Abstract
Previous research indicates that the Ñanchoc Valley in northern Peru was an important locus of early and middle Holocene human settlement, and that between 9200 and 5500 14C yr B.P. the valley inhabitants adopted major crop plants such as squash (Cucurbita moschata), peanuts (Arachis sp.), and cotton (Gossypium barbadense). We report here an examination of starch grains preserved in the calculus of human teeth from these sites that provides direct evidence for the early consumption of cultivated squash and peanuts along with two other major food plants not previously detected. Starch from the seeds of Phaseolus and Inga feuillei, the flesh of Cucurbita moschata fruits, and the nuts of Arachis was routinely present on numerous teeth that date to between 8210 and 6970 14C yr B.P. Early plant diets appear to have been diverse and stable through time and were rich in cultivated foods typical of later Andean agriculture. Our data provide early archaeological evidence for Phaseolus beans and I. feuillei, an important tree crop, and indicate that effective food production systems that contributed significant dietary inputs were present in the Ñanchoc region by 8000 14C yr B.P. Starch grain studies of dental remains document plants and edible parts of them not normally preserved in archaeological records and can assume primary roles as direct indicators of ancient human diets and agriculture.
Keywords: early diets, South America, food production
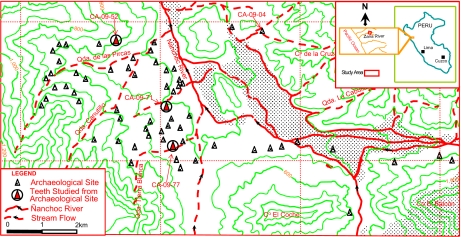
The Late Pleistocene to Middle Holocene Ñanchoc preceramic culture is known from 46 sites in the Ñanchoc Valley, a tributary of the Zaña Valley located at 500 m above sea level on the lower western slopes of the Andes (1–7) (Fig. 1). Recovered from hearths and floors in 13 excavated house structures dating from ∼10,100 to 5500 14C yr B.P. (∼11,200 to 6000 calendar years ago or cal. B.P.) were the macrobotanical remains of a variety of plant cultivars along with gathered wild plants and the bones of various large and small animal species, providing evidence for a broad spectrum and mixed subsistence economy in a tropical dry forest setting (1–3, 6). Between 10,000 and 7000 14C yr B.P. during the Late Paijan and Las Pircas phases, crop production was practiced close to small, permanent, circular houses. Associated with the plant remains during the subsequent Tierra Blanca phase between 7000 and 5000 14C yr B.P. were stone hoes, larger grinding stones, plots for planting crops, small-scale irrigation canals and public mounds (site CA-09–04, Fig. 1), and rectangular houses (1–3, 6).
Fig. 1.
Location of the Ñanchoc Valley in northern Peru and archaeological sites discussed in this study.
We examined 39 human teeth from Las Pircas occupations at four different sites [Table 1, supporting information (SI) Table S1]. The teeth were mostly isolated remains from adults and subadults that were securely embedded within intact and sealed house floors and directly associated with radiocarbon dates made on human bone, macrobotanical plant remains, and wood charcoal ranging from 8210 ± 180 to 6970 ± 60 14C yr B.P. (Methods; Table 1; Tables S1 and S2; see ref. 1 for a more complete list of 14C dates from the sites). Three teeth were submitted to Beta Analytic Inc. for accelerator mass spectrometer (AMS) radiocarbon dating but insufficient protein remained for an age determination (Table 1). The isolated teeth come from an estimated minimum of six to eight individuals, probably from both sexes; three complete individuals are also represented (Table 1).
Table 1.
Types and Numbers of Starch Grains Recovered from the Ñanchoc Teeth
| Sample/14C Age |
Phaseolus |
Arachis |
Cucurbita moschata |
Inga* |
Legume |
Globular |
Bell |
U #1, 2, 3, 4, 5, 6 |
Other |
Total |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | (x̄ Size; range) | n | (x̄ Size; range) | n | n | n | n | n | n | n | |
| Site CA-09–77 | Microns | Microns | ||||||||||
| 2A, 6970 ± 60 HB | 14 | 3 | (8.7; 6–12) | 11 | (8.3; 6–12) | 26 | 5 | 0 | 0 | 1 - - - - - | 11(1) | 71 I |
| 29, 6970 ± 60 HB | 55 | 2 | (6; 5–7) | 2 | (7; 5–9) | 4 | 13 | 5 | 0 | 5 - - - - - | 10(5) | 91 M |
| 19, 6970 ± 60 HB | 24 | 0 | 1 | (12) | 1 | 0 | 2 | 0 | - - - - - - | 7(4) | 35 M | |
| 68, #1, 7840 ± 40 PH+ | 93 | 0 | 4 | (9.8; 8–11) | 0 | 1 | 2 | 0 | 2 - - - - - | 12(2) | 114 M | |
| 68, #2, 7840 ± 40 PH | 37 | 4 | (7–7 ) | 2 | (13; 11–15) | 2 | 13 | 9 | 1 | - - - - - - | 12(8) | 80 M |
| 7, #2, 7840 ± 40 PH | 3 | 5 | (10.2; 7–14) | 0 | 0 | 0 | 1 | 0 | - 2 - - - - | 2 | 13 M | |
| 50, #1, 7840 ± 40 PH+ | 14 | 41 | (11.9; 7–18) | 0 | 2 | 0 | See note | 0 | - - - - - - | 41(8) | 98 M | |
| 50, #4, 7840 ± 40 PH | 22 | 6 | (9; 6–13) | 2 | (6–6) | 14 | 1 | 12 | 1 | 15 2 2 - - - | 13(3) | 90 M |
| 56, #4 7840 ± 40 PH+ | 5 | 2 | (13; 8–17) | 0 | 0 | 0 | 0 | 0 | - - - - - - | 11(9) | 18 M | |
| **59, 7840 ± 40 PH | 23 | 3 | (13.3;12–15) | 0 | 0 | 4 | 0 | 0 | 1 2 - 1 - 1 | 4 | 42 M | |
| 58, #1, 7840 ± 40 PH | 22 | 1 | (12) | 0 | 0 | 0 | 0 | 0 | - 2 - 2 2 - | 4 | 27 M | |
| 58, #3 7840 ± 40 PH | 3 | 3 | (8.7; 8–9) | 4 | (8.5; 6–11) | 14 | 0 | 7 | 1 | - 2 - 2 2 - | 17 | 55 M |
| Site CA-09–28 | ||||||||||||
| 1A 8210 ± 180 WC | 4 | 1 | (9) | 9 | (9.8; 7–12) | 1 | 0 | 0 | 0 | - - - - - - | 9 | 15M |
Note: 14C dates from CA-09–77 are derived from materials (HB = human bone, PH = charred peanut hull) directly associated with teeth from hearths sealed in individual intact floors (ca. 3 cm thick) of buried house structures (see Table S2 and refs. 1, 6 for more details). For CA-09–28, the associated date is on wood charcoal.
+Teeth were submitted to Beta Analytic Inc. for AMS radiocarbon dating but insufficient protein remained for an age determination.
*Identified as Inga feuillei. The category ″Legume″ represents oval grains that occur in Phaseolus and also in other Fabaceae genera. ″Globular″ grains are consistent with those in Arachis and Inga and also occur in a variety of non-legume taxa.
**Three grains typical of underground organs occurred. In sample 50,
#1 many of the ″Other″ grains occurred in clumps and were difficult to describe in detail but they were globular in shape as in peanut and/or pacay. ″Bell″ grains that occurred in small numbers on some teeth are consistent with manioc but a positive identification is not possible. Numbers in parentheses in the ″Other″ category represent gelatinized grains that were too damaged from heat to identify. Letters in bold after Total n indicate type of tooth (I = incisor, M = molar). Teeth 50 and 58 are from sub-adults. Others are indeterminate as to the approximate age of the individual. Tooth 1A is from a complete skeleton.
We cannot state with much precision how much of an individual's lifetime the tooth calculus that was studied represents. Little information is available on the rate of calculus formation but because it accumulates over an individual's life if not removed, at least several years of diet is probably represented for each tooth studied. To remove dental calculus and examine and identify the starch in it we used nondestructive methods developed by ourselves and large modern reference collections housed in D.R.P.'s laboratory (Methods and SI; we also searched for phytoliths in the calculus preparations and found none, not surprising in view of the fact that the plants evidenced on the teeth and many others that were potentially available in the study region do not produce phytoliths in their edible parts).
Results
Most teeth yielded some starch grains and 13 contained them in significant quantities. Table 1 contains the kinds and number of grains retrieved from these specimens. Table S1 contains data on the teeth with fewer grains. Ubiquitous and consistently common over the 1,000-year period represented are four taxa, Phaseolus, Arachis, Cucurbita moschata, and Inga feuillei, indicating they were major dietary sources (Table 1) (Fig. 2 a–e). We cannot determine conclusively whether the Phaseolus starch is from a domesticated bean or whether it has a greater affinity to P. lunatus or P. vulgaris (Fig. 1 a and b for archaeological Phaseolus; Fig. S1 A and B for modern comparisons). It is generally considered that single domestications of the Andean lima and common bean occurred, respectively, in southern Ecuador/northern Peru and southern Peru/Bolivia (8–12). Three species of wild Phaseolus closely related to domesticated species, all of which were analyzed here, grow in Peru: P. augusti, P. pachyrrhizoides, and wild P. vulgaris. The first two probably should be merged taxonomically and are thought to be the lima's wild ancestor (9, 12).
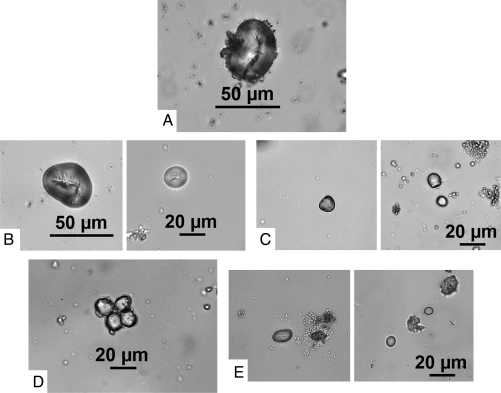
Fig. 2.
(a) An oval starch grain from Phaseolus with a longitudinal fissure and lamellae from tooth 29. (b) Left, an oval starch grain from Phaseolus with a ragged longitudinal fissure and lamellae. Right, a round starch grain from Phaseolus with a stellated fissure and lamellae concentrated near the edge. Both grains are from tooth 50, no. 4. (c) Bell-shaped grains from teeth 1A (Left) and 2A (Right) with “pleats” (unique kinds of lamellae) and pressure facets from Cucurbita moschata fruit flesh. (d) A cluster of four starch grains from peanut nuts from tooth 50, no. 1. The grains are globular in shape and have projections/knobs at the edge, slight fissures, and tiny starch grains attached at the periphery. (e) Oval (Left) and elliptical (Right) starch grains with white longitudinal fissures from Inga feuillei from samples 50, no. 4 and 58, no. 3.
In most teeth samples, average and maximum Phaseolus grain size is considerably larger than in wild common bean, which can be excluded from representation on this basis (Table 2 and Table S3 for comparative modern data). However, there is a broad range of size overlap between wild and domesticated lima bean starch, and size values for all but one of the teeth fall within this overlap zone. In sample 19, dated to 6970 14C yr B.P., average length and width of Phaseolus grains exceed those of all modern wild beans studied. Given the size similarities between wild and domesticated lima bean starch, we prefer to exercise caution at this time with the interpretation of this single tooth showing a domesticated grain size. Starch from wild and domesticated Phaseolus species is too similar in morphology to attempt a species-specific identification on this basis (Methods and Fig. S1 A and B).
Table 2.
Phaseolus starch grain morphology and size on the Ñanchoc teeth
| Site and specimen | Age | Oval, no damage |
Oval damaged |
Round with lamellae |
Round with fissures |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Length, μm | Width, μm | n | Length, μm | Width, μm | n | Diameter, μm | n | Diameter, μm | n | ||
| CA-09–77 | |||||||||||
| 19 | 6970 ± 60 | 39 (32–53) | 29 (23–39) | 5 | 37 (28–52) | 30 (20–51) | 15 | 30 (29–30) | 2 | 14 (11–17) | 2 |
| 29, #1 | 6970 ± 60 | 29 (18–50) | 23 (9–36) | 5 | 27 (19–47) | 21 (8–31) | 19 | 24 (20–29) | 4 | 19 (15–23) | 2 |
| 27, #2 | 6970 ± 60 | 44 | 38 | 1 | 0 | 0 | 0 | ||||
| 10, #1 | 6970 ± 60 | 20 (19–21) | 16 (14–17) | 2 | 0 | 0 | |||||
| 2A | 6970 ± 60 | 35 (26–41) | 25 (21–32) | 7 | 24 (19–31) | 17 (15–20) | 4 | 0 | 23 (13–32) | 3 | |
| 29, #2 | 6970 ± 60 | 0 | 29 (26–32) | 2 | 0 | 0 | |||||
| 29, #3 | 6970 ± 60 | 0 | 34 | 27 | 1 | 0 | 0 | ||||
| 68, #1 | 7840 ± 40 | 29 (19–44) | 25 (17–37) | 13 | 29 (16–41) | 24 (13–36) | 17 | 27 (20–36) | 7 | 15 | 1 |
| 68, #2 | 7840 ± 40 | 29 (23–39) | 23 (17–33) | 9 | 29 (16–46) | 24 (12–42) | 18 | 21 (15–26) | 9 | 22 | 1 |
| 7, #1 | 7840 ± 40 | 0 | 39 (36–43) | 26 (24–27) | 3 | ||||||
| 7, #2 | 7840 ± 40 | 27 | 24 | 1 | 28 | 22 | 1 | 0 | 26 | 1 | |
| 50, #1 | 7840 ± 40 | 33 (27–39) | 23 (22–26) | 3 | 23 (18–27) | 21 (16–24) | 7 | 41 | 1 | 14 (10–16) | 3 |
| 50, #4 | 7840 ± 40 | 34 (21–41) | 25 (15–32) | 4 | 28 (18–42) | 21 (13–29) | 12 | 28 (26–29) | 2 | 15 (12–19) | 4 |
| 50, #2 | 7840 ± 40 | 20 | 1 | 22 | 18 | 2 | 26 | 1 | 18 (16–21) | 3 | |
| 56, #4 | 7840 ± 40 | 0 | 36 (30–45) | 27 (24–31) | 4 | 31 | 1 | 0 | |||
| 59 | 7840 ± 40 | 0 | 32 (22–52) | 24 (17–44) | 20 | 47 | 1 | 0 | |||
| 58 | 7840 ± 40 | 29 (17–48) | 24 (12–41) | 19 | 0 | 0 | |||||
| 58, #1 | 7840 ± 40 | 0 | 29 (17–48) | 24 (12–41) | 19 | 16 (14–17) | 2 | 37 | 1 | ||
| 58, #3 | 7840 ± 40 | 28 (27–29) | 23 (22–23) | 2 | 0 | 37 | 1 | 0 | |||
| CA-09–52 | |||||||||||
| 117 | 8080 ± 70 | 0 | 0 | 19 | 1 | 0 | |||||
| 113 | 7920 ± 120 | 27 | 16 (13–19) | 2 | 0 | ||||||
| 15 | 7920 ± 120 | 46 | 32 | 1 | 31 (24–41) | 24 (14–31) | 5 | 36 | 1 | 22 | 1 |
| 3A | 7920 ± 120 | 24 | 18 | 1 | 34 | 29 | 1 | 0 | 0 | ||
| CA-09–28 | |||||||||||
| 1A | 8210 ± 180 | 38 | 22 | 1 | 29 (22–36) | 21 (20–21) | 2 | 33 | 1 | 10 | 1 |
Phaseolus oval grains in the ″no damage″ category exhibit no signs of damage from heat or other sources. Oval grains in the ″damaged″ category exhibit heat damage such as altered or no extinction crosses and damaged fissures (Figs. S3 a–d and S4 a–d). ″Round with lamellae″ grains are those without fissures and with lamellae covering the entire grain. ″Round with fissures″ are the smaller round grains that have a variety of fissure types, including stellate, and are without lamellae or have lamellae concentrated in a thin, dense band at the periphery of the grain. The presence and proportions of these different grain types on the teeth were also matches for the genus Phaseolus and no other legume taxon. In the size categories, the first number is the mean and the numbers in parentheses are size ranges for the grains.
The ecology of wild Phaseolus does strongly suggest that the starch grains represent cultivars. Wild lima and common beans are found today in dry and thorny or humid forest at elevations between 1,800 and 3,000 m above sea level (asl) (9). The Ñanchoc sites, located at 500 m asl in dry tropical forest, are considerably outside of the elevational range and native habitats of these plants. The previous earliest record for Phaseolus in South America was the domesticated P. lunatus from Chilca, southern coastal Peru, dated to 5600 14C yr B.P. (ca. 6400 cal. B.P.) (13). The earliest common bean remains, from Guitarrero Cave in central highland Peru (13), presently date to ca. 4600 14C yr B.P. (ca. 5000 cal. B.P.). Considering this evidence along with the greater proximity of the Ñanchoc region to the probable hearth of lima domestication, it may be more likely that the Phaseolus starch is from a lima rather than a common bean.
Previous work identified the early presence of a domesticated squash species, Cucurbita moschata, which likely is native to lowland northern South America, possibly Colombia. Distinctive, elliptical-shaped seeds dark brown in color that are unique to C. moschata were recovered from sites CA-09–77 and CA-09–27 where they were directly dated to 9240 ± 50 14C yr B.P. and 7660 ± 40 14C yr B.P., respectively (1). Starch grain data provide an independent line of evidence for this crop and broaden our understanding of how Cucurbita was consumed during early periods. Many teeth contained distinctive bell-shaped grains diagnostic of the flesh of C. moschata fruits (Fig. 2c; Fig. S2a) (seeds from the genus have no starch). In morphology and size the grains are identical to those found in the flesh of traditional modern fruits of C. moschata and they are unlike those of other Cucurbita species, including C. sororia, a wild taxon closely related to C. moschata (14) (Methods; SI). Cucurbita flesh produces significantly less starch than legume seeds, thus it is likely to be underrepresented in the records. The evidence firmly indicates that at Ñanchoc early domesticated Cucurbita was routinely consumed and not used primarily as a utilitarian or industrial plant (e.g., as containers and fishnet floats). Moreover, as all wild Cucurbita species have extremely bitter, nonedible flesh, human selection for the major domestication gene that codes for nonbitter fruits by 8000 14C yr B.P. is implied.
Another plant evidenced through previous research is the peanut. Hulls from Arachis sp. recovered from CA-09–77 were directly dated to 7840 ± 40 14C yr B.P. (1). The hulls were not exact morphological matches for modern A. hypogaea but neither did they conform to a known wild species. They almost certainly represent cultivars because they occur far outside the natural distribution of the genus in southern South America (15). Peanut nuts were not preserved in the macrobotanical records; however, starch grains diagnostic of Arachis nuts (hulls contain no starch) are present on many teeth (Table 1; Fig. 2d; Fig. S2b). In their morphological and size characteristics they are matches for modern Arachis hypogaea (compare peanut data in Table 1 with that for modern varieties in Table S3). We also studied two peanut hulls retrieved from sites CA-09–77 and CA-09–50 that date to ∼8000 to 7800 14C yr B.P. and 7000 14C yr B.P., respectively, to ascertain whether any starch grains from decayed nuts survived in them. These specimens also yielded grains like those in A. hypogaea.
We examined a diploid wild species, A. williamsi, which is closely related to A. hypogaea. It has starch grains with a mean length of 6.6 μm and a maximum length of between 4 and 10 μm. This length is much smaller than grain size on the teeth and in different varieties of modern A. hypogaea, where maximum length was ≥18 μm and average length was from 9.5 to 10.1 μm (Table S3). Grains from A. williamsi also lack surface decorations found in A. hypogaea. We also studied three wild Arachis species, A. burchelli, A. marginata, and A. retusa, which are from a different taxonomic section of the genus that is more distantly related to A. hypogaea. They all exhibited markedly different starch morphological attributes (bell shaped instead of globular) when compared with A. hypogaea, A. williamsi, and the archaeological grains, providing more evidence that the Ñanchoc peanuts were closely related to A. hypogaea. This may be a case where different traits indicative of domestication, such as hull morphology and nut starch grain qualities in these peanut examples, were not transformed from their wild states simultaneously or at the same rate by early human selection pressure, resulting in early cultivars that have a mixture of wild- and domesticated-type features. Analysis of the direct wild progenitors of the peanut, thought to be A. ipaensis and A. duranensis or A. helodes (15, 16), will shed more light on this issue.
Inga feuillei (pacay) is an important tree crop that was well used on the Peruvian coast by 4500 14C yr B.P (17–19). It is probably native to the lowland eastern slopes of the Andes and it can be grown at much higher elevations, which made it important to the Inca (20). Pacay has large edible pods with a sweet, white pulp. Starch identified from this plant derives from the seeds; the pulp does not contain starch (Fig. 2e; Fig. S2c). This is clear evidence for early tree crop exploitation in the Ñanchoc region.
A variety of other starch grains occurred in lower quantities and just a few of them appear to be from underground organs (Table 1). Three grains were consistent with those from manioc roots although a positive identification is not possible from the grain types present (21). Macrobotanical remains of manioc were retrieved from the sites (6). Low overall frequencies of starch from underground organs suggest they were infrequently consumed. Other grains that occurred in low numbers, including six types of unknown but distinctive-looking starch (U nos. 1–6), are conceivably from foods such as cacti and tree fruits that are not represented in our modern collections. It also should be pointed out that some potentially important food sources that were well used during the later pre-Columbian period in the study region do not produce starch or have nondiagnostic grains, thus their dietary contribution cannot be studied. They include domesticated tree fruits of various Annona species (guanabana, custard apple) and guava (Psidium guajava), other fruits (Bunchosia armeniaca and hartwegiana; Solanum quitoense or naranjilla), and the lupine bean (Lupinus mutabilis).
Discussion
Starch grain data from dental remains can inform a number of important issues concerning early human diets and the transition from hunting and gathering to agriculture. It has long been discussed, for example, whether the earliest plants taken under cultivation in some areas of the world were dietary items or used for nonfood purposes. In the Americas, at least four different species of Cucurbita native to North America, Mesoamerica, and South America are now known to have been among the first crop plants in these regions, and it has recently been proposed that early cultivation and diffusion of Cucurbita pepo ssp. ovifera in the eastern United States were motivated much more by the utility of the plant for its nonfood uses than by its role in the diet (22, cf. ref. 23). In contrast, the Ñanchoc evidence clearly shows that C. moschata was commonly eaten by some of the first farmers in Peru.
Overall the evidence indicates that by ca. 8000 14C yr B.P. (ca. 8600 cal. B.P.) an effective farming system employing a range of seed, vegetable, tree, and root crops provided balanced, nutritious, and stable diets to the inhabitants of the tropical western slopes of the Peruvian Andes. The ubiquity of plants at Ñanchoc that would come to typify later agricultural systems suggests that regional dietary patterns began to emerge not long after food production and crop dispersals began. The multifaceted botanical, settlement (sedentary and seminucleated communities), landscape (irrigation canals and furrowed fields), and artifactual data (e.g., stone hoes, grinding stones, storage pits) (1–7) show that Ñanchoc societies cannot be characterized as casual food producers who were deriving only negligible dietary inputs from cultivated plants. The evidence from here and some other well-studied regions in the Neotropics (17, 24, 25, 26) instead indicates an early Holocene development of food production together with an emergence not long afterward—at ∼8000 to 7000 14C yr B.P.—of subsistence economies that included significant amounts of cultivated and domesticated products. Thus, although large sedentary and nucleated villages do not occur in the Americas until after 5000 14C yr B.P., earlier food production in some areas was practiced by people who, while still integrating planting with foraging, were committed farmers who had taken the first steps along the pathway to full-fledged agriculture.
A routine cultivation (a persistent cycle of sowing and harvesting) of plants that provide stable and significant dietary inputs, but that do not acquire all or even some of the phenotypic traits typical of the domestication syndrome for hundreds to thousands of years (known as predomestication cultivation), is a pattern increasingly being documented at the beginning of agriculture in the Old World (27, 28). The issue has received less attention thus far in the New World, in part because early macrobotanical remains from the tropics are rare, but some potential examples exist from highland Mexico (25). The peanut evidence from Ñanchoc points to an instance of predomestication cultivation (hulls lack features of modern A. hypogaea) possibly together with a time-transgressive appearance of different domesticated traits in this plant (starch grains are matches for modern A. hypogaea and not wild Arachis studied), suggesting that domestication in some major New World crops may also have been a protracted and complex process. Possible instances of predomestication cultivation should be sought more widely in the New World using multifaceted archaeobotanical data sets and allied paleoecological evidence when possible. Much more evidence is needed but it is possible that as in the Old World, traditional, domestication-based concepts of agricultural origins—those that posit that a suite of fully domesticated plants formed the basis for the first effective food production systems and initial spread of crops—may have to be adjusted.
In conclusion, the Ñanchoc evidence elucidates in a number of ways early agricultural lifeways in the Andean region. While, on the present evidence, most of the Central Andes between 10,000 and 8000 C14 yr B.P. were occupied by a wide variety of maritime and terrestrial foragers that were still organized in relatively small, mobile groups, some populations such as those in the Ñanchoc Valley were adopting more sedentary life styles and becoming farmers. The incorporation into subsistence economies of domesticated plants and other cultigens during this period did not immediately lead to a full-scale agricultural economy, as Ñanchoc people continued to hunt, fish, and gather wild plants. Managing a wide range of food sources across several closely juxtaposed ecological zones may have helped to reduce subsistence risk. By ∼6500 to 6000 C14 years ago, the emergence of a planned aggregated community and small-scale public monuments in the area are evident, anchored by more intensive food production systems that now included irrigation canals and crop field systems (2, 5). These developments, in turn, created new ways of life, new perceptions of public and private spaces, and new relationships between members of a community, which represent some of the initial pulses toward the rise of Andean civilization (5). More complex ideological, technological, and subsistence advances that took place after 5500 14C yr B.P. in Peru (18–19), including a shift to agricultural dependence, are probably direct extensions of the Ñanchoc developments.
Methods
Starch Grain Sampling of Teeth.
Our sampling strategy used methods nondestructive to the teeth and the starch (29). First, teeth were brushed with a soft tooth brush and water to remove adherent soil and other particles. A dental pick was used to scrape areas of teeth with visible calculus, and the residue was transferred directly to a microscope slide where a few drops of water had been placed. On many teeth this procedure was done from three to five separate times by sampling different areas of the specimen such as the crowns of molars and the gum line and making new microscope mounts each time. The plaque could at times be seen to be yellowish in color when it was transferred to the slide. Sampling was stopped when no additional starch grains were recovered. Before the coverslip was put on, one drop of 50% water/glycerine was added to the residue-water suspension to retard drying and allow grains to be more easily rotated when encountered. This light solution of glycerine does not alter starch morphology. The non-use of chemicals ensures that starch grains will not suffer damage during and after their removal from dental remains.
Archaeological peanut hulls were examined by adding a few drops of water to the inside of the specimens using a pasteur pipette, gently washing the hull wall with the water, and transferring the liquid to a microscope slide.
Starch Grain Identification.
We used a large modern reference collection numbering ∼500 economic and other plant species from 50 different families along with published sources (21, 24, 30–35). Our modern collection includes all major and many now-minor crops and other plants known or thought to have been used in Latin America/the Andean region during prehistory and many wild species closely related to crop plants such as: (1) the wild progenitors of lima and common beans from South America and Mexico; (2) other wild Fabaceae including Inga, Canavalia, Vigna, and Macropitilium; (3) wild Arachis; and (4) wild Cucurbita native to southern Central America and South America (C. maxima ssp. andreana, which is the wild ancestor of C. maxima and C. argyrosperma ssp. sororia) (see also ref. 21 for further details on the collection). C. sororia is closely related to the as yet undiscovered wild ancestor of C. moschata (14).
We studied a diverse collection of modern, traditional lima and common bean land races from Peru, Mexico, and other areas of Latin America. Our results indicate the following: (1) there is considerable overlap in grain size of wild lima beans, domesticated lima beans, and domesticated P. vulgaris; (2) grain size in wild common beans appears to be significantly smaller than in both domesticated common and lima bean, and (3) there is considerable morphological similarity among wild and domesticated species in Phaseolus, making species-specific identification difficult on this basis (Table S3; Fig. S1 A and B).
Our starch keys and classification system emphasize shape, surface, and size attributes known to be useful in identification (21, 24, 30–35) (see also Figs. S1–S5). To confirm identifications of Phaseolus, Arachis, C. moschata, and I. feuillei we took advantage of the fact that each, along with genera and species closely related to them, produce more than one type of starch grain, resulting in taxon-unique starch population signatures when a multiple grain analysis is undertaken. Archaeological sample sizes from individual teeth often permitted such an analysis, and these population signatures in addition to the occurrence of diagnostic, individual grain types robustly indicate the presence of these four major taxa (Figs. S1–S5).
We encountered partially and completely gelatinized grains, presumably a result of cooking, on many of the teeth. Some were identifiable because they retained their shape and surface characteristics, and some still exhibited an extinction cross. Recognition of these grains was based on cooking experiments we carried out (below) and other research (34–36).
Cooking Experiments.
We cooked whole common and lima beans by placing them in a test tube and then into a hot water bath for times ranging from 15 to 45 min. After 15 min many grains expanded to three times their normal size, lost their lamellae and fissures, and exhibited no or damaged extinction crosses. However, many retained their characteristic shape, size, and surface attributes and their extinction crosses, showing no signs of damage (Fig. S3 a and b). Additionally, a small proportion of damaged grains still exhibited the characteristic oval to kidney Phaseolus shape and had lamellae and remnant fissures. The majority of starch grains in samples that were cooked for 45 min lost lamellae and fissures, but many retained the characteristic Phaseolus shapes, and lamellae and remnant fissures were still visible on a few (Fig. S3c). Most still polarized, exhibiting the diffuse extinction cross arms that are characteristic of heat damage (Fig. S3d). Cooked grains that retain Phaseolus shapes and have lamellae or remnant fissures are recognizable as Phaseolus, and they routinely occurred on the teeth (Fig. S4 a–d).
We made comparisons of starch grain size in uncooked and cooked beans, in the latter measuring only grains that were recognizable as Phaseolus (Table S3). Cooked grains were either marginally larger or smaller than uncooked specimens. Our analysis of roasted and unroasted peanuts indicates that starch from roasted specimens does not suffer morphological damage or size alteration, presumably because the hull offers a good deal of protection. We expect we will achieve a better understanding of these issues when experiments are carried out using cooking methods more analogous to prehistoric techniques, such as pit roasting and hot rock boiling. Nonetheless, it is clear that starch grains from cooked foods are surviving in an identifiable form on ancient human teeth. They have also been recovered from blackened food residues in Early Formative ceramic cooking vessels from Ecuador and stone tools from Ecuador and New Guinea (34–36).
Supplementary Material
Acknowledgments.
This research was supported by the National Museum of Natural History, Washington, DC, Smithsonian Tropical Research Institute (Balboa, Panama), National Science Foundation, University of Kentucky, Vanderbilt University, Earthwatch, Guggenheim Foundation, National Geographic Society, and the Fulbright Commission. We thank the Instituto Nacional de Cultura of Peru for permits to carry out the work and International Center for Tropical Agriculture (CIAT), Cali, Colombia and David Williams for providing many of the modern Phaseolus and peanut samples, respectively. We are grateful to our colleagues Jack Rossen, John Verano, and Sonia Guillen who provided unpublished data, and Patricia Netherly, Greg Maggard, and Kary Stackelbeck who assisted with the research. We thank three anonymous reviewers who provided helpful comments on the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808752105/DCSupplemental.
References
- 1.Dillehay TD, Rossen J, Andres TC, Williams DE. Preceramic adoption of peanut, squash, and cotton in northern Peru. Science. 2007;316:1890–1893. doi: 10.1126/science.1141395. [DOI] [PubMed] [Google Scholar]
- 2.Dillehay TD, Eling HH, Jr, Rossen J. Preceramic irrigation canals in the Peruvian Andes. Proc Natl Acad Sci USA. 2007;102:17241–17244. doi: 10.1073/pnas.0508583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillehay TD, Netherly PJ, Rossen J. Middle preceramic and residential sites on the forested slopes of the western Andes, northern Peru. Am Antiq. 1989;54:733–759. [Google Scholar]
- 4.Dillehay TD, Rossen J, Maggard G, Stackelbeck K, Netherly P. Localization and possible social aggregation in the Late Pleistocene and Early Holocene on the north coast of Peru. Quat Inter. 2003;109–110:3–11. [Google Scholar]
- 5.Dillehay TD, Rossen J, Netherly PJ. The Ñanchoc tradition: The beginnings of Andean civilization. Am Sci. 1997;85:46–55. [Google Scholar]
- 6.Rossen J, Dillehay TD. Ancient cultigens or modern intrusions?: Evaluating plant remains in an Andean case study. J Archaeol Sci. 1996;23:391–407. [Google Scholar]
- 7.Dillehay TD, Rossen J, Netherly PJ. Middle preceramic household, ritual, and technology in northern Peru. Chungara. 1999;30:111–124. [Google Scholar]
- 8.Maquet A, Vekemans X, Baudoin J-P. Phylogenetic study on wild allies of Lima bean, Phaseolus lunatus (Fabaceae), and implications on its origin. Plant Syst Evol. 1999;218:43–54. [Google Scholar]
- 9.Caicedo AL, Gaitán MC, Duque P, Toro O, Debouck DG, Tohme J. AFLP fingerprinting of Phaseolus lunatus L. and related wild species from South America. Crop Sci. 1999;39:1497–1507. [Google Scholar]
- 10.Fofana B, du Jardin P, Baudoin JP. Genetic diversity in the Lima bean (Phaseolus lunatus L. ) as revealed by chloropast DNA (cpDNA) variations. Genet Resour Crop Evol. 2001;48:437–445. [Google Scholar]
- 11.Chacón SMI, Pickersgill B, Debouck DG. Domestication patterns in common bean (Phaseolus vulgaris L. ) and the origin of the Mesoamerican and Andean cultivated races. Theor Appl Genet. 2005;110:432–444. doi: 10.1007/s00122-004-1842-2. [DOI] [PubMed] [Google Scholar]
- 12.Fofana B, Baudoin JP, Vekemans X, Debouck DG, du Jardin P. Molecular evidence for an Andean origin and a secondary gene pool for the Lima bean (Phaseolus lunatus L. ) using chloroplast DNA. Theor Appl Genet. 1999;98:202–212. [Google Scholar]
- 13.Kaplan L, Lynch TF. Phaseolus (Fabaceae) in archaeology: AMS radiocarbon dates and their significance for pre-Columbian agriculture. Econ Bot. 1999;53:262–272. [Google Scholar]
- 14.Sanjur O, Piperno DR, Andres TC, Wessel-Beaver L. Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitochondrial gene: Implications for crop plant evolution and areas of origin. Proc Natl Acad Sci USA. 2002;99:535–540. doi: 10.1073/pnas.012577299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milla SR, Isleib TG, Stalker HT. Taxonomic relationships among Arachis sect. Arachis species as revealed by AFLP markers. Genome. 2005;48:1–11. doi: 10.1139/g04-089. [DOI] [PubMed] [Google Scholar]
- 16.Bertoti de Cunha F, et al. Genetic relationships among Arachis hypogaea L. (AABB) and diploid Arachis species with AA and BB genomes. Genet Resour Crop Evol. 2008;55:15–20. [Google Scholar]
- 17.Piperno DR, Pearsall DM. The Origins of Agriculture in the Lowland Neotropics. San Diego: Academic; 1998. [Google Scholar]
- 18.Solis RS, Haas J, Creamer W. Dating Caral, a preceramic site in the Supe Valley on the central coast of Peru. Science. 2001;292:723–726. doi: 10.1126/science.1059519. [DOI] [PubMed] [Google Scholar]
- 19.Haas J, Creamer W, Ruiz A. Dating the Late Archaic occupation of the Norte Chico region in Peru. Nature. 2004;432:1020–1023. doi: 10.1038/nature03146. [DOI] [PubMed] [Google Scholar]
- 20.Lost Crops of the Incas. Washington, DC: National Academy Press; 1989. [Google Scholar]
- 21.Piperno DR. In: Documenting Domestication. Zeder M, Bradley DG, Emshwiller E, Smith BD, editors. Berkeley: Univ of California Press; 2006. pp. 46–67. [Google Scholar]
- 22.Fritz GJ. Gender and the early cultivation of gourds in eastern North America. Am Antiq. 1999;64:417–429. [Google Scholar]
- 23.Hart JP, Daniels RA, Sheviak CJ. Do Cucurbita pepo gourds float fishnets? Am Antiq. 2004;69:141–148. [Google Scholar]
- 24.Piperno DR, Ranere AJ, Holst I, Hansell P. Starch grains reveal early root crop horticulture in the Panamanian tropical forest. Nature. 2000;407:894–897. doi: 10.1038/35038055. [DOI] [PubMed] [Google Scholar]
- 25.Piperno DR. In: Behavioral Ecology and the Transition to Agriculture. Kennett DJ, Winterhalder B, editors. Berkeley: Univ of California Press; 2006. pp. 137–166. [Google Scholar]
- 26.Iriarte J. In: Rethinking Agriculture. Denham T, Iriarte J, Vrydaghs L, editors. Walnut Creek, CA: West Coast Press; 2008. pp. 167–188. [Google Scholar]
- 27.Weiss E, Kislev ME, Hartmann A. Autonomous cultivation before domestication. Science. 2006;312:1608–1610. doi: 10.1126/science.1127235. [DOI] [PubMed] [Google Scholar]
- 28.Fuller D. Contrasting patterns in crop domestication and domestication rates: Recent archaeological insights from the Old World. Annals Bot. 2007;100:903–924. doi: 10.1093/aob/mcm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry AG, Piperno DR. Using plant microfossils from dental calculus to recover human diet: A case study from Tell al-Raqa'i, Syria. J Archaeol Sci. 2008;35:1943–1950. [Google Scholar]
- 30.Reichert ET. The Differentiation and Specificity of Starches in Relation to Genera, Species, etc. Washington, DC: Carnegie Institution of Washington; 1913. [Google Scholar]
- 31.Torrence R, Barton H, editors. Ancient Starch Research. Walnut Creek, CA: Left Coast Press; 2006. [Google Scholar]
- 32.Dickau R, Ranere AJ, Cooke RG. Starch grain evidence for the preceramic dispersals of maize and root crops into tropical dry and humid forests of Panama. Proc Natl Acad Sci USA. 2007;104:3651–3656. doi: 10.1073/pnas.0611605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearsall DM, Chandler-Ezell K, Zeidler JA. Maize in ancient Ecuador: Results of residue analysis of stone tools from the Real Alto site. J Archaeol Sci. 2004;31:423–442. [Google Scholar]
- 34.Zarillo S, Pearsall DM, Raymond JS, Tisdale MA, Quon DJ. Directly dated starch residues document early formative maize (Zea mays L.) in tropical Ecuador. Proc Natl Acad Sci USA. 2008;105:5005–5111. doi: 10.1073/pnas.0800894105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandler-Ezell K, Pearsall DM, Zeidler J. Root and tuber phytoliths and starch grains document manioc (Manihot esculenta), arrowroot (Maranta arundinacea), and llerén (Calathea sp. ) at the Real Alto site, Ecuador. Econ Bot. 2006;60:103–120. [Google Scholar]
- 36.Barton H. Starch residues on museum artifacts: Implications for determining tool use. J Archaeol Sci. 2007;34:1752–1762. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.