Abstract
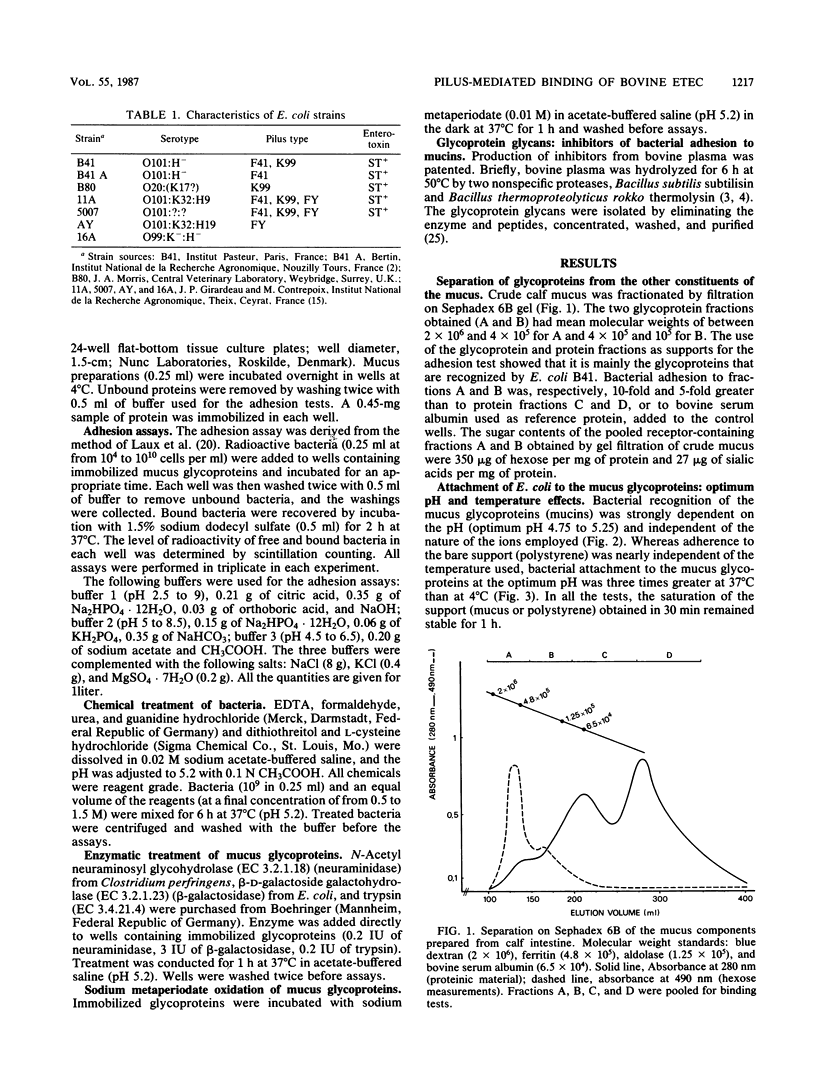
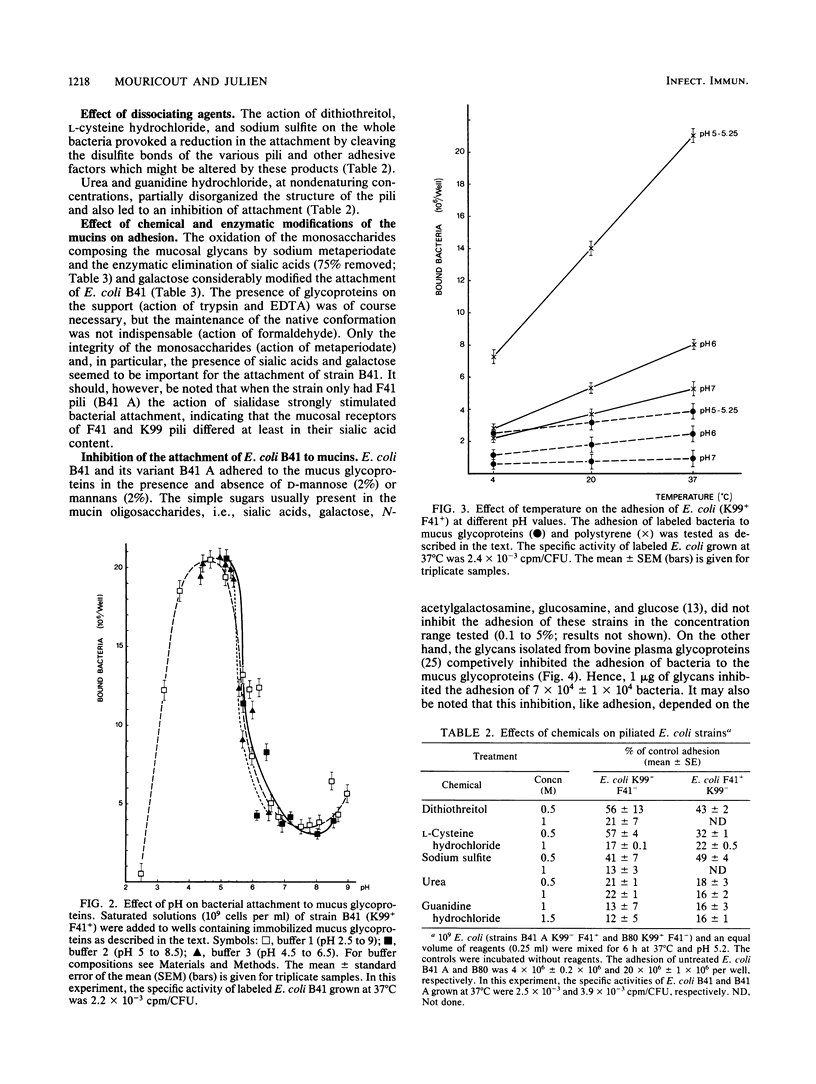
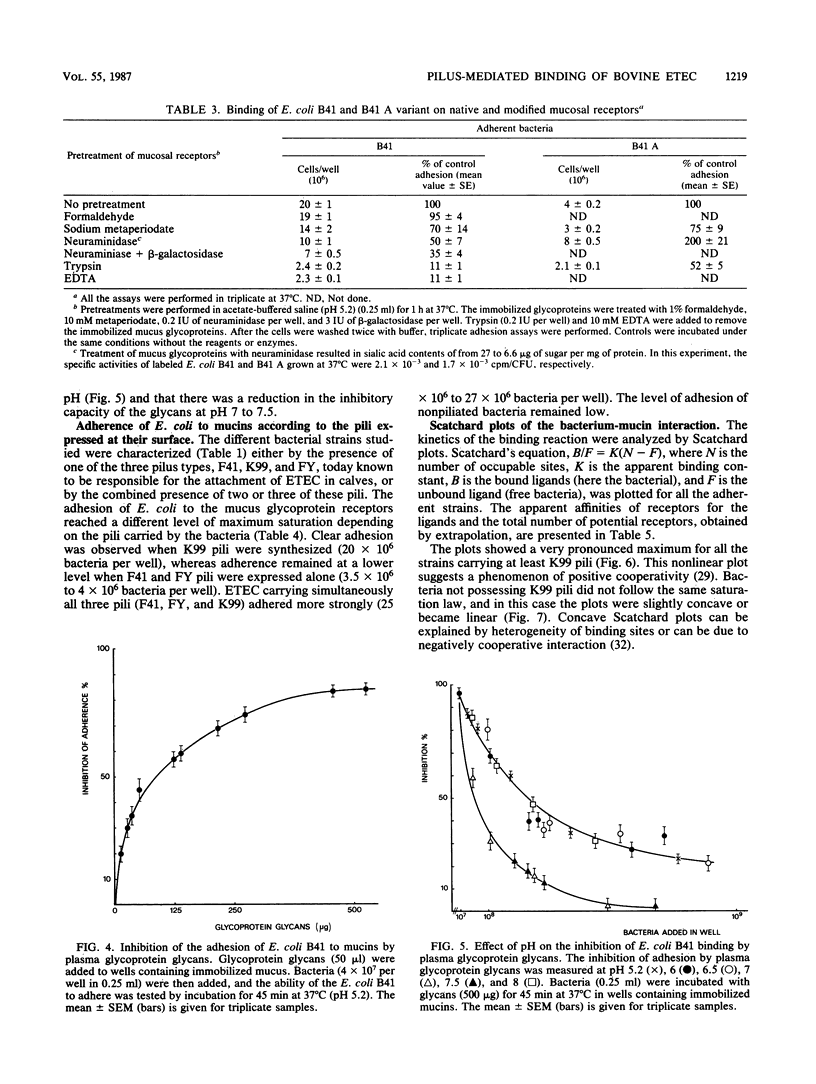
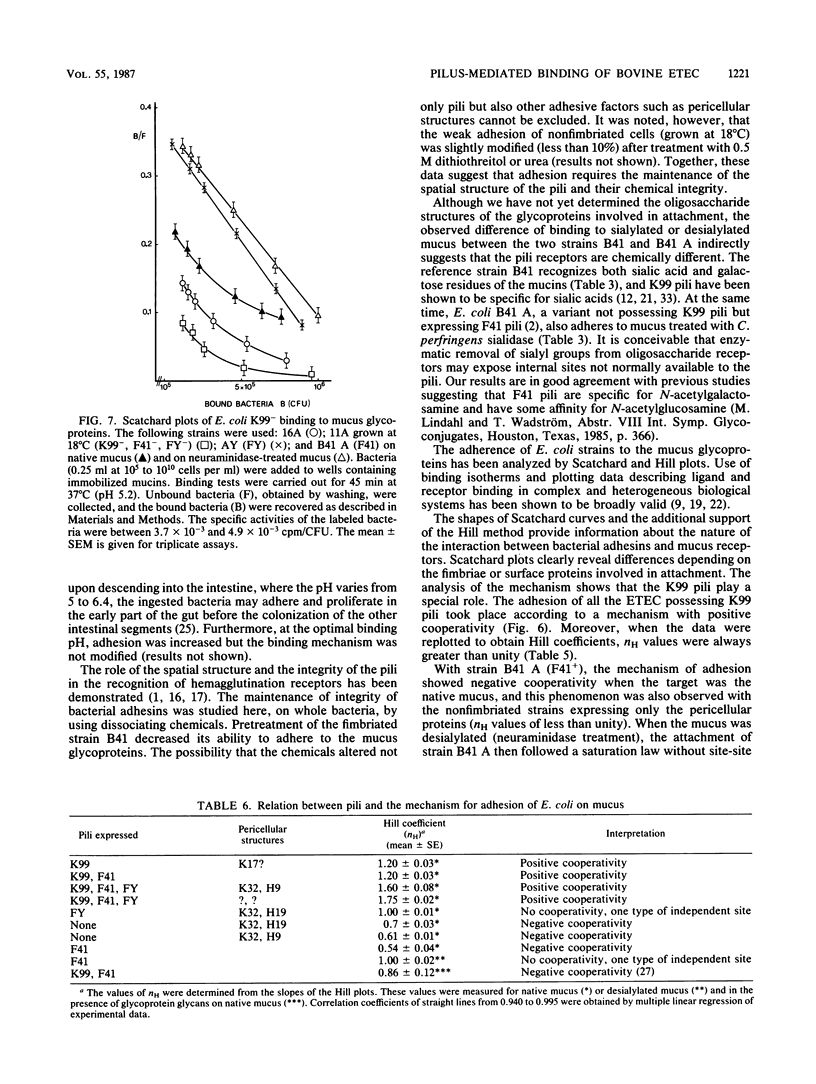
In this study we show that the adhesion to mucus of the enterotoxigenic Escherichia coli strains responsible for diarrhea in calves involves a bacterium-mucin recognition phenomenon in which the bacterial pili and specific mucus receptors carried by the glycoproteins (2,000 to 400 kilodalton) play a major role. An adhesion maximum was observed at a pH of less than 6 (4.75 to 5.25). The sialic acids and galactose appeared to be at least partly responsible for the attachment of K99 pili, whereas F41 pili preferentially recognized desialylated receptors. The attachment of different strains of E. coli characterized by the presence of the three main pili, K99, F41, and FY, known to be responsible for the binding of enterotoxigenic E. coli to the intestinal epithelium of the calf, was studied using Scatchard and Hill analyses. The attachment mechanism of bacteria carrying K99 pili showed positive cooperativity. FY and F41 pili recognized independent receptor sites, the first on sialylated mucus and the second on sialidase-treated mucus. Moreover, F41 pili were found to bind the native mucus according to a negative cooperativity phenomenon. Finally, the recognition sites carried by bacterial pilins may be saturated by some animal glycoprotein glycans which are therefore adhesion inhibitors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartus H., Actor P., Snipes E., Sedlock D., Zajac I. Indications that the erythrocyte receptor involved in enterotoxigenic Escherichia coli attachment is a sialoglycoconjugate. J Clin Microbiol. 1985 Jun;21(6):951–954. doi: 10.1128/jcm.21.6.951-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A. F41 antigen as a virulence factor in the infant mouse model of Escherichia coli diarrhoea. J Gen Microbiol. 1985 Nov;131(11):3037–3045. doi: 10.1099/00221287-131-11-3037. [DOI] [PubMed] [Google Scholar]
- Burrows M. R., Sellwood R., Gibbons R. A. Haemagglutinating and adhesive properties associated with the K99 antigen of bovine strains of Escherichia coli. J Gen Microbiol. 1976 Oct;96(2):269–275. doi: 10.1099/00221287-96-2-269. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrepois M. G., Girardeau J. P. Additive protective effects of colostral antipili antibodies in calves experimentally infected with enterotoxigenic Escherichia coli. Infect Immun. 1985 Dec;50(3):947–949. doi: 10.1128/iai.50.3.947-949.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist F. W. The meaning of Scatchard and Hill plots. Methods Enzymol. 1978;48:270–299. doi: 10.1016/s0076-6879(78)48015-2. [DOI] [PubMed] [Google Scholar]
- Forstner J. F. Intestinal mucins in health and disease. Digestion. 1978;17(3):234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- Freter R. Mechanisms of association of bacteria with mucosal surfaces. Ciba Found Symp. 1981;80:36–55. doi: 10.1002/9780470720639.ch4. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., van Mechelen J. R., de Graaf F. K. Effect of chemical modifications on the K99 and K88ab fibrillar adhesins of Escherichia coli. Biochim Biophys Acta. 1985 Nov 29;832(2):148–155. doi: 10.1016/0167-4838(85)90326-7. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., van den Berg P. A., Bak H. J., de Graaf F. K. Localization of lysine residues in the binding domain of the K99 fibrillar subunit of enterotoxigenic Escherichia coli. Biochim Biophys Acta. 1986 Jul 25;872(1-2):92–97. doi: 10.1016/0167-4838(86)90151-2. [DOI] [PubMed] [Google Scholar]
- Klotz I. M. Number of receptor sites from scatchard and klotz graphs: a constructive critique. Science. 1983 May 27;220(4600):981–981. doi: 10.1126/science.220.4600.981. [DOI] [PubMed] [Google Scholar]
- Klotz I. M. Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science. 1982 Sep 24;217(4566):1247–1249. doi: 10.1126/science.6287580. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Wadström T. K99 surface haemagglutinin of enterotoxigenic E. coli recognize terminal N-acetylgalactosamine and sialic acid residues of glycophorin and other complex glycoconjugates. Vet Microbiol. 1984 Jul;9(3):249–257. doi: 10.1016/0378-1135(84)90042-7. [DOI] [PubMed] [Google Scholar]
- Minton A. P. On the interpretation of binding isotherms in complex biological systems. Apparent homogeneity of some heterogeneous systems. Biochim Biophys Acta. 1979 Dec 4;558(2):179–186. doi: 10.1016/0005-2736(79)90058-0. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Thorns C. J., Sojka W. J. Evidence for two adhesive antigens on the K99 reference strain Escherichia coli B41. J Gen Microbiol. 1980 May;118(1):107–113. doi: 10.1099/00221287-118-1-107. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Number of receptor sites from Scatchard and Klotz graphs: a constructive critique. Science. 1983 May 27;220(4600):979–981. doi: 10.1126/science.6302842. [DOI] [PubMed] [Google Scholar]
- Roosendaal B., van Bergen en Henegouwen P. M., de Graaf F. K. Subcellular localization of K99 fimbrial subunits and effect of temperature on subunit synthesis and assembly. J Bacteriol. 1986 Mar;165(3):1029–1032. doi: 10.1128/jb.165.3.1029-1032.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels P. L., Moon H. W., Schneider R. A. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1980 Apr;28(1):298–300. doi: 10.1128/iai.28.1.298-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellwood R. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim Biophys Acta. 1980 Oct 1;632(2):326–335. doi: 10.1016/0304-4165(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Smit H., Gaastra W., Kamerling J. P., Vliegenthart J. F., de Graaf F. K. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun. 1984 Nov;46(2):578–584. doi: 10.1128/iai.46.2.578-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Verseveld H. W., Bakker P., van der Woude T., Terleth C., de Graaf F. K. Production of fimbrial adhesins K99 and F41 by enterotoxigenic Escherichia coli as a function of growth-rate domain. Infect Immun. 1985 Jul;49(1):159–163. doi: 10.1128/iai.49.1.159-163.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


