number of cells expressing GnRH-I mRNA and protein in the POA supports the hypothesis that the dove GnRH-I neurons in this region play a central role in mediating the effects of these stimuli on gonadal activity during the prelaying phase of the breeding cycle.
Previous studies have shown that male doves paired with females during the pre-laying phase of the breeding cycle show sustained elevations in plasma LH (Cheng and Follett, 1976; Goldsmith et al., 1981; Silver et al., 1980) and androgens (Feder, et al., 1977; O’Connell et al., 1981). This neuroendocrine response depends upon the gonadal state of the female and involves auditory stimulation, since deafened males exposed to intact females and males exposed to other males or to ovariectomized females fail to show elevations in plasma androgen (Feder et al., 1977; O’Connell et al., 1981) The fact that androgen levels begin to rise in the plasma of male doves within 4 h of pairing with an intact female (Feder et al., 1977) is consistent with the results of previous studies on the effects of mating-related stimuli on gonadotropin secretion and/or hypothalamic GnRH activity in male and female mammals, which indicate that neuroendocrine responses to such cues may occur within minutes of the onset of stimulation (see introduction). Although we cannot determine directly from our data how quickly GnRH is released in male doves in response to exposure to females, the fact that GnRH mRNA and protein expression were elevated within 1–2 h of courtship onset suggests that GnRH neurons in the POA respond rapidly to courtship-related stimuli. Nevertheless, plasma LH was not increased at 1 h after courtship initiation, which corroborates earlier findings by Silver et al. (1980). Plasma LH levels were also low at 2 h after courtship interactions began. Since plasma androgens begin to increase at 4 h after courtship onset (Feder et al., 1977), and testosterone secretion is known to occur after increased LH release (Sterling et al., 1978), it is likely that the elevation in plasma LH responsible for this gonadal activation is stimulated by an increase in GnRH secretion that occurs between 2 h and 4 h after pairing. Although further studies are needed to verify this assumption, it is interesting that female white-crowned sparrows show a similar delay in plasma LH elevations in response to male vocalizations (Maney et al., 2007). In part, this lag between the initiation of GnRH synthesis and the deduced onset of GnRH release may reflect the time required for further processing of proGnRH and transport of newly synthesized GnRH to terminals in the median eminence.
The fact that courtship interactions increased the number of POA cells exhibiting GnRH-I mRNA by over 40% at 1 hr after pairing and the number of GnRH-ir cells in the POA by 60% at 2 hr after pairing suggests that these cues exert potent and rapid effects on GnRH synthesis. As previously discussed (see introduction), activation of the HPG axis by social signals is not accompanied by increased GnRH synthesis in most of the mammalian species that have been studied in this context. While the functional significance of these differences in patterns of socially-induced GnRH synthesis remain to be determined, we hypothesize that the rapid increase in GnRH synthesis that male doves show when exposed to females supports a sustained increase in GnRH release that is necessary to maintain continued LH secretion and gonadal activation over the breeding pair’s extended (7–10 day) period of courtship and nest building activity.
Acknowledgments
This research was supported by NIMH grant MH41447 to J.B. We thank Dr. Henryk Urbanski for the generous gift of GnRH antiserum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand S, Lossee-Olson S, Turek FW, Horton TH. Differential regulation of luteinizing hormone and follicle-stimulating hormone in male Siberian hamsters by exposure to females and photoperiod. Endocrinology. 2002;143:2178–2188. doi: 10.1210/endo.143.6.8839. [DOI] [PubMed] [Google Scholar]
- Bakker J, Rubin BS, Baum MJ. Changes in mediobasal hypothalamic gonadotropin-releasing hormone messenger ribonucleic acid levels induced by mating or ovariectomy in a reflex ovulator, the ferret. Endocrinology. 1999;140:595–602. doi: 10.1210/endo.140.2.6519. [DOI] [PubMed] [Google Scholar]
- Ball GF, Hahn TP. GnRH neuronal systems in birds and their relation to the control of seasonal reproduction. In: Pahar IS, Sakuma Y, editors. GnRH Neurons: Gene to Behavior. Tokyo: Brain Shuppan; 1997. pp. 325–342. [Google Scholar]
- Belle MDC, Sharp PJ, Lea RW. Aromatase inhibition abolishes courtship behaviours in the ring dove (Streptopelia risoria) and reduces androgen and progesterone receptors in the hypothalamus and anterior pituitary gland. Mol. Cell. Biochem. 2005;276:193–204. doi: 10.1007/s11010-005-4060-6. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Moore IT, Sower SA, Wingfield JC. Evidence for a novel gonadotropin-releasing hormone in hypothalamic and forebrain areas in songbirds. Brai Behav. Evol. 2004;63:34–46. doi: 10.1159/000073758. [DOI] [PubMed] [Google Scholar]
- Brown MB, Forsythe AB. Robust tests for the equality of variances. J. Amer. Stat. Assoc. 1974;69:364–367. [Google Scholar]
- Cheng M-F. Ovarian development in the female ring dove in response to stimulation by intact and castrated male doves. J. Endocrinol. 1974;63:43–53. doi: 10.1677/joe.0.0630043. [DOI] [PubMed] [Google Scholar]
- Cheng M-F. Progress and prospects in ring dove research: a personal view. Adv. Study Behav. 1979;9:97–129. [Google Scholar]
- Cheng M-F. Audio-vocal pathways controlling GnRH release. In: Dawson A, Sharp PJ, editors. Functional Avian Endocrinology. New Delhi: Narosa Publishing House PVT. LTD; 2005. pp. 113–129. [Google Scholar]
- Cheng M-F, Follett BK. Plasma luteinizing hormone during the breeding cycle of the female ring dove. Horm. Behav. 1976;7:199–205. doi: 10.1016/0018-506x(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Dunn IC, Chen Y, Hook C, Sharp PJ, Sang HM. Characterization of the chicken preprogonadotrophin-releasing hormone-I gene. J. Mol. Endocrinol. 1993;11:19–29. doi: 10.1677/jme.0.0110019. [DOI] [PubMed] [Google Scholar]
- Feder HH, Storey A, Goodwin D, Reboulleau C, Silver R. Testosterone and “5α-Dihydrotestosterone ” levels in peripheral plasma of male and female ring doves (Streptopelia risoria) during the reproductive cycle. Biol. Reprod. 1977;16:666–677. doi: 10.1095/biolreprod16.5.666. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Social regulation of the brain: sex, size and status. Novartis Found. Symp. 2002;244:169–184. [PubMed] [Google Scholar]
- Goldsmith AR, Edwards C, Koprucu M, Silver R. Concentrations of prolactin and luteinizing hormone in plasma of doves in relation to incubation and development of the crop gland. J. Endocrinol. 1981;90:437–443. doi: 10.1677/joe.0.0900437. [DOI] [PubMed] [Google Scholar]
- Gore AC, Wersinger SR, Rissman EF. Effects of female pheromones on gonadotropin-releasing hormone gene expression and luteinizing hormone release in male wild-type and oestrogen receptor-alpha knockout mice. J. Neuroendocrinol. 2000;12:1200–1204. doi: 10.1046/j.1365-2826.2000.00578.x. [DOI] [PubMed] [Google Scholar]
- Hahn TP, Ball GF. Changes in brain GnRH associated with photorefractoriness in house sparrows (Passer domesticus) Gen. Comp. Endocrinol. 1995;99:349–363. doi: 10.1006/gcen.1995.1119. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Hodos W. Stereotaxic Atlas of the Brain of the Pigeon. Baltimore: Johns Hopkins University Press; 1967. [Google Scholar]
- Lambert GM, Rubin BS, Baum MJ. Sex difference in the effect of mating on c-fos expression in luteinizing hormone-releasing hormone neurons of the ferret forebrain. Endocrinology. 1992;131:1473–1480. doi: 10.1210/endo.131.3.1505478. [DOI] [PubMed] [Google Scholar]
- Lehrman DS, Brody PN, Wortis RP. The presence of the mate and nesting material as stimuli for the development of incubation behavior and for gonadotropin secretion in the ring dove. Endocrinology. 1961;68:507–516. doi: 10.1210/endo-68-3-507. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Deviche PJ, Crain RD, Ball GF, Hahn TP. Seasonal changes in brain GnRH immunoreactivity and song-control nuclei volumes in an opportunistically breeding songbird. Brain Behav. Evol. 2001;58:38–48. doi: 10.1159/000047260. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lake JI, Lange HS, O’Brien S. Rapid neuroendocrine responses to auditory courtship signals. Endocrinology. 2007;148:5624–5623. doi: 10.1210/en.2007-0879. [DOI] [PubMed] [Google Scholar]
- Mikami SI. Immunocytochemistry of the avian hypothalamus and adenohypophysis. Int. Rev. Cytol. 1986;103:189–248. doi: 10.1016/s0074-7696(08)60836-0. [DOI] [PubMed] [Google Scholar]
- O’Connell ME, Reboulleau C, Feder HH, Silver R. Social interactions and androgen levels in birds. Gen. Comp. Endocrinol. 1981;44:453–463. doi: 10.1016/0016-6480(81)90332-4. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Nelson AL, Ahmed EI, Parfitt DB, Romeo RD, Sisk CL. Female pheromones stimulate release of luteinizing hormone and testosterone without altering GnRH mRNA in adult male Syrian hamsters (Mesocricetus auratus) Gen. Comp. Endocrinol. 2004;138:211–217. doi: 10.1016/j.ygcen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rissman EF. Behavioral regulation of the GnRH system. In: Pahar IS, Sakuma Y, editors. GnRH Neurons: Gene to Behavior. Tokyo: Brain Shuppan; 1997. pp. 325–342. [Google Scholar]
- Sharp PJ, Dunn IC, Talbot RT. Sex differences in LH response to chicken LHRH-I and -II in the domestic fowl. J. Endocrinol. 1987;115:323–331. doi: 10.1677/joe.0.1150323. [DOI] [PubMed] [Google Scholar]
- Silver R, Goldsmith AR, Follett BK. Plasma luteinizing hormone in male ring doves during the breeding cycle. Gen. Comp. Endocrinol. 1980;42:19–24. doi: 10.1016/0016-6480(80)90252-x. [DOI] [PubMed] [Google Scholar]
- Silver R, Ramos C, Machuca H, Silverin B. Immunocytochemical distribution of GnRH in the brain of adult and posthatching great tit Parus major and ring dove Streptopelia roseogrisea. Ornis Scand. 1992;23:222–232. [Google Scholar]
- Silverman A, Asarian L, Khalil M, Silver R. GnRH, brain mast cells and behavior. Prog. Brain. Res. 2002;141:315–325. doi: 10.1016/S0079-6123(02)41102-8. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ubuka T, Yin H, Osugi T, Ukena K, Bentley GE, Ciccone N, Inoue K, Chowdhury VS, Sharp PJ, Wingfield JC. Mode of action and functional significance of avian gonadotropin inhibitory hormone [GnIH]: a review. J. Exp. Zool. A. Comp. Exp. Biol. 2006;305:801–806. doi: 10.1002/jez.a.305. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Kim SO, Connolly ML. Influence of photoperiod and 6-methoxybenzoxazolinone on the reproductive axis of inbred LSH/Ss Lak male hamsters. J. Reprod. Fertil. 1990;90:157–163. doi: 10.1530/jrf.0.0900157. [DOI] [PubMed] [Google Scholar]
- White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. J. Exp. Biol. 2002;205:2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- Yang S, Pau KF, Hess DL, Spies HG. Sexual dimorphism in secretion of hypothalamic gonadotropin-releasing hormone and norepinephrine after coitus in rabbits. Endocrinology. 1996;137:2683–2693. doi: 10.1210/endo.137.7.8770887. [DOI] [PubMed] [Google Scholar]