Abstract
The anti-inflammatory activity of intravenous Ig (IVIG) results from a minor population of the pooled IgG molecules that contains terminal α2,6-sialic acid linkages on their Fc-linked glycans. These anti-inflammatory properties can be recapitulated with a fully recombinant preparation of appropriately sialylated IgG Fc fragments. We now demonstrate that these sialylated Fcs require a specific C-type lectin, SIGN-R1, (specific ICAM-3 grabbing non-integrin-related 1) expressed on macrophages in the splenic marginal zone. Splenectomy, loss of SIGN-R1+ cells in the splenic marginal zone, blockade of the carbohydrate recognition domain (CRD) of SIGN-R1, or genetic deletion of SIGN-R1 abrogated the anti-inflammatory activity of IVIG or sialylated Fc fragments. Although SIGN-R1 has not previously been shown to bind to sialylated glycans, we demonstrate that it preferentially binds to 2,6-sialylated Fc compared with similarly sialylated, biantennary glycoproteins, thus suggesting that a specific binding site is created by the sialylation of IgG Fc. A human orthologue of SIGN-R1, DC-SIGN, displays a similar binding specificity to SIGN-R1 but differs in its cellular distribution, potentially accounting for some of the species differences observed in IVIG protection. These studies thus identify an antibody receptor specific for sialylated Fc, and present the initial step that is triggered by IVIG to suppress inflammation.
Keywords: autoimmune disease, DC-SIGN, rheumatoid arthritis, sialylated IgG Fc, SIGN-R1
Since their initial discovery by von Behring and Kitasato over a century ago, immunoglobulins have served as prototypical effector molecules of immune defense. Antigen binding, a property of the variable domains of the molecule, confers on immunoglobulins their remarkable diversity, while the constant region of the molecule functions to couple antigenic recognition to cellular effector pathways. It is through these Fc domains that immunoglobulins engage specific cellular receptors, translating the specificity of antigen recognition into in vivo responses. Thus, IgG molecules are able to trigger proinflammatory responses, such as phagocytosis and tumor cell killing through the engagement and cross-linking of cognate Fc receptors for IgG (FcγRs) (1). Similarly, IgG immune complexes, when deposited in end organs such as the kidney, synovium or lung, can induce an inflammatory response initiated by the activation of FcγRs on inflammatory cells, such as macrophages and neutrophils, and trigger the tissue pathology observed in diseases such as systemic lupus erythematosis and arthritis. Systematic analysis of the Fc domain interactions with FcγRs and the resulting in vivo biological properties of IgG antibodies has highlighted the critical role of these interactions to the efficacy of antibodies in diverse settings, including therapeutic IgGs developed for the treatment of neoplastic diseases, developed in defense against microbial pathogens, and in understanding the mechanisms of tissue pathology in autoantibody mediated autoimmune diseases (2).
However, IgG has also been demonstrated to mediate anti-inflammatory activity when administered as very high doses to patients suffering from autoimmune diseases (3). Monomeric IgG, purified from the serum of thousands of healthy donors (IVIG) is a commonly administered at high doses (1–2 g/kg) for the treatment of a number of autoimmune diseases, including immune-mediated thrombocytopenia, chronic inflammatory demyelinating polyneuropathy, Kawasaki Disease and Guillain-Barre syndrome, and is widely used in other autoimmune disorders (4–6).
A number of hypotheses have been advanced to explain the paradoxical activity of high dose IgG, and include models that attribute the activity to the polyclonal binding specificities, encoded in the variable domains of the administered antibodies that may counteract the activity of autoantibodies or inflammatory mediators (6). Others have focused on the IgG Fc portion as the anti-inflammatory component, and are supported by early clinical studies in which preparations of Fc fragments were active in restoring platelet levels in autoimmune thrombocytopenia comparable to that of intact IgG (7). Numerous mechanisms have been proposed to account for this activity of high dose IgG Fc fragments, including competition for cellular FcγRs, saturation of FcRn, and modulation of inhibitory pathways (6). Attempts to distinguish among these models have been hampered by a lack of in vivo models that recapitulate the anti-inflammatory activity of high dose IgG, and an incomplete understanding of the biochemical composition of the active components within the polyclonal therapeutic required for in vivo activity.
To address these shortcomings, we have developed murine inflammatory disease models that are attenuated by the anti-inflammatory activity of high-dose IVIG or its Fc fragments, including immune thrombocytopenia (8), serum-induced arthritis (9) and nephrotoxic nephritis (10). Thus, mice deficient in the macrophage growth/differentiation factor CSF-1 or mice lacking the inhibitory FcγRIIB receptor fail to respond to IVIG treatment to attenuate thrombocytopenia, arthritis, or nephritis (8–10). Other pathways, such as the classical pathway of complement activation, for example, appear to be dispensible for IVIG protection (8–10). These results have lead us to propose a model in which Fc fragments of IgG interact with a regulatory macrophage population in the spleen, which, in turn, mediates the stimulation of an anti-inflammatory pathway, ultimately increasing surface expression of the inhibitory Fc receptor on effector macrophages found at sites of immune complex deposition (3, 6).
The high-dose requirement for the anti-inflammatory activity of IVIG (1–2 g/kg), suggested that the preparations of purified IgG from normal human donors contain a small fraction of active therapeutic. We recently reported on the biochemical characterization of IVIG preparations that display anti-inflammatory activity in the animal models described above, and demonstrated an absolute requirement for the Fc fragment and its unique, N-linked complex, biantennary glycan attached at Asn 297 (11). Further characterization demonstrated that the anti-inflammatory activity in IVIG preparations depended on a minor fraction of IgG Fc that contained the fully processed N-linked glycan terminated in sialic acid, linked in an α2,6 linkage to the penultimate galactose (12). This fully processed glycan is found in 1–3% of IgG in IVIG, thus explaining the high dose requirement of IVIG. As expected, enrichment of this fraction by lectin affinity chromatography or in vitro sialylation, resulted in a concomitant reduced dose requirement for in vivo activity. Glycoproteins displaying similar complex, biantennary glycans terminating in sialic acid failed to demonstrate anti-inflammatory activity, indicating that both the amino acid backbone of the IgG Fc and the associated glycan are required. The biantennary glycan found at Asn 297 in the Fc fragment is unusual in that it is structurally constrained within the cavity formed by the A and B chains of the IgG Fc, adopting a highly ordered conformation that contrasts to similar glycans exposed on the solvent face of proteins (13). This biochemical analysis of the active fraction of IVIG was confirmed by the preparation of a fully recombinant, human IgG1 Fc with a biantennary glycan terminating in α2,6-sialic acid-galactose linkages that recapitulated the in vivo anti-inflammatory activity of intact IVIG, albeit at a much reduced dose (12). The in vivo activity of this recombinant preparation was enhanced 35-fold compared with the activity of IVIG.
In the present study, we sought to define the mechanism by which the 2,6-sialylated Fc mediates an anti-inflammatory response, and identify the properties of the regulatory macrophage population and a receptor required for initiating this pathway in response to 2,6-sialylated Fc. A population of splenic marginal zone macrophages that express the C-type lectin SIGN-R1 are required for the anti-inflammatory activity of IVIG, and this receptor and its human ortholog, DC-SIGN, specifically interact with sialylated Fc's. These studies thus provide the framework for the dissection of a property of the IgG Fc region, whereby its modification by sialylation converts it from a proinflammatory molecule to an anti-inflammatory one.
Results
Requirement for a Splenic Marginal Zone Macrophage Population.
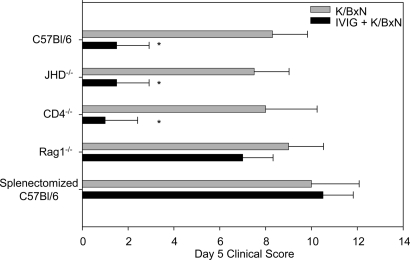
To further examine the properties of the regulatory macrophage population required for IVIG-mediated immune suppression, a panel of mouse strains with defined defects in specific immune cell populations were treated with IVIG one hour before receiving arthritis inducing sera (14, 15) (K/BxN, Fig. 1). Consistent with previous results, wild-type C57BL/6 mice were protected from inflammation by IVIG (Fig. 1), as were mice deficient in B cells (JHD−/−) or CD4+ T cells (CD4−/−). However, IVIG was not effective at protecting mice deficient in both B and T cells (Rag1−/−) that lack organized follicular structures (16), or splenectomized mice. Previously, IVIG was shown to be ineffective at protecting op/op mice (9) that are defective in CSF-1 dependent macrophage populations (17), including those found in the marginal zone of the spleen.
Fig. 1.
A splenic macrophage population is required for IVIG anti-inflammatory activity. Wild-type C57BL/6 mice, mice deficient in B cells (JHD−/−), mice deficient in CD4+ T cells (CD4−/−), mice deficient in both B and T cells (Rag1−/−), and splenectomized mice were treated with IVIG and K/BxN sera, and footpad swelling was monitored. Means and standard deviation of day 6 clinical scores from 4–5 mice per group are plotted, and are representative of three separate experiments; *, P < 0.05 as determined by a Student t test.
The results of these experiments suggested that specific macrophage populations in the splenic marginal zone might be required for the anti-inflammatory effect of the 2,6 sialylated Fc found in IVIG. Next, we set out to determine which of these populations were involved in the anti-inflammatory activity of IVIG.
The C-type Lectin, SIGN-R1, Is Required for IVIG Protection.
A variety of receptors are known to be expressed by marginal zone macrophages that are capable of interacting with glycopeptides and include the scavenger receptor MARCO (18), the sialic acid binding receptor sialoadhesin (CD169 (19)) and SIGN-R1 (20), a C-type lectin involved in the binding of S. pneumonia and dextran (21, 22).
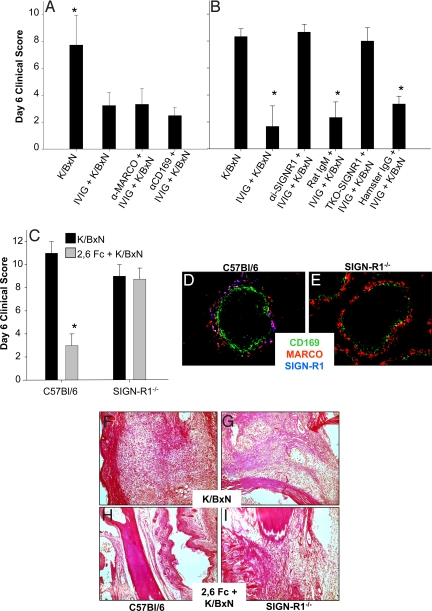
The ability of IVIG to attenuate K/BxN-induced tissue pathology was examined in the presence of antibodies that blocked the interactions of marginal zone macrophage receptors MARCO, sialoadhesin, and SIGN-R1 with their respective ligands. Although α-MARCO (23, 24) or α-CD169 (19) antibodies had no effect on the ability of IVIG to mediate its anti-inflammatory response (Fig. 2A), both the SIGN-R1 blocking antibody (22) (α-SIGN-R1) and an antibody that results in the transient down-regulation of SIGN-R1 expression (21) (TKO-SIGN-R1) abrogated the protective capacity of IVIG (Fig. 2B). The requirement for SIGN-R1 expression in mediating the anti-inflammatory effect of IVIG was further demonstrated by the loss of 2,6-sialylated Fc-mediated protection in mice with a targeted disruption of SIGN-R1 (25) (SIGN-R1−/−, Fig. 2 C–E). SIGN-R1 deficient mice treated with K/BxN serum alone displayed joint inflammation with characteristic neutrophil infiltration comparable to that seen in wild-type mice but are unable to attenuate this inflammatory response when pretreated with 2,6 sialylated Fc (Figs. 2 F–I).
Fig. 2.
The marginal zone macrophage receptor SIGN-R1 is required for IVIG anti-inflammatory activity. (A) Mice were administered blocking antibodies to marginal zone receptors CD169 (α-CD169), and MARCO (α-MARCO), treated with IVIG followed by K/BxN sera, and footpad swelling monitored. Mean and standard deviation of day 6 clinical scores from 4–5 mice per group are plotted; *, P < 0.05 as determined by ANOVA followed by a Tukey post-hoc test. (B) In a separate experiment, mice were treated with SIGN-R1 blocking antibodies (α-SIGNR1), isotype control antibodies for α-SIGNR1 (Rat IgM), antibodies that transiently knockdown SIGN-R1 expression (TKO-SIGN-R1), or an appropriate isotype control for TKO-SIGN-R1 (Hamster IgG). Next, IVIG and K/BxN were administered, and footpad swelling was monitored over the following week; means and standard deviations of 4–5 mice per group are plotted. *, P < 0.05 as determined by Anova followed by Tukey post-hoc. (C) C57BL/6 and SIGN-R1−/− mice were administered arthritis inducing sera (K/BxN, black bars), some of which received 2,6-Fcs 1 h earlier (2,6-Fc + K/BxN, gray bars). Footpad swelling was monitored over the next seven days in terms of clinical scores. Means and standard deviations of 34 mice per group are plotted. *, P < 0.05 as determined by ANOVA followed by Tukey post-hoc. The splenic marginal zones of C57BL/6 wild type (D) and SIGN-R1−/− (E) mice were examined by immunofluorescence for CD169 (green), MARCO (red), and SIGN-R1 (blue). Monocyte and neutrophil infiltration in the ankle bones of C57BL/6 or SIGN-R1−/− mice were examined in H&E-stained sections 7 days after treatment. Darkly stained nuclei of monocytes and neutrophils infiltrate the ankle bones of both C57BL/6 (F) and SIGN-R1−/− (G) mice treated with K/BxN sera; the inflammatory infiltration is reduced in C57BL/6 mice receiving 2,6-Fc treatment (H) but not in 2,6-Fc-treated SIGN-R1−/− (I) mice.
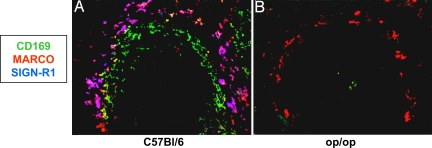
Op/op mice are unable to mediate the anti-inflammatory activity of IVIG and lack MOMA-1+ macrophages (9, 17), which could suggest that MOMA-1+ metallophillic macrophages might be additionally required by IVIG for its anti-inflammatory activity. To reconcile this observation with the data presented in Fig. 2, we examined whether, in addition to the lack of MOMA-1+ cells, the expression of SIGN-R1 was perturbed in these mice. Consistent with the results shown in Fig. 2, op/op mice lack the expression of SIGN-R1 on the MARCO+ cells in the marginal zone (Fig. 3), thus providing a consistent explanation for the loss of IVIG protection we had previously observed in this strain (9).
Fig. 3.
Op/op mice lack SIGN-R1 expression on marginal zone macrophages. Spleen sections from wild type C57BL/6 (A) and op/op mice (B) were examined for marginal zone macrophage receptor expression by immunofluorescent staining for CD169 (green), MARCO (red), and SIGN-R1 (blue). Imaging conditions for each fluorescent channel were identical for each sample.
SIGN-R1 Binds 2,6-Sialylated Fc.
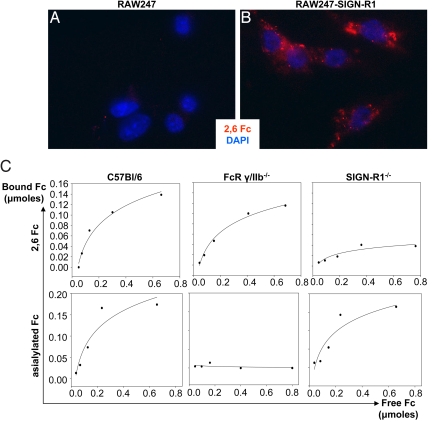
SIGN-R1 is a calcium dependent lectin with a glycan binding profile that includes mannan and dextran (22, 26). Previous studies have not suggested a binding specificity that included glycans that terminate in sialic acid (26). However, the glycan associated with IgG Fc fragments is found in an unusual, highly ordered conformation (27), and the protective activity of 2,6-sialylated Fc requires both the amino acid backbone and the 2,6-sialylated glycan (11), and the interaction of SIGN-R1 and IgG antibodies had not been examined. We next set out to determine whether SIGN-R1 had the ability to bind to this sialylated glycoprotein ligand. A transfected macrophage cell line that expressed SIGN-R1 (RAW-SIGN-R1) selectively bound sialylated Fcs compared with untransfected cells (Fig. 4 A and B). To demonstrate that 2,6 sialylated Fcs and asialylated Fcs bound to specific, nonoverlapping receptors on macrophages, we harvested resident peritoneal, SIGN-R1+ macrophages derived from wild type C57BL/6 mice, from mice lacking all IgG Fc receptors (28, 29) (FcR γ/IIB−/−) or from SIGN-R1 deficient mice (25) (SIGN-R1−/−) for quantitative binding assays. Fcγ receptor-deficient macrophages (FcR γ/RIIb−/−) bound α2,6-Fcs, while SIGN-R1−/− macrophages preferentially bound asialylated Fcs (Fig. 4C). These results are consistent with our previous results that canonical IgG Fc receptors bind to sialylated Fc with a 10-fold lower affinity than asialylated Fc (6, 11, 12). Thus, the 2,6-sialylation of the IgG Fc glycan converts the molecule from one able to productively engage FcγRs and mediate an inflammatory response, to a species that has reduced FcγR binding but acquires the ability to engage a macrophage expressed lectin, SIGN-R1, and mediate an anti-inflammatory response.
Fig. 4.
SIGN-R1 expressing cells preferentially bind 2,6-sialylated Fc's. RAW (A) and RAW-SIGN-R1 cells (B) were pulsed with fluorochrome-labeled 2,6-Fcs (red), stained with DAPI (blue), and imaged at 400× with identical exposure times and intensities. (C) Resident peritoneal macrophages isolated from C57BL/6 (left column), FcR γ/IIb−/− (middle column), and SIGN-R1−/− (right column) mice were pulsed with increasing concentrations of 2,6-sialylated Fcs (top row) or asialylated Fcs (bottom row). The amount of bound Fcs were determined and are plotted verses the free, unbound Fcs, and are representative of two separate experiments.
A Human Orthologue, DC-SIGN, Binds 2,6-Sialylated Fc.
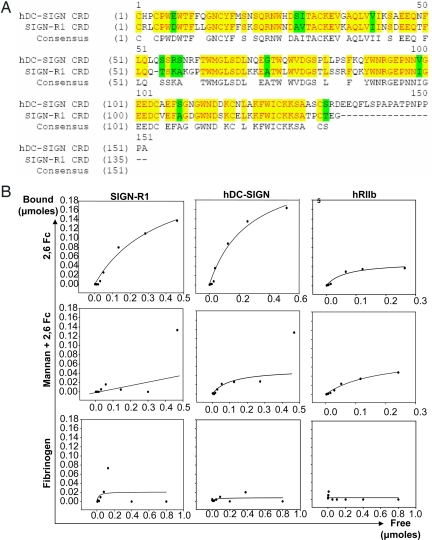
A human orthologue for SIGN-R1, based on sequence homology of the CRD (Fig. 5A) and its carbohydrate binding specificity (26) is the C-type lectin DC-SIGN (20). Like SIGN-R1, DC-SIGN has been shown to bind to mannosylated glycans, such as the gp120 glycoprotein of HIV (30) and is thought to be involved in the suppression of immunity triggered by this virus by inducing an anti-inflammatory response (31, 32). In contrast to SIGN-R1, DC-SIGN is expressed on dendritic cells (33), a ubiquitous cellular population found throughout the body. We examined the binding specificity of DC-SIGN in transfected CHO cells compared with SIGN-R1 expressing CHO cells. Both DC-SIGN and SIGN-R1 expressing CHO cells bound 2,6-sialylated Fc (Fig. 5B and Table 1). Mannan, a known ligand for DC-SIGN, was able to compete with 2,6-sialylated Fc for its binding to the transfected CHO cells, demonstrating that the binding sites for these two ligands on the CRD are likely to be overlapping. No binding was observed for fibrinogen, an abundant serum glycoprotein with a sialylated biantennary glycan composition similar to that found on the IgG Fc, but lacking the highly ordered structure seen for the Fc linked glycan (Fig. 5B), indicating that the interactions between 2,6-sialylated Fc and these lectins required both the glycan and amino acid backbone for their specificity.
Fig. 5.
Human DC-SIGN and SIGN-R1 display similar binding profiles of sialylated Fcs. (A) Amino acid sequences of the carbohydrate recognition domains (CRD) of human DC-SIGN and SIGN-R1 are aligned. Yellow and green highlights indicate identical and similar amino acids, respectively. (B) CHO cells expressing SIGN-R1 (left column), hDC-SIGN (middle column), or hFcγRIIb (right column) were pulsed with 2,6-Fcs (top row), incubated with mannan before 2,6-Fc pulse (middle row), or pulsed with fibrinogen (bottom row). The amount of bound glycoproteins was determined, and is plotted verses the free, unbound protein. Ka values determined by linear regression analysis are displayed in Table 1.
Table 1.
Kas of SIGN-R1, hDC-SIGN, and hFcγRIIb for sialylated and asialylated IgG Fcs
| Receptor | 2,6 Fc | Asialylated Fc |
|---|---|---|
| SIGN-R1 | 2.7 × 10−6 | n.b. |
| hDC-SIGN | 3.6 × 10−6 | n.b. |
| hFcγRIIb | 1.5 × 10−5 | 1.6 × 10−6 |
Ka values were determined by linear regression analysis of the equilibrium binding curves shown in Fig. 4B. n.b., no binding.
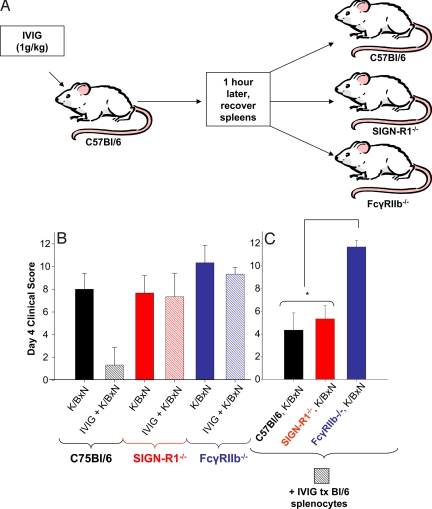
IVIG Protection Is Mediated by Splenocytes.
The results presented above suggest that 2,6-sialylated Fc interacts with a lectin expressed on marginal zone macrophages to initiate an anti-inflammatory pathway that ultimately modifies the ability of effector macrophages to trigger activation FcγRs in response to autoantibody deposition. One pathway by which this may be accomplished is through enhanced expression of the inhibitory FcR, FcγRIIB, on these effector macrophages. The consequence of enhanced expression of FcγRIIB is to raise the threshold required to cross-link activation FcγRs, such as FcγRIII and IV. To investigate this hypothesis, we treated wild-type C57BL/6 mice with IVIG and one hour later isolated the splenocytes from these animals. The treated cells were introduced into untreated animals, which were then challenged with the K/BxN serum to induce arthritis (Fig. 6A). As shown in Fig. 6B, protection from K/BxN induced arthritis could be transferred by these treated splenocytes, even when the recipients lacked SIGN-R1. However, the absence of FcγRIIB in the recipient prevented the protection afforded by these splenocytes, consistent with the role of FcγRIIB in setting a threshold for the triggering of activation FcγR on effector macrophages.
Fig. 6.
IVIG treated splenocytes can transfer anti-inflammatory activity, but require inhibitory FcγRIIb expression in recipient mice. (A) A schematic diagram of an IVIG-adoptive transfer system, where C57BL/6 mice are administered IVIG, killed 1 h later, splenocytes recovered and administered to recipient C57BL/6, SIGN-R1−/−, FcγRIIb−/− mice, that are subsequently given K/BxN sera. (B) C57BL/6 (black), SIGN-R1−/− (red), and FcγRIIb−/− (blue) mice were administered K/BxN (solid bars) or IVIG and K/BxN (hatched bars), and footpad swelling monitored. (C) In parallel, IVIG treated splenocytes from C57BL/6 mice were transferred to C57BL/6 (black), SIGN-R1−/− (red), and FcγRIIb−/− (blue) mice, which were administered K/BxN and footpad swelling monitored. Clinical scores of 4–5 mice per group four days after treatment are plotted; P < 0.05 as determined by ANOVA followed by Tukey post-hoc test.
Discussion
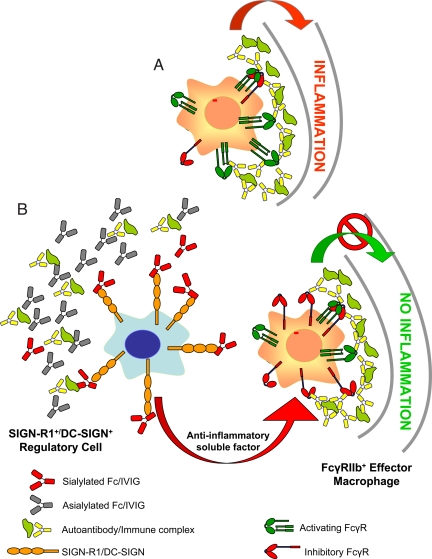
The results presented here establish SIGN-R1 as a receptor required for the anti-inflammatory activity of IVIG and its active component, α2,6-Fc, This binding interaction is presumed to initiate a pathway in which sialylated IgG promotes an anti-inflammatory state and ultimately results in the up-regulation of FcγRIIB on effector macrophages (6, 8). This model, summarized in Fig. 7, has been presented previously (6) and can now be further refined to indicate the identity of a receptor required for initiating the anti-inflammatory response of IVIG. In support of this multistep mechanism, we have observed that splenocytes isolated from IVIG-treated wild-type mice transfer protection to K/BxN serum-treated mice (Fig. 6). This protection does not require the presence of SIGN-R1 in the recipient animal, but does require FcγRIIB expression in the recipient. Thus, despite the presence of SIGN-R1+ macrophages in the periphery, it is the splenic SIGN-R1+ cells that are involved in binding sialylated Fc and initiating the anti-inflammatory response. The binding of 2,6-sialylated Fc to SIGN-R1 has been demonstrated by saturation binding studies on cells expressing this lectin. This requirement for cell surface expression is consistent with previous reports that failed to detect binding of carbohydrate ligands to monomeric SIGN-R1 (26). Because SIGN-R1 likely forms a homotetramer (34), these results suggest that the binding interactions are of low affinity and high avidity (Table 1). The ligand, 2,6-sialylated Fc, is composed of both the amino acid and glycan structure, again consistent with previous reports that failed to demonstrate SIGN-R1 binding to sialic acid containing glycans. It is likely that the 2,6 sialic acid-galactose linkage generates a conformation in the IgG Fc that is involved in binding to SIGN-R1. Modifications to the IgG N-linked core glycan have been demonstrated to modify binding to other receptors; de-fucosylation of the N-linked glycan at Asn 297 in human IgG1 enhances binding to human FcγRIIIA (35). Similarly, de-fucosylation of mouse IgG2b Fc enhances binding to mouse FcγRIV (36). However, while these modifications to the core glycan have been shown to modify the binding affinity to receptors, such as the FcγRs, 2,6-sialylation results in the acquisition of binding to a previously unrecognized partner, SIGN-R1. The consequence of this modification is dramatic—IgG is converted from an state in which binding to FcγRs can trigger a inflammatory response through macrophage activation, to a state in which binding to FcγRs is reduced and binding to SIGN-R1 is acquired.
Fig. 7.
A model for the anti-inflammatory activity of 2,6-sialylated Fc. Features of this model have been presented previously. (A) Under inflammatory and autoimmune conditions, immune complexes formed by autoantigens and self-reactive antibodies bind activating FcR expressed by inflammatory macrophages, leading to their activation. (B) 2,6-Sialylated Fc, found in preparations of i.v. gamma globulin (IVIG), engages a lectin on the surface of a regulatory macrophage population in the spleen found in the marginal zone, now identified as SIGN-R1. Engagement of this lectin induces a cellular program that results in the secretion of anti-inflammatory, soluble mediators that target effector macrophages found at the site of tissue inflammation where pathogenic immune complexes are deposited. These effector macrophages respond to the anti-inflammatory mediators by increasing surface expression of the inhibitory FcgRIIB receptor, thereby altering the threshold concentration of immune complexes necessary to trigger macrophage activation and subsequent inflammation. The net result of this pathway then is to attenuate autoantibody mediated inflammation and tissue pathology. The homologous pathway in the human differs in that the lectin is DC-SIGN and the regulatory cells are dendritic cells and thus, not restricted to the spleen.
The anti-inflammatory pathway triggered by 2,6-sialylated Fc is conserved in both mice and humans by virtue of the specificity of the lectin binding to α2,6-Fc, albeit through different target cells. The presumptive human orthologue of SIGN-R1, DC-SIGN, demonstrates the same binding specificity for carbohydrate ligands as does mouse SIGN-R1 and, most notably, binds to 2,6-sialylated Fc with a Ka comparable to SIGN-R1. However, DC-SIGN differs from SIGN-R1 in its pattern of cellular expression. It is expressed on dendritic cells and is thus more broadly distributed than mSIGN-R1, whose expression on splenic marginal zone macrophages is required for the activity of IVIG. This difference in anatomical requirement may account for the clinical observation that IVIG is active as an anti-inflammatory therapeutic in the treatment of splenectomized patients, in contrast to the situation in mice (see Fig. 1).
Although the precise features of the anti-inflammatory pathway triggered by 2,6-sialylated Fc remain to be defined, the model we have put forward to account for the anti-inflammtory activity of sialylated Fc (Fig. 7) postulates that soluble mediators are released from the 2,6-sialylated Fc activated regulatory cells. These mediators would act as anti-inflammatory cytokines to modify effector cell responses to autoantibodies at distal sites, such as the joint, kidney or spleen, attenuating inflammation and resulting tissue pathology. Our data are also consistent with a model in which 2,6-sialylated Fc activated regulatory cells mediate their anti-inflammatory response by cell to cell contact with effector cells or an intermediate cellular population. The end result of either model is the enhanced expression of the inhibitory FcγRIIB molecule on effector cells.
We have previously demonstrated that 2,6-sialylated IgG is found in the serum of naïve animals, as well as in the serum of healthy adults, and may function to maintain an anti-inflammatory environment in the steady state (11). Upon antigenic stimulation, as would occur in response to a microbial pathogen, IgGs directed to the inoculated antigen were found to be hyposialylated and thus able to mediate pathogen directed cytotoxicity and phagocytosis. This switching between sialylated IgG and asialylated IgG suggests a mechanism by which the immune response can distinguish between IgG antibodies in the steady state and those generated in response to a specific antigenic challenge, thereby protecting the host against coincidental activation of inflammatory pathways in the absence of a pathogenic challenge. It is worth noting that both SIGN-R1 and hDC-SIGN (22, 26) interact with viral and bacterial (30, 37) pathogens directly through their specificity for high mannose oligosaccharides, which can result in internalization and clearance of these organisms. Taken in light of the data presented here it suggests that lectins, such as SIGN-R1 and DC-SIGN, can bind to multiple, discrete ligands that can result in different cellular responses. The topology of these binding sites on SIGN-R1/DC-SIGN are likely to be overlapping, based on our observations that mannan competes with 2,6-sialylated Fc for binding to SIGN-R1 and DC-SIGN. This competition between different ligands capable of mediating different cellular responses may provide another mechanism by which these organisms can compete with 2,6-sialylated Fc for binding to SIGN-R1/DC-SIGN and thus shift the host response away from the anti-inflammatory steady state to an active, inflammatory one, thereby favoring pathogen elimination. Subversion of this pathway by some pathogens, such as HIV, may inappropriately maintain an anti-inflammatory state and thus prevent effective immunity from becoming established. Previous observations on the induction of molecules associated with anti-inflammatory responses, like IL-10 and ATF-3 (38, 39) and the persistence of an immature dendritic cell phenotype after DC-SIGN engagement by HIV, are consistent with this proposed pathway.
The studies reported here further highlight the protean activities mediated by the IgG molecule and identify yet another activity associated with the IgG Fc domain. The identification of SIGN-R1/DC-SIGN as a lectin required for the anti-inflammatory activity of IVIG suggests new therapeutic approaches by which inflammation can be suppressed in autoimmune diseases through the manipulation of this pathway.
Materials and Methods
Mice.
C57BL/6, NOD, JHD−/−, CD4−/−, and Rag1−/− mice were purchased from the Jackson Laboratory. KRN TCR C57BL/6 mice were gifts from D. Mathis and C. Benoist (Harvard Medical School, Cambridge, MA) and bred with NOD mice to generate K/BxN mice. SIGN-R1−/− mice were provided by A. McKenzie (Medical Research Council, Cambridge, United Kingdom) and C. G. Park (The Rockefeller University, New York). FcγRIIb−/− (29) and FcR γ chain−/− mice (28) were previously generated in this laboratory and bred to generate FcR γ/RIIb−/− double knockout mice. Age-matched female mice at 5–8 weeks of age were used for all experiments and maintained at the Rockefeller University animal facility. All experiments were done in compliance with federal laws and institutional guidelines and have been approved by the Rockefeller University. K/BxN serum was prepared as described previously (9). IVIG or α2,6-Fcs (1 g/kg or 0.033 g/kg, respectively) were injected 1 h before K/BxN serum. Inflammation was scored 0–3 for each paw (0, no swelling; 3, maximal swelling) and added for total clinical score per individual mouse. Mice receiving blocking antibody treatment received 100 μg of antibody for 1 h (for α-SIGN-R1 (ERTR-9, AbD Serotec), α-MARCO (ED31, AbD Serotec), α-CD169 (3D6, AbD Serotec), or 24 h (for TKO-SIGN-R1 (22D1, eBioscience) before IVIG. For surgical procedures, mice were anestitized and spleens cauterized under sterile conditions, wounds stapled, and mice allowed to recover for one week.
IVIG Fc Preparations.
Sialylated 2,6-Fc fragments were generated as previously described (11, 12), either by enriching the 2,6-sialylated Fc from Fc fragments derived from IVIG preparations by SNA chromatography or by in vitro sialylation of Fc fragments derived either from IVIG or a recombinantly expressed IgG1 mAb. Equivalent results were obtained with each preparation. Preparations were confirmed by lectin blotting using LCA-biotin (Vector) for N-linked glycans, SNA-biotin (Vector) for α2,6-sialic acid linkages (11). Some Fc preparations were treated with neuraminidase (New England Biolabs) to remove all sialic acid residues. Proteins were labeled with Alexa-647 or biotin according to manufacturer's instructions (Invitrogen).
Saturation Binding Experiments.
Binding studies were conducted as previously described (26), with the following modifications. 2 × 105 cells per well were plated in 24-well plates and allowed to adhere overnight. The next day, the media was removed, and the cells were pulsed with increasing concentrations of biotinylated glycoprotein for 1 h at 37°C in PBS with 1 mM CaCl2 and 1 mM MgCl2. After the incubation, the supernatants were collected, and the concentration of glycoproteins were determined by sandwich ELISA, capturing human IgG (Bethyl), detecting with an anti-biotin-HRP antibody (Bethyl), and developed with TMB development reagent (KPL). Alternatively, the amount of biotin was determined as previously described (26). For inhibition binding experiments, the cells were incubated with 20 μg/ml mannan for 20 min before treatment with Fcs.
Histology.
Spleens were removed, frozen in OCT freezing media (Sakura Finetek), and 4-μm sections were cut, fixed in cold acetone, stained with α-SIGN-R1-Alexa647 (eBioscience), α-MARCO (Serotec), α-CD169 (Serotec), and imaged on a Zeiss Axiovert fluorescent microscope. To image RAW cells, the same numbers of cells were plated onto circular coverslips placed in 24-well plates overnight, these cells were pulsed with 1 μg of Alexa647-labeled (Invitrogen) sialylated Fc in 100 μl, stained with DAPI, and the coverslips transferred to slides and analyzed by using an Axiovert fluorescent microscope (Zeiss). All exposure times and contrast adjustments were held identical for corresponding fluorescent channels between samples. Ankle joint histology was preformed as previously described (9), and imaged at 100× by using an Axiovert light microscope (Zeiss).
Adoptive Transfers.
Wild-type C57BL/6 mice were administered IVIG (1 g/kg), and one hour later spleens were removed, digested with Liberase Blendzyme 3 (Roche) for 30 min at 37°C and single cell suspensions generated. Treated splenocytes (1 × 108 total) were administered to naïve C57BL/6, SIGN-R1−/−, or FcγRIIb−/− recipient mice, which were treated with K/BxN serum 1 h later
Acknowledgments.
We thank C. G. Park for providing SIGN-expressing cell lines and helpful discussions, J. Pollard (Albert Einstein College of Medicine, Bronx, NY) for providing op/op mice, A. McKenzie (Medical Research Council, Cambridge, United Kingdom) for providing SIGN-R1−/− mice, and J. Pagan and P. Smith for excellent technical support. R.M.A. is an Irvington Institute Fellow of the Cancer Research Institute. This work was supported by National Institutes of Health grants (to J.V.R.), Virdante Pharmaceutical, Inc., and Swedish Research Council and the Fernstrom Foundation grants (to M.C.K. and F.W.).
Footnotes
Conflict of interest statement: J.V.R is a founder and shareholder of Virdante Pharmaceutical, Inc.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 25, 2006.
References
- 1.Ravetch JV, Nimmerjahn F. In: Fundamental Immunology. Paul WE, editor. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 684–705. [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 3.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 4.Clynes R. Protective mechanisms of IVIG. Curr Opin Immunol. 2007;19:646–651. doi: 10.1016/j.coi.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Negi VS, et al. Intravenous immunoglobulin: An update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27:233–245. doi: 10.1007/s10875-007-9088-9. [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: The intravenous IgG paradox. J Exp Med. 2007;204:11–15. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debre M, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342:945–949. doi: 10.1016/0140-6736(93)92000-j. [DOI] [PubMed] [Google Scholar]
- 8.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 9.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med. 2006;203:789–797. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 12.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 14.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 15.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. A new mouse model of rheumatoid arthritis: Organ-specific disease provoked by systemic autoimmunity. Ryumachi. 1997;37:147. [PubMed] [Google Scholar]
- 16.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 17.Cecchini MG, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 18.Elomaa O, et al. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 19.Crocker PR, et al. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991;10:1661–1669. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park CG, et al. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- 21.Kang YS, et al. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang YS, et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- 23.Palecanda A, et al. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med. 1999;189:1497–1506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinheiro da Silva F, et al. CD16 promotes Escherichia coli sepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- 25.Lanoue A, et al. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2004;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galustian C, et al. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- 27.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 28.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 29.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 30.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 31.Engering A, Geijtenbeek TB, van Kooyk Y. Immune escape through C-type lectins on dendritic cells. Trends Immunol. 2002;23:480–485. doi: 10.1016/s1471-4906(02)02296-2. [DOI] [PubMed] [Google Scholar]
- 32.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geijtenbeek TB, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 34.Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- 35.Natsume A, et al. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded antibody comprising a single-chain antibody linked the antibody constant region. J Immunol Methods. 2005;306:93–103. doi: 10.1016/j.jim.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 37.Tailleux L, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caparros E, et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 39.Hodges A, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]