Abstract
Treatment of neuropathic pain is a major clinical problem. This study shows expression of phospholipase ß3 (PLCß3) in mouse and human DRG neurons, mainly in small ones and mostly with a nonpeptidergic phenotype. After spared nerve injury, the pain threshold was strongly reduced, and systemic treatment of such animals with the unselective PLC inhibitor U73122 caused a rapid and long-lasting (48-h) increase in pain threshold. Thus, inhibition of PLC may provide a way to treat neuropathic pain.
Keywords: galanin receptor 2, nerve injury, neuropeptide, pain treatment, sensory neuron
The phospholipase C (PLC) family consists of several isoforms, such as PLCβ, γ, δ, and ε, which are linked to membrane receptors mediating intracellular signaling cascades (1–3). PLC has been demonstrated in dorsal root ganglion (DRG) neurons (4–6). Of the 4 major PLCβ isoforms, PLCβ1, -β3, and -β4 expressed in DRGs, the PLCβ3 transcript shows the clearly highest levels (5).
Involvement of PLCβ3 in regulation of pain and related sensations at the spinal level has been demonstrated in several studies. For example, PLCβ3−/− mice show enhanced morphine responsiveness (7) and have a deficient scratching (“itching”) behavior (5). Bradykinin- and nerve growth factor-induced hypersensitivity involves PLCβ3 activation (8), and PLCβ3 is important for PKC2-mediated acute and chronic inflammatory pain (6). Other isoforms of PLCβ have also been associated with pain. Thus, there is evidence that PLCβ1 is involved in the thermal nociceptive response (10), and PLCβ4−/− mice show attenuated nociceptive behavior in the second phase of the formalin test, resulting from the tissue inflammation (11). Moreover, inhibition of PLC has been shown to attenuate acute and chronic inflammatory hyperalgesia (9).
In the present study, we have monitored pain thresholds and the effect of an unselective PLC inhibitor (U73122) in the spared nerve injury (SNI) model of neuropathic pain in mouse (12, 13). In parallel we have analyzed the localization of PLCß3 and a number of transmitter related markers in DRGs and spinal cord and the effect of SNI. Human DRGs were also studied.
Results
SNI-Induced Hyperalgesia.
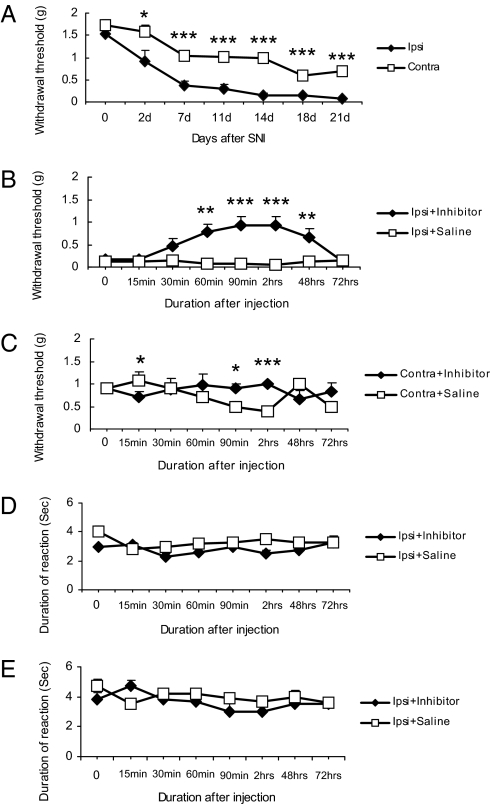
After SNI, mice developed mechanical allodynia-like behavior as shown by the decrease in withdrawal threshold of the hindpaw ipsilateral to the nerve injury. This was seen 2 days after the surgery, with a pronounced effect between 7 and 21 days (Fig. 1A). A decrease, albeit less pronounced, was also seen in the contralateral hindpaw between day 7 and 21 after nerve injury (Fig. 1A).
Fig. 1.
The PLC inhibitor U73122 (30 mg/kg) increases pain threshold after SNI. (A) Time course of mechanical threshold measured by the von Frey hair filaments after unilateral SNI (n = 12). Ipsilateral hindpaw displays a strong and long-lasting reduction in threshold with a less pronounced decrease contralaterally. (B) Fourteen days after SNI, a single dose of U73122 causes a long-lasting, ipsilateral increase in mechanical threshold as compared with saline-treated mice (n = 6 per group). (C) The transient effects of inhibitor on mechanical threshold is also seen in the contralateral paw compared with saline-treated group 14 days after SNI (n = 6 per group). (D) Pin-prick test after SNI. (E) Cold test after SNI. The withdrawal response duration (in seconds) after nociceptive mechanical stimulation or cold stimulation (acetone) is not changed in inhibitor-treated group (n = 6) compared with saline-treated group (n = 6). Data are expressed as mean ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with the vehicle-treated group.
Effects of a PLC Inhibitor.
Gross examination revealed that U73122 (30 mg/kg, i.p.) neither caused sedation nor impaired motor function when compared with vehicle-treated animals (data not shown). When given 14 days after SNI, U73122 (30 mg/kg, i.p.) significantly increased ipsilateral withdrawal threshold 60 min after injection, as monitored with mechanical stimulation with von Frey hairs (n = 6; **, P < 0.01, compared with vehicle), an effect still observed after 48 h, but returning to vehicle levels at 72 h (Fig. 1B). A small but significant effect was seen contralaterally at 90 and 120 min (n = 6; *, P < 0.05; ***, P <0.001; compared with vehicle) (Fig. 1C). Also, when given 18 days after SNI, U73122 significantly affected withdrawal threshold (Fig. S1a), both ipsi- and contralaterally (Fig. S1b). Pin-prick hyperalgesia and cold allodynia were examined 14 days after SNI. The withdrawal response duration (in seconds) after nociceptive mechanical stimulation or cold stimulation were not significantly different, when comparing inhibitor-treated and saline-treated groups (Fig. 1 D and E).
Expression of PLCβ3-LI in DRGs.
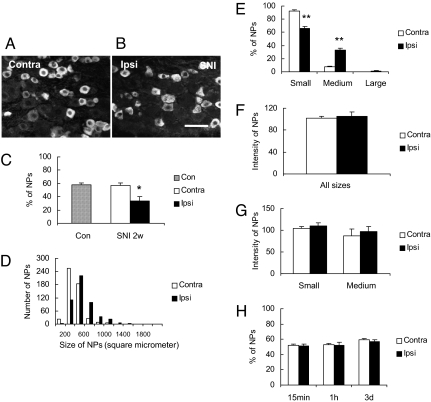
In normal mouse DRGs, ≈60% of all neuron profiles (NPs) were PLCβ3 immunoreactive (IR) (Fig. 2 A and C), with a range of 100–1,200 μm2 (majority 200–600 μm2) (Fig. 2D), that is mainly representing small neurons. After SNI there was a significant decrease in the percentage of PLCβ3-IR NPs in the ipsilateral DRGs (Fig. 2 B vs. A and C). Two weeks after SNI ≈35% of all NPs were stained, whereas no change could be seen in the contralateral DRGs when compared with controls (Fig. 2C). The analysis of the size distribution of PLCβ3-IR NPs 2 weeks after SNI revealed a shift within the category of medium-sized NPs, that is there was a higher proportion of PLCβ3-IR, medium-sized NPs in the ipsilateral DRGs as compared with contralateral ones, and this change was significant (**, P < 0.01, Fig. 2E). The intensity of PLCβ3-like immunoreactivity (LI) (fluorescence levels) in NPs did not change in ipsilateral as compared with contralateral DRGs (Fig. 2F), neither when considering all PLCβ3-IR NPs nor in subpopulations, such as small vs. medium-sized PLCβ3-IR NPs (Fig. 2G). After ipsilateral injection of carrageenan into the hind paw no significant change could be seen at any time interval (15 min, 1 h or 3 days) (Fig. 2H).
Fig. 2.
Expression of PLCβ3-LI in DRGs. (A and B) Immunofluorescence micrographs showing PLCβ3-IR neurons in contra- (A) and ipsilateral DRGs (B) 14 days after SNI. (C) Percentage of PLCβ3-IR NPs in control DRGs and 2 weeks after SNI. The lesion causes an almost 50% decrease. (D) Size distribution of PLCβ3-IR NPs in contra- or ipsilateral DRGs 2 weeks after SNI (500 NPs were measured in each group). There is a trend toward expression of PLCß3 in larger NPs after lesion. (E) Proportion of PLCβ3-IR NPs in small (<600 μm2), medium (600–1,400 μm2), or large (>1,400 μm2) NPs. (F and G) Immunofluorescence levels (intensity) of PLCβ3-IR NPs in contra- or ipsilateral DRG neurons of different size categories [all sizes (E); small <600 μm2 and medium-sized 600-1400 μm2 (F)] 2 weeks after SNI. No significant effects are seen. (H) Percentage of PLCβ3-IR NPs in the contra- and ipsilateral DRGs after carrageenan injection. No significant effects are seen. Error bars represent standard error of the mean (SEM). Significant differences are indicated by *, P < 0.05; **, P < 0.01 compared with contralateral DRGs. (Scale bar: 50 μm, A–B.)
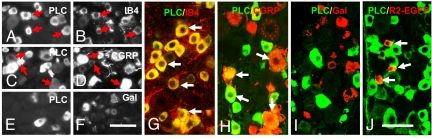
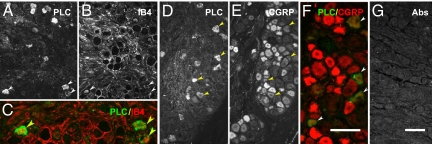
In normal control DRGs a high proportion (>80%) of PLCβ3-IR NPs expressed isolectin B4 (IB4), a neuronal marker mostly present in nonpeptidergic neurons (Fig. 3A, B, and G and Fig. S2a). Approximately 40% of the PLCβ3-IR NPs were colocalized with calcitonin gene-related peptide (CGRP)-LI (Fig. 3 C, D, and H; Fig. S2a), an accepted marker for peptidergic neurons. Two weeks after SNI a very small proportion (≈5%) of the PLCβ3-IR NPs expressed galanin (Fig. 3 E, F, and I; Fig. S2a), a peptide that is strongly up-regulated after nerve injury, mainly in small and medium-sized neurons. In ipsilateral DRGs, 80% and 55% of the PLCß3-IR NPs were IB4- and CGRP-positive, respectively, and conversely IB4- (80%) and CGRP- (55%) positive NPs were PLCß3-IR (Fig. S2b). In a mouse with a galanin receptor 2 (GalR2)-EGFP construct (14) GalR2-positive neurons expressed PLCß3-LI (Fig. 3J).
Fig. 3.
Expression of PLCβ3 in several neuronal subpopulations in mouse DRGs. Shown are immunofluorescence micrographs of control (A–D, G, H, J) or ipsilateral DRGs (E, F, and I) 2 weeks after SNI, incubated with antiserum to PLCβ3 (A, C, E, G–J), CGRP (D and H), galanin (F and I), or EGFP (J) or stained for IB4 (B and G). A and B, C and D, and E and F show, respectively, the same section. Arrows indicate coexistence of PLCβ3 with IB4 (A, B, and G), CGRP (C, D, and H), or GalR2-EGFP (J), most pronounced for PLCß3 plus IB4 (A, B, and G). G–J are merged sections from double-staining. [Scale bars: 50 μm, A–F; G–J.]
Expression of PLCβ3 in Spinal Cord.
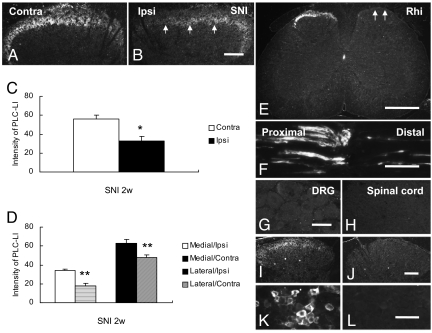
PLCβ3-LI was present in a dense fiber plexus in the superficial layers, mainly lamina II, in the contralateral dorsal horn of the L4–5 segments (Fig. 4A). No PLCß3-IR cell bodies could be detected. SNI induced an ipsilateral reduction (Fig. 4 B vs. A and C), but more so in the medial than in the lateral dorsal horn (*, P < 0.05; **, P < 0.01; Fig. 4 B vs. A and 4D). In contrast, no obvious differences were observed at any time interval (15 min, 1 h or 3 days) after carrageenan injection (Fig. S3). In the lumbar spinal cord, dorsal rhizotomy induced a virtually complete ipsilateral depletion of PLCβ3-LI in the dorsal horn (Fig. 4E). Eight hours after a nerve crush, PLCβ3-LI had accumulated around the lesion, but mainly on the proximal side (Fig. 4F).
Fig. 4.
Expression of PLCβ3 in spinal cord, sciatic nerve, and control experiments. (A and B) Immunofluorescence micrographs of PLCβ3-LI in lumbar spinal cord 2 weeks after SNI. PLCβ3-IR fibers are mainly located in lamina II of the contralateral dorsal horn (A), with a strong ipsilateral reduction after SNI (B, arrows). No PLCβ3-IR cell bodies can be seen. (C) Quantitative evaluation of spinal dorsal horn shows a significant reduction of PLCβ3-LI (gray levels) 2 weeks after SNI (*, P < 0.05 compared with contralateral side). (D) SNI results in reduction of PLCβ3-LI (gray levels) in both lateral and medial portions of ipsilateral lamina I-II. (E) PLCβ3-LI is strongly reduced ipsilaterally 2 weeks after dorsal rhizotomy (Rhi) (arrows). (F) PLCβ3-LI strongly accumulates on the proximal side of a crush. (G and H) After incubation with control serum, no fluorescent positive neurons or fibers can be observed, in neither the DRG (G) nor spinal dorsal horn (H). (I–L) PLCβ3 signal is absent in dorsal horn (J) and DRG (L) of PLCß3−/− mouse as compared with wild type mouse (I and K). Significant differences are indicated by *, P < 0.05; **, P < 0.01 compared with contralateral side. (Scale bars: 100 μm, A–B; G–H; I–J; K–L).
Expression of PLCβ3 in Human DRGs.
In general, all three markers analyzed (PLCß3: 17% of DRG NPs; IB4: 15%; CGRP: 60%) were found to be expressed in human DRGs. Thus, PLCβ3-LI was mainly found in small-sized neurons (Fig. 5A, C, D, and F). Moreover, 70% of PLCß3-IR NPs were IB4-positive (Fig. 5 A–C), and almost all PLCβ3-IR NPs were CGRP-IR, whereas only 20% of CGRP-IR NPs expressed PLCβ3 (Fig. 5 D–F).
Fig. 5.
Expression of PLCβ3 in human DRG and double-staining experiments as well as absorption control. (A–F) PLCβ3-IR cell bodies are present in human DRGs (A and D) and express IB4 (A–C) or CGRP (D–F). Arrowheads indicate coexistence between PLCβ3 and IB4 (C) or CGRP (F), respectively. (G) After incubation with control serum, no fluorescent neurons can be observed. (Scale bars: 100 μm, A–B–D–E– G; C–F).
Control Experiments.
Preabsorption of the PLCβ3 antiserum with the immunogenic PLCβ3 peptide caused a complete disappearance of all staining patterns described above, including DRGs (Fig. 4G) and spinal dorsal horn (Fig. 4H). Furthermore, the analysis of DRGs and spinal cord of PLCß3−/− mice (15) revealed a complete absence of the PLCß3 signal when compared with wild type mice (Fig. 4 I and K vs. J and L). Also the PLCß3 staining in human DRGs could not be seen after incubation with PLCß3 antiserum preabsorbed with the immunogenic peptide (Fig. 5 G vs. A and D).
Discussion
The present results strongly suggest that PLC plays an important role in neuropathic pain. Thus, when the unselective PLC inhibitor U73122 is given as a single dose 2 weeks after SNI, the threshold is increased within 60 min and remains elevated for 48 h. However, no effect of the inhibitor could be detected in the pin-prick test for hyperalgesia, or after cold stimulation (acetone), suggesting modality specificity.
The antinociceptive effect of U73122 is in agreement with a study by Galeotti et al. (16) showing that this PLC inhibitor dose-dependently prevents the thermal hypernociception monitored in the hot plate test induced by a very low dose of morphine. Moreover, inhibition of PLCβ3 by local injection of U73122 into the hind paw has been shown to attenuate acute and chronic inflammatory hyperalgesia induced by unilateral carrageenan injection at the same site (9). These models clearly differ from the SNI used in the present study, which monitors neuropathic pain (12, 13) and applies the inhibitor systemically. In fact, in our study no effect of U73122 was seen in the thermal (cold) test. The apparent pronociceptive effect of PLCß3 was also evident in studies on the role of this enzyme in μ opiate-mediated responses. Thus, mice lacking PLCβ3 exhibit an up to 10-fold decrease in the ED50 value for morphine in producing antinociception, providing the first evidence that PLCß3 is “pronociceptive” (7). These mice also present an attenuated histamine-induced scratching behavior mediated by a subset of C-fiber nociceptors expressing histamine H1 receptor and PCLβ3 (5).
The site(s) and mechanism(s) of action remains to be established. With regard to site, the study by Joseph et al. (6) suggests that DRG neurons could be one target, since not only hindpaw injection of U73122, but also intrathecal injection of PLCβ3 antisense ODN, reduce hyperalgesia. In addition to transduction via many other G-protein-coupled receptors, including the just-mentioned opiate receptors, also galanin (17) could be involved. This neuropeptide exerts its action via 3 G-protein-coupled receptors, GalR1-R3 (18, 19), and may, like opioid peptides, represent an endogenous analgesic molecule (20). Galanin's expression in DRG neurons is dramatically increased after peripheral nerve injury (21), mediating antinociception probably via GalR1 (20). However, galanin has also pronociceptive actions (21–23), possibly via GalR2 and enhancement of release of excitatory transmitter(s) from primary afferent nerve terminals in the dorsal horn (24). This may be mediated by an intracellular pathway involving PLC and Ca2+ mobilization (19). GalR2 is found in many rat (25) and in mouse (present results) DRG neurons, as is also PLCß3, both in rat (4, 6) and, as shown here, in mouse and human. Thus, it may be speculated that pronociception through GalR2 involves PLC, and that inhibition of this enzyme contributes to the strong and long-lasting antinociception by the PLC inhibitor.
The present results also show that in the mouse, a population of mainly small, mostly IB4-positive, less often CGRP-IR DRG neurons express PLCβ3, confirming a study by Han et al. (5). However, hardly any coexistence was seen between PLCß3 and galanin after SNI. This is probably because galanin is up-regulated in those lesioned neurons in which PLCß3 has been down-regulated. There is a dense PLCß3-IR fiber network mainly in lamina II of the dorsal horn, which disappears after dorsal rhizotomy. The enzyme is also transported into the peripheral branches of DRG neurons, in agreement with the Western blot analysis of Han et al. (5). PLCß3 could in addition be visualized in human DRG neurons. Other studies have shown a similarly frequent occurrence in rat DRGs (4, 6). Thus, the enzyme is present, and could be active, in all parts of the DRG neurons of several species.
Two weeks after SNI, but not after inflammation, the percentage of PLCβ3-IR neurons was significantly decreased (by 50%), as was the PLCβ3-LI in the superficial dorsal horn, in a similar manner as shown for thiamine monophosphatase staining in the dorsal horn after various types of SNI (26). These findings suggest that peripheral nerve injury reduces PLCβ3 levels in all parts of DRG neurons. No change in PLCß3 levels in DRG neurons were analyzed in any of the other published immunohistochemical/in situ hybridization studies (5, 6).
It is, however, important to note that in the SNI model the sural nerve, and thus the DRG neurons projecting into this nerve and into spinal cord, have been spared (12). Our results show that PLCβ3-positive fibers remain in the lateral dorsal horn, that is the projection territory of the sural nerve as shown with thiamine monophosphatase staining after SNI (26). Thus, it is likely that PLCβ3 levels in the sural population of DRG neurons are unchanged and that these neurons are targets for the PLC inhibitor.
The present study also shows that a similar PLCβ3 mechanism may operate in human ganglia. With regard to galanin, its transcript is normally present in ≈10–15% of all human DRG NPs (27), as also seen here, whereas little is known about the expression of the galanin receptors. Therefore, a key question for understanding a potential role of PLCβ3 in pain and in GalR2 transduction in human DRGs is to what extent GalR2 is expressed in human ganglia.
Taken together, the present results suggest that inhibition of PLC isoforms may offer a new and efficacious treatment of neuropathic pain, which still is a major clinical problem (28, 29).
Materials and Methods
Animals and Human Tissue.
Male C57BL/6J mice were used. For control, a PLCβ3−/− mouse was examined. Human DRGs were harvested from children with obstetric brachial plexus lesions who underwent reconstructive nerve surgery. The studies were approved by local Ethical Committees, and parental consent had been obtained for the human DRGs.
Surgeries and Drugs.
Surgical procedures were performed under anesthesia with isoflurane. Unilateral, SNI was made as described by Decosterd et al. (12), and survival times were 14 and 21 days. For dorsal rhizotomy, animals were anesthetized, and the left L4 to L6 dorsal roots were transected; survival time was 14 days. Intraaxonal transport was studied 8 h after compression of the sciatic nerve carried out under anesthesia. The effect of inflammation was studied in animals receiving an injection of carrageenan into the plantar surface of the left hindpaw, and survival for 15 min, 1 h and 3 days. The PLC inhibitor U73122 (Tocris) (30 mg/kg, dissolved in 0.5% DMSO) (9) was administered i.p. as a single dose 14 or 18 days after SNI. The animals were tested 15 min after injection.
Behavioral Tests.
Mechanical allodynia was tested in transparent plastic domes on a metal mesh floor, and the threshold for paw withdrawal (both ipsi- and contralateral side) was measured by graded-strength von Frey monofilaments to assess mechanical allodynia (12, 13, 26). For mechanical hyperalgesia (pin- prick test), a safety pin, was used, and the duration of paw withdrawal was recorded (30). Cold allodynia was tested with a drop of acetone solution, and the duration of the withdrawal response was recorded (31).
Immunohistochemistry and Quantifications.
Animals were deeply anesthetized and transcardially perfused with picric acid-formalin. The L5 DRGs and L4-L5 segments of spinal cord were dissected and cut in a cryostat. The sections were processed using a commercial kit (TSA Plus; NEN Life Science Products), that is, incubated with guinea pig anti-PLCβ3 antiserum (15) (1: 4,000). Double-staining experiments were carried out for CGRP, galanin, isolectin B4 (IB4) from Griffonia Simplicifolia I (GSA I), or GFP. The sections were analyzed in a confocal scanning microscope. The human tissue was immersion-fixed in formalin, rinsed in 10% buffered sucrose, sectioned and processed for immunohistochemistry as described above.
The percentage of PLCß3-IR NPs were counted and the extent of their colocalization with CGRP-, galanin- or IB4-LI. The relative PLCß3 fluorescence levels (intensity) was measured in DRGs and spinal dorsal horn (lamina I-II), and the size of PLCβ3-IR NPs (small, medium-sized and large).
Statistics.
Student's t test and the Kruskal–Wallis ANOVA test (one-way ANOVA on ranks) was used for the comparison of data among groups. P < 0.05 was chosen as the significant level.
More information is available in SI Material and Methods.
Supplementary Material
Acknowledgments.
We thank Professor Lars Terenius (Center for Molecular Medicine, Karolinska Institutet, Stockholm) and Professor Elvar Theodorsson (Department of Clinical Chemistry, Linköping University, Linköping, Sweden) for their generous donations of CGRP and galanin antiserum, respectively. This work was supported by Swedish Medical Research Council Grant 04X-2887, the Marianne and Marcus Wallenberg Foundation and the Knut and Alice Wallenberg Foundation. We thank Drs. Isabella Decosterd (Lausanne University Hospital, Lausanne, Switzerland) and Camilla I. Svensson (Karolinska Institutet) for valuable advice.
Note added in proof.
Double-staining with antiserum to activating transcription factor 3 (ATF3), a marker for lesioned DRG neurons (32), revealed that, 14 days after SNI, all PLCβ3-positive NPs lacked ATF3-LI. Conversely, ATF3 staining was never associated with PLCβ3-LI. This supports the assumption that only unlesioned neurons projecting into the sural nerve are likely to be affected by the PLC inhibitor U73122.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810899105/DCSupplemental.
References
- 1.Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu Rev Pharmacol Toxicol. 2006;46:355–379. doi: 10.1146/annurev.pharmtox.46.120604.141223. [DOI] [PubMed] [Google Scholar]
- 4.Lagercrantz J, Piehl F, Nordenskjöld M, Larsson C, Weber G. Expression of the phosphoinositide-specific phospholipase Cbeta3 gene in the rat. Neuroreport. 1995;6:2542–2544. doi: 10.1097/00001756-199512150-00022. [DOI] [PubMed] [Google Scholar]
- 5.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Xie W, et al. Genetic alteration of phospholipase C beta3 expression modulates behavioral and cellular responses to mu opioids. Proc Natl Acad Sci USA. 1999;96:10385–10390. doi: 10.1073/pnas.96.18.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang HH, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 9.Hou C, et al. In vivo activity of a phospholipase C inhibitor, 1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole -2,5-dione (U73122), in acute and chronic inflammatory reactions. J Pharmacol Exp Ther. 2004;309:697–704. doi: 10.1124/jpet.103.060574. [DOI] [PubMed] [Google Scholar]
- 10.Liu NJ, vonGizycki H, Gintzler AR. Phospholipase Cbeta1 modulates pain sensitivity, opioid antinociception and opioid tolerance formation. Brain Res. 2006;1069:47–53. doi: 10.1016/j.brainres.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 11.Miyata M, et al. Role of thalamic phospholipase Cβ4 mediated by metabotropic glutamate receptor type 1 in inflammatory pain. J Neurosci. 2003;23:8098–8108. doi: 10.1523/JNEUROSCI.23-22-08098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 13.Bourquin AF, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Xia S, et al. Visualization of a functionally enhanced GFP-tagged galanin R2 receptor in PC12 cells: Constitutive and ligand-induced internalization. Proc Natl Acad Sci USA. 2004;101:15207–15212. doi: 10.1073/pnas.0406571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomura S, Fukaya M, Tsujioka T, Wu D, Watanabe M. Phospholipase Cbeta3 is distributed in both somatodendritic and axonal compartments and localized around perisynapse and smooth endoplasmic reticulum in mouse Purkinje cell subsets. Eur J Neurosci. 2007;25:659–672. doi: 10.1111/j.1460-9568.2007.05334.x. [DOI] [PubMed] [Google Scholar]
- 16.Galeotti N, Stefano GB, Guarna M, Bianchi E, Ghelardini C. Signaling pathway of morphine induced acute thermal hyperalgesia in mice. Pain. 2006;123:294–305. doi: 10.1016/j.pain.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Tatemoto K, Rökaeus Å, Jörnvall H, McDonald TJ, Mutt V. Galanin—a novel biologically active peptide from porcine intestine. FEBS. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 18.Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux JF. Molecular cloning of a functonal human galanin receptor. Proc Natl Acad Sci USA. 1994;91:9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharm Sci. 2000;21:109–116. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- 20.Xu X-J, Hökfelt T, Wiesenfeld-Hallin Z. Galanin and spinal pain mechanisms: Where do we stand in 2008. Cell Mol Lifes Sci. 2008;65:1813–1819. doi: 10.1007/s00018-008-8155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hökfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci Lett. 1987;83:217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- 22.Reeve AJ, Walker K, Urban L, Fox A. Excitatory effects of galanin in the spinal cord of intact, anaesthetized rats. Neurosci Lett. 2000;295:25–28. doi: 10.1016/s0304-3940(00)01576-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu HX, et al. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: Selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu HX, Hökfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci. 2002;23:468–474. doi: 10.1016/s0165-6147(02)02074-6. [DOI] [PubMed] [Google Scholar]
- 25.Kerekes N, Mennicken F, O'Donnell D, Hökfelt T, Hill RH. Galanin increases membrane excitability and enhances Ca2+ currents in adult, acutely dissociated dorsal root ganglion neurons. Eur J Neurosci. 2003;18:2957–2966. doi: 10.1111/j.1460-9568.2003.03057.x. [DOI] [PubMed] [Google Scholar]
- 26.Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: A behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 27.Landry M, et al. Galanin expression in adult human dorsal root ganglion neurons: Initial observations. Neuroscience. 2003;117:795–809. doi: 10.1016/s0306-4522(02)00965-x. [DOI] [PubMed] [Google Scholar]
- 28.Jensen TS, Finnerup NB. Management of neuropathic pain. Curr Opin Support Palliat Care. 2007;1:126–131. doi: 10.1097/SPC.0b013e3282eeb45f. [DOI] [PubMed] [Google Scholar]
- 29.Dray A. Neuropathic pain: Emerging treatments. Br J Anaesth. 2008;101:48–58. doi: 10.1093/bja/aen107. [DOI] [PubMed] [Google Scholar]
- 30.Decosterd I, et al. Intrathecal implants of bovine chromaffin cells alleviate mechanical allodynia in a rat model of neuropathic pain. Pain. 1998;76:159–166. doi: 10.1016/s0304-3959(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 31.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 32.Tsujino H, et al. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.